Published online Jan 26, 2023. doi: 10.12998/wjcc.v11.i3.669
Peer-review started: October 14, 2022
First decision: December 13, 2022
Revised: December 28, 2022
Accepted: January 9, 2023
Article in press: January 9, 2023
Published online: January 26, 2023
Processing time: 104 Days and 2 Hours
Heterotopic pregnancy (HP) is a rare condition in which both ectopic and intrauterine pregnancies occur. HP is uncommon after natural conception but has recently received more attention due to the widespread use of assisted reproductive techniques (ART) such as ovulation promotion therapy.
Here, we describe a case of HP that occurred after ART with concurrent tubal and intrauterine singleton pregnancies. This was treated successfully with surgery to preserve the intrauterine pregnancy, resulting in the birth of a low-weight premature infant. This case report aims to increase awareness of the possibility of HP during routine first-trimester ultrasound examinations, especially in pregnancies resulting from ART and even if multiple intrauterine pregnancies are present.
This case alerts us to the importance of comprehensive data collection during regular consultations. It is important for us to remind ourselves of the possibility of HP in all patients presenting after ART, especially in women with an established and stable intrauterine pregnancy that complain of constant abdominal discomfort and also in women with an unusually raised human chorionic gonadotropin level compared with simplex intrauterine pregnancy. This will allow symptomatic and timeous treatment of patients with better results.
Core Tip: Extrauterine pregnancies are collectively known as ectopic pregnancies. Heterotopic pregnancy (HP) is a rare type of ectopic pregnancy where both ectopic and intrauterine pregnancies occur. The more frequent use of assisted reproductive technologies (ART) leads to a rise in ectopic pregnancy, consequently leading to an increase in the incidence of HP. We report a case of HP that occurred after ART. Combined with the analysis of the cases indexed in PubMed, we concluded several possible factors related to the correlation. Symptomatic and timeous treatment of patients could lead to improved outcomes.
- Citation: Wang YN, Zheng LW, Fu LL, Xu Y, Zhang XY. Heterotopic pregnancy after assisted reproductive techniques with favorable outcome of the intrauterine pregnancy: A case report. World J Clin Cases 2023; 11(3): 669-676
- URL: https://www.wjgnet.com/2307-8960/full/v11/i3/669.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i3.669
Ectopic pregnancies occur in 1%–2% of all pregnancies. Heterotopic pregnancy (HP) is a rare type of ectopic pregnancy that involves the coexistence of both intrauterine and ectopic pregnancies. A recently estimated incidence of HP is about 1/30000[1] in spontaneous pregnancies, increasing to 1/360 to 1/100[2] in pregnancies resulting from assisted reproductive techniques (ART). ART can result in pelvic inflammatory disease which also contributes to HP. Here, we describe a case of HP after ART in a 26-year-old woman, and through a literature review of previous cases, we summarize the possible causes and related mechanisms accounting for the higher rate of HP after ART.
A 26-year-old Chinese woman presented to the gynecology clinic with a complaint that led to the suspicion of HP.
Suspicion of heterotopic pregnancy after in vitro fertilization and embryo transfer cycles (IVF-ET).
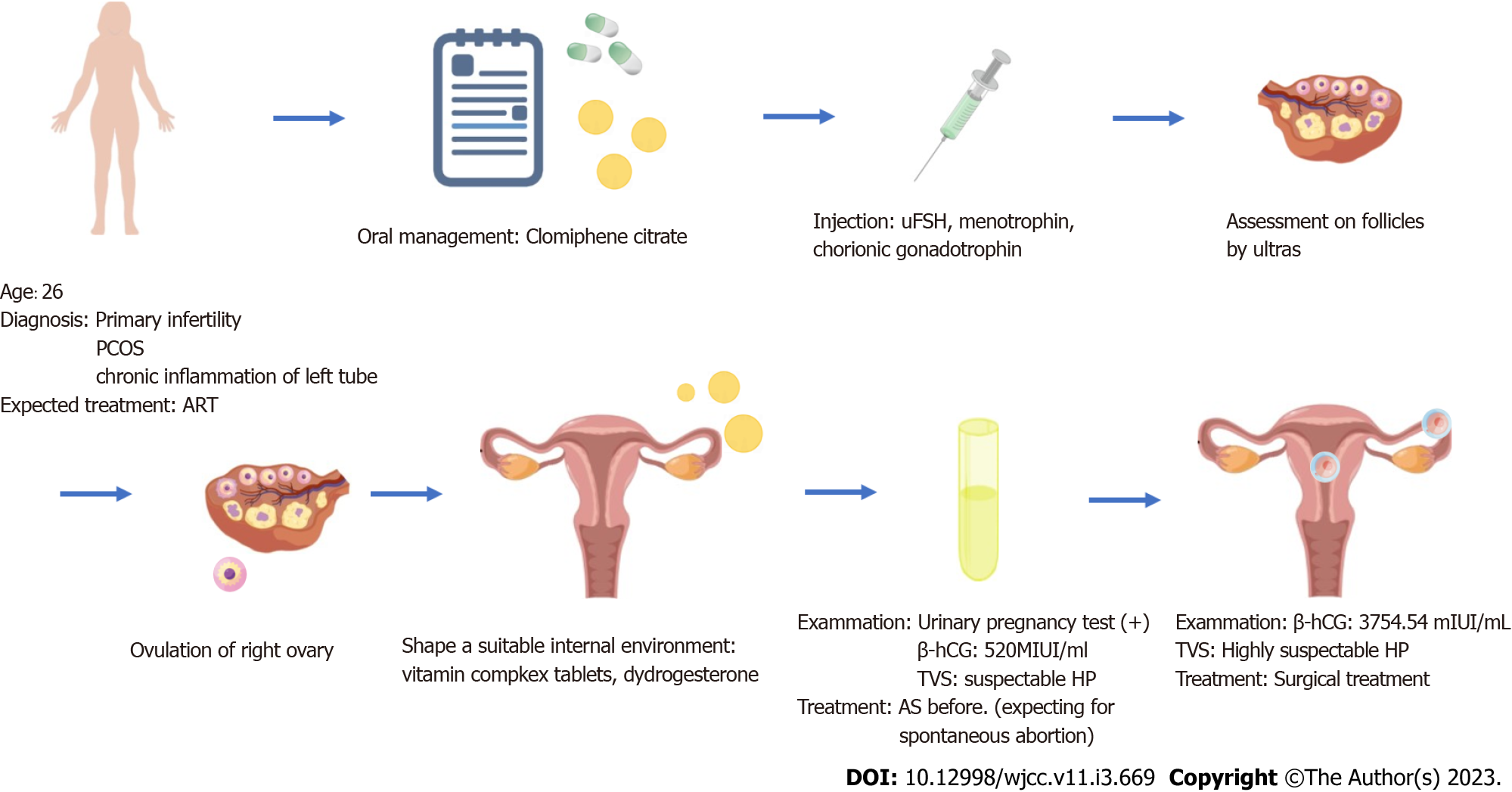
Three months before, the patient presented to our reproductive department with a complaint of infertility. After a detailed examination, she was diagnosed with primary infertility, polycystic ovary syndrome, and compound chronic inflammation of the left fallopian tube. Her husband’s semen analysis revealed mild asthenospermia. After obtaining their consent, ART was performed.
The patient was pretreated with oral medroxyprogesterone pills (2 mg per pill) given at a dose of 20 mg per day for seven days until the following menses. Controlled ovarian hyperstimulation (COH) was performed on the third day of the period with the use of clomiphene citrate pills (100 mg qd) for five days. After completion of this oral management, the COH was continued with urofollitropin for injection (uFSH) (75 units qd) for four days, with the addition of the same dose of menotrophin for the following four days. Follicle maturation was monitored by ultrasonography. After three days, instead of repeated COH by injection, the patient received further treatment with chorionic gonadotrophin given at a dose of 10000 units and it was suggested that she had sex on that day with the expectation of natural conception.
Two days later, ovulation of the right ovary was detected on the ultrasound scan, and we suggested that the patient take vitamin complex tablets from then onward as well as dydrogesterone starting two days hence to form a habitable place for a fetus and create a suitable internal environment to nurture a growing child.
The urinary pregnancy test was positive 15 days after ovulation and the serum human chorionic gonadotropin (hCG) level was 520 mIU/mL. A transvaginal ultrasound examination showed an intrauterine pregnancy with a 10-mm-thick endometrium at the C stage, with six follicles in the right ovary, four echoless regions in the left ovary, and mild pelvic effusion. Early intrauterine pregnancy with additional ectopic pregnancy in the right tube was strongly suspected due to the enhanced clinical clues. As the patient was strongly in favor of continuing the pregnancy, they decided to anticipate spontaneous abortion of the ectopic pregnancy and treated the intrauterine pregnancy as before.
Six days later, the follow-up serum hCG level was 3754.54 mIU/mL and transvaginal sonography reexamination showed an intrauterine singleton without a clearly visible yolk sac and multiple echoless areas in the bilateral adnexa. Finally, after another six days had passed, the detection of an intrauterine gestational sac (GS) of 14 mm × 6.0 mm, an embryonic bud of 3 mm, an embryonic heartbeat, and a GS of 9 mm × 7 mm with a yolk sac beside the right ovary confirmed the presence of HP. These findings combined with the clinical factors provided the main indications for surgery. (Figure 1) Having taken our advice, the patient (1 gravida, 0 para) presented to the gynecology clinic.
The patient denied any family history of malignant tumors.
On physical examination, the vital signs were as follows: Body temperature, 36.5℃; blood pressure, 115/85 mmHg; heart rate, 97 beats per min; respiratory rate, 19 breaths per min.
The human chorionic gonadotropin level was 20295.14 mIU/mL.
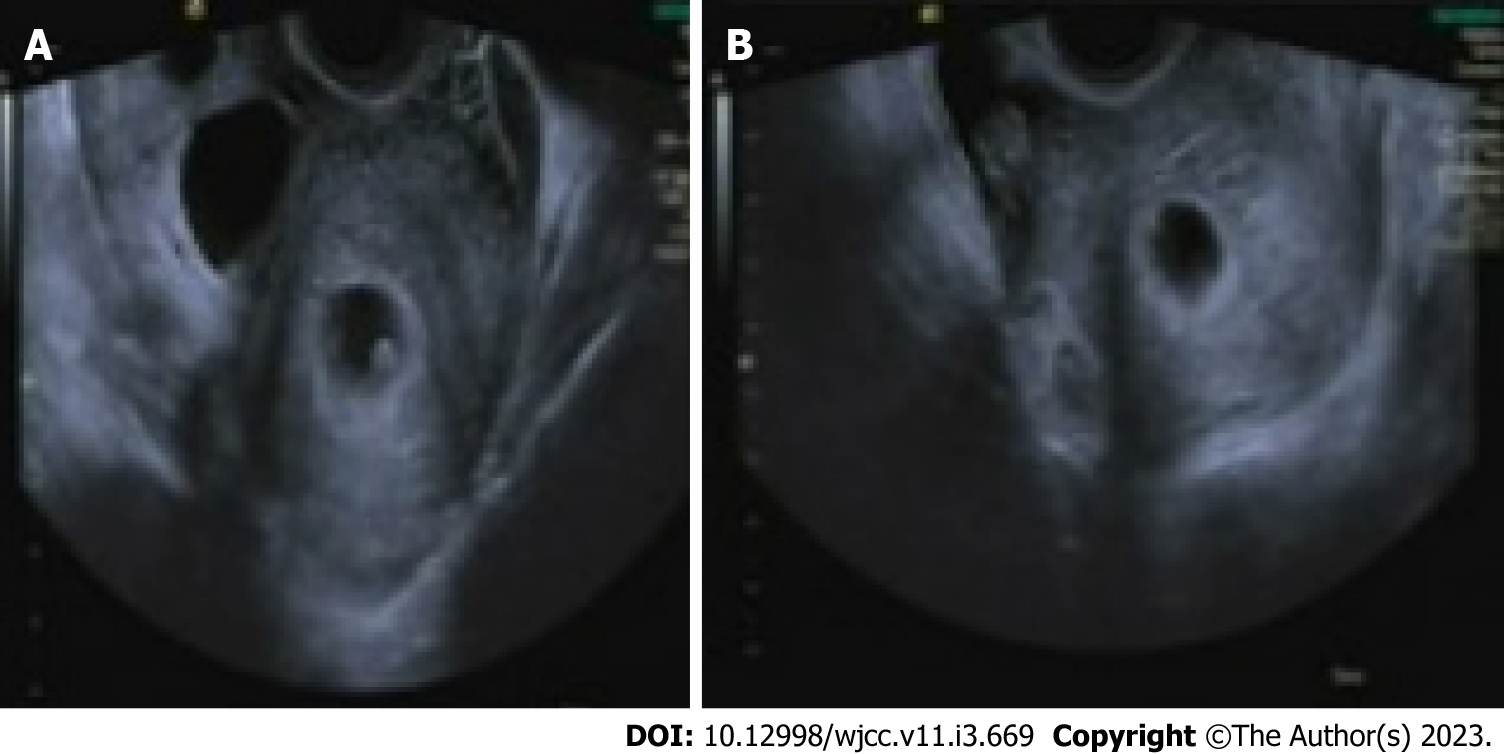
An intrauterine gestational sac appropriate for seven weeks of pregnancy was seen on transvaginal sonography (Figure 2A). Both ovaries were larger than normal, with dimensions of 11 cm × 5 cm for the right and 8 cm × 4 cm for the left. A mass in the right ovary suggested the presence of an ectopic pregnancy (Figure 2B).
Combined with the patient’s medical history and intraoperative findings, the final diagnosis was HP.
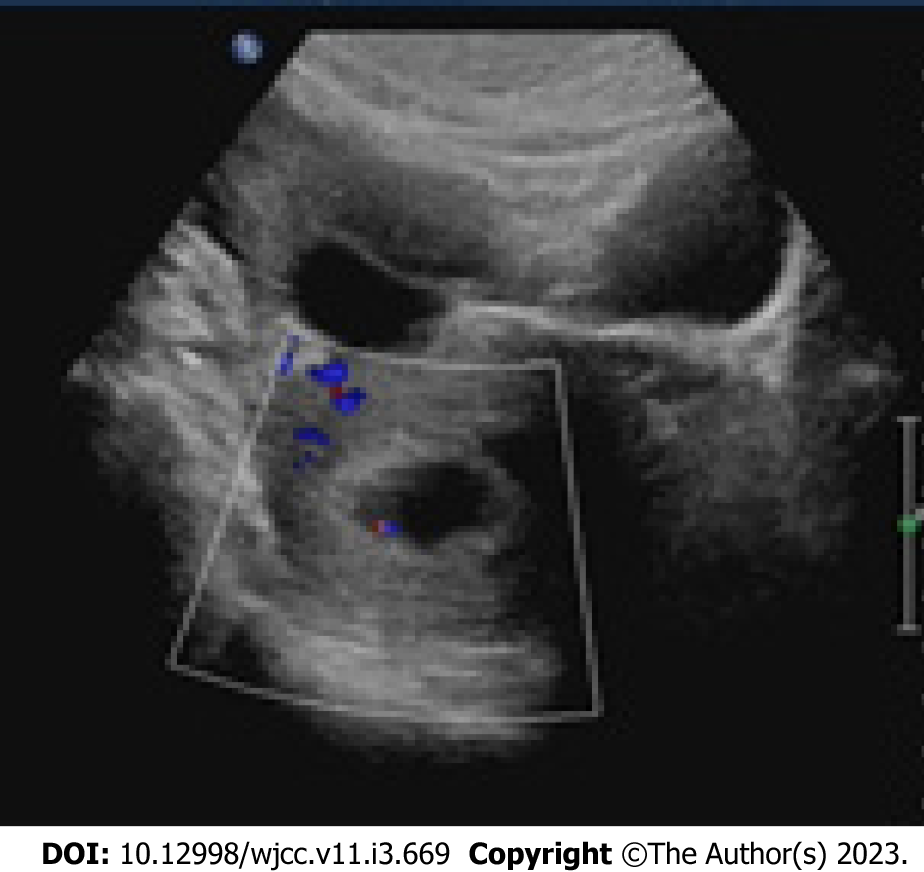
Laparoscopic surgery was performed. On removal of the blood in the peritoneal cavity (approximately 300 mL), the bilateral ovaries were immediately visible, clearly larger than normal as had been seen on the transvaginal sonography. The right fallopian tube was thickened with a bluish-purple lump of approximately 5.0 cm × 3.0 cm. The left fallopian tube was normal. The uterus was regular in shape and normal in size. After sufficient and short intraoperative communication with her medical authorizer, the surgeon excised the swollen right fallopian tube. During the operation, the estimated blood extravasation was approximately 50 mL. After the operation, the patient was given Ceftizoxime Sodium injection (0.2 g bid) to prevent inflammation which was discontinued after normal WBC counts were obtained on routine blood test reexamination. The patient recovered well during the remaining hospitalization and her reexamination by transvaginal sonography was satisfactory (Figure 3). She was discharged six days after surgery and was monitored for the duration of her pregnancy.
The patient’s obstetric follow-ups and fetal assessments were normal showing good fetal growth of her intrauterine singleton. The course of pregnancy was unremarkable until the patient experienced contractions at 30+5 wk of gestation. She was admitted to the hospital and, after confirming premature rupture of membranes, cervical effacement, and complete cervical dilatation, we continued with the delivery process, resulting in a baby boy with a birth weight of 1430 g. He was admitted to the neonatal unit. The postpartum course was uneventful for both the mother and baby.
Many recent articles on ART have paid particular attention to the link between ART and an increased incidence of HP, with some reports indicating HP rates as high as 8.6% after ART[3].
HP is diagnosed when ultrasound findings demonstrate an intrauterine pregnancy and a concurrent ectopic pregnancy[4]. As discussed in the Introduction, there is inescapable evidence that the incidence of HP is higher in pregnancies resulting from ART than in spontaneous pregnancies. However, many cases are still clinically misdiagnosed during first-trimester examinations due to poor awareness of this fact[5]. The differences in HP incidence between natural HP and HP after IVF-ET are apparent and present an additional problem, namely, how to explain this phenomenon and how to prevent it.
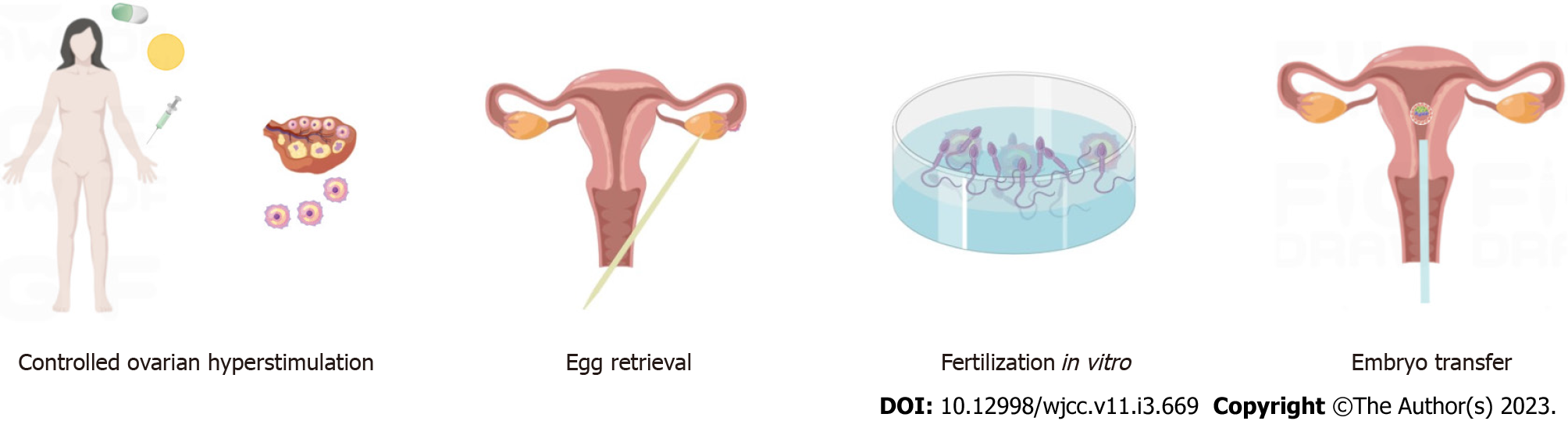
To answer this question, we first need to understand the ART procedure itself, which includes all clinical fertility treatments. In this respect, after analysis of earlier studies, we focus on the IVF-ET, typically performed by culturing embryos for a few days before transferring them through the cervix to the uterus. IVF-ET consists of four successive steps, commonly stated as stimulation of the growth of multiple ovarian follicles, egg retrieval from the woman’s ovaries, fertilization in vitro, and, finally, the transfer of viable embryos into the uterine cavity (Figure 4). However, in many cases, these four steps are not fully performed due to reasons such as allowing the possibility of natural conception, as in the present case[6]. Furthermore, during the steps described above, there are several factors that predispose to higher rates of HP in pregnant women after ART[7].
Firstly, as in the case of the present patient, ovarian induction is widely used for the retrieval of multiple follicles, indicating the presence of often more than one follicle in the body ready to be fertilized after the intervention. Therefore, in the period following attempts at either natural or in vitro fertilization, multiple zygotes are often present, implying the availability of more than one embryo ready for implantation, which could occur spontaneously in different places. An experimental study has demonstrated that the adhesion of mouse embryos to the human endometrium reduces gradually with increasing concentrations of estradiol (E2)[8]. It has been shown that very high levels of serum E2 influence implantation[9,10]. Recent studies have also shown that normal gene expression in endometrial tissue is disturbed during IVF-ET cycles as a result of high levels of serum E2 or increases in follicular progesterone[11,12].
Secondly, the majority of patients undergoing IVF-ET suffer from one or more types of reproductive dysfunction, with tubal dysfunction accounting for approximately 95% of these[13,14]. Various processes, including ovum and sperm transport, fertilization, and early stages of embryogenesis, occur in the fallopian tubes[15]. Gametes and fertilized embryos are propelled along the tubes by the action of smooth muscles and ciliary beating[5,15], and dysfunction of these systems may affect the correct homing of the oocyte and embryo. A perusal of the recent literature supports the hypothesis that tubal implantation is a result of tubal malfunction, specifically caused by alterations both in tubal transport mechanisms and expression of factors normally responsible for preventing implantation within the fallopian tube[16-19]. Such malfunctions can result from disease, such as chronic salpingitis or the effects of surgery, as well as endocrine disruptions during ovulation induced by hyperstimulation by exogenous gonadotropins or the chronic administration of low-dose progestogen[15]. It is presumed that the amount of administered estrogen is sufficient to prevent increases in progesterone or its tubal effects, delaying the passage of the ovum through the ampulla-isthmus junction for sufficient time to allow hatching of the blastocyst in the ampulla. These mechanisms appear to be associated with an increased risk of HP[14,15].
However, some scientists hold a different perspective, namely, that the increased incidence of embryonic implantation outside the uterus is determined by the embryo itself rather than by tubal factors. E-cadherin is known to be responsible for the anchorage of placental villi and its expression is reduced when differentiation occurs outside of the villi. Previous studies on E-cadherin-knockout mice have shown that the protein is required for normal implantation, as knockout embryos did not form functional trophectoderm and died during implantation[20]. In normal embryos, insulin-like growth factor 1 receptor (IGF-1R) is present throughout the membrane but on activation, is found exclusively at cell contact sites, colocalizing with E-cadherin. It has been found that abrogation of IGF-1R signaling with specific inhibitors blocks trophectoderm formation and compromises embryo survival during murine blastocyst formation and that E-cadherin is required to maintain the proper activation of IGF-1R. Perturbation of E-cadherin extracellular integrity, independent of its cell-adhesion function, was shown to block IGF-1R signaling, inducing cell death in the trophectoderm, and thus indicating a crucial and non-substitutable role of E-cadherin for these processes[21]. Strong E-cadherin expression is only seen at tubal implantation sites in patients after IVF-ET[13]. E-cadherin staining in the fallopian tubes of IVF-ET patients has been found to be restricted to the trophoblast, supporting the hypothesis of the dominant influence of the embryo in ectopic pregnancy after IVF-ET treatment[13]. Thus, it is possible that ectopic pregnancies in IVF-ET patients with normal fallopian tubes may be associated with reduced abilities of the embryos for implantation.
Furthermore, ARTs, such as ovarian induction and oocyte retrieval, are known to enhance uterine contraction, which could also impede embryonic implantation[22].
The recognized risks of ectopic pregnancy have led clinicians to turn their attention to ways in which these risks can be reduced in clinical practice.
Firstly, to reduce the risk of multiple births, milder and more physiological approaches to ovarian stimulation during IVF-ET are being considered; these have been shown to have several advantages despite the likelihood of producing fewer embryos. The term mild-stimulation IVF (MS-IVF) is defined as the administration of follicle-stimulating hormone or human menopausal gonadotropin at lower doses or shorter durations during a cycle with co-treatment of gonadotropin-releasing hormone antagonists or the administration of oral anti-estrogens or aromatase inhibitors either individually or combined with gonadotropins to collect fewer oocytes[23]. The advantages of MS-IVF, namely, improved safety, tolerance, and affordability, in IVF-ET cycles have been demonstrated, and many studies have shown an association between this gentle stimulation and improved perinatal outcomes[24].
Secondly, to reduce the effects of reproductive disorders such as tubal dysfunction that hinder oocyte and zygote transport within the fallopian tube, it is advisable to instigate treatment to restore the system to its normal state as far as possible before ART[25]. In addition, considering prognosis and perinatal outcomes, salpingectomy is considered a better treatment for women diagnosed with ectopic pregnancy compared with conservative treatment due to the reduced likelihood of future ectopic pregnancies after treatment[26].
Furthermore, it is suggested that the optimum time for embryo transferal is five to six days after oocyte retrieval when the embryo is exactly in the blastocyst stage. This is supported not only by basic experimental studies but also by the consistent results of clinical trials[25-27].
In addition, options for embryo transfer are not only limited to the cleavage or blastocyst phases but also include techniques such as fresh or frozen embryo transfer. It has been found that the transfer of fresh blastocysts does not affect perinatal outcomes in singleton live births compared with the transfer after the cleavage stage[28]. No significant differences were observed in the incidence of ectopic pregnancy between fresh and frozen/thawed cycles in a large patient sample[22]. The risk of recurrent ectopic pregnancy was found to be lower with fresh embryo transfer[26]. However, no definite conclusions on the effects of fresh or frozen embryos on the risk of ectopic pregnancy can be made at this time, and further robust evidence is required.
The increased incidence of HP is considered a clinical disadvantage of ART and also alerts us to the importance of collecting comprehensive data during regular consultations. It is important for us to remind ourselves of the possibility of HP in all patients presenting after ART, especially in women with an established and stable intrauterine pregnancy that complain of constant abdominal discomfort and also in women with unusually raised hCG levels compared with simplex intrauterine pregnancy. This will allow symptomatic and timeous treatment of patients leading to improved outcomes.
| 1. | Dendas W, Schobbens JC, Mestdagh G, Meylaerts L, Verswijvel G, Van Holsbeke C. Management and outcome of heterotopic interstitial pregnancy: Case report and review of literature. Ultrasound. 2017;25:134-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 2. | Guimarães AC, Reis LDO, Leite FC, Reis CFDD, Costa AP, Araujo WJB. Spontaneous Heterotopic Triplet Pregnancy with a Two Viable Intrauterine Embryos and an Ectopic One with Right Tubal Rupture. Rev Bras Ginecol Obstet. 2019;41:268-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 3. | Clayton HB, Schieve LA, Peterson HB, Jamieson DJ, Reynolds MA, Wright VC. Ectopic pregnancy risk with assisted reproductive technology procedures. Obstet Gynecol. 2006;107:595-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 160] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 4. | Elson CJ, Salim R, Potdar N, Chetty M, Ross JA, Kirk EJ. Diagnosis and management of ectopic pregnancy. BJOG. 2016;123:e15-e55. [RCA] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 161] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 5. | Lin EP, Bhatt S, Dogra VS. Diagnostic clues to ectopic pregnancy. Radiographics. 2008;28:1661-1671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 152] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 6. | Van Voorhis BJ. Outcomes from assisted reproductive technology. Obstet Gynecol. 2006;107:183-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 47] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 7. | Fauque P. Ovulation induction and epigenetic anomalies. Fertil Steril. 2013;99:616-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 8. | Chang KT, Su YT, Tsai YR, Lan KC, Hsuuw YD, Kang HY, Chan WH, Huang FJ. High levels estradiol affect blastocyst implantation and post-implantation development directly in mice. Biomed J. 2022;45:179-189. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 9. | Takeuchi M, Seki M, Furukawa E, Takahashi A, Saito K, Kobayashi M, Ezoe K, Fukui E, Yoshizawa M, Matsumoto H. Improvement of implantation potential in mouse blastocysts derived from IVF by combined treatment with prolactin, epidermal growth factor and 4-hydroxyestradiol. Mol Hum Reprod. 2017;23:557-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 10. | Fauser BC, Devroey P. Reproductive biology and IVF: ovarian stimulation and luteal phase consequences. Trends Endocrinol Metab. 2003;14:236-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 150] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 11. | Haouzi D, Assou S, Dechanet C, Anahory T, Dechaud H, De Vos J, Hamamah S. Controlled ovarian hyperstimulation for in vitro fertilization alters endometrial receptivity in humans: protocol effects. Biol Reprod. 2010;82:679-686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 91] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 12. | Labarta E, Martínez-Conejero JA, Alamá P, Horcajadas JA, Pellicer A, Simón C, Bosch E. Endometrial receptivity is affected in women with high circulating progesterone levels at the end of the follicular phase: a functional genomics analysis. Hum Reprod. 2011;26:1813-1825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 233] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 13. | Revel A, Ophir I, Koler M, Achache H, Prus D. Changing etiology of tubal pregnancy following IVF. Hum Reprod. 2008;23:1372-1376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 14. | Farquhar CM. Ectopic pregnancy. Lancet. 2005;366:583-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 327] [Cited by in RCA: 342] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 15. | Jansen RP. Endocrine response in the fallopian tube. Endocr Rev. 1984;5:525-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 125] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 16. | Refaat B, Dalton E, Ledger WL. Ectopic pregnancy secondary to in vitro fertilisation-embryo transfer: pathogenic mechanisms and management strategies. Reprod Biol Endocrinol. 2015;13:30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 92] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 17. | Shaw JL, Dey SK, Critchley HO, Horne AW. Current knowledge of the aetiology of human tubal ectopic pregnancy. Hum Reprod Update. 2010;16:432-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 221] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 18. | Refaat B. Role of activins in embryo implantation and diagnosis of ectopic pregnancy: a review. Reprod Biol Endocrinol. 2014;12:116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 19. | Refaat B, Simpson H, Britton E, Biswas J, Wells M, Aplin JD, Ledger W. Why does the fallopian tube fail in ectopic pregnancy? Fertil Steril. 2012;97:1115-1123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 20. | Larue L, Ohsugi M, Hirchenhain J, Kemler R. E-cadherin null mutant embryos fail to form a trophectoderm epithelium. Proc Natl Acad Sci U S A. 1994;91:8263-8267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 655] [Cited by in RCA: 690] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 21. | Bedzhov I, Liszewska E, Kanzler B, Stemmler MP. Igf1r signaling is indispensable for preimplantation development and is activated via a novel function of E-cadherin. PLoS Genet. 2012;8:e1002609. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 22. | Decleer W, Osmanagaoglu K, Meganck G, Devroey P. Slightly lower incidence of ectopic pregnancies in frozen embryo transfer cycles versus fresh in vitro fertilization-embryo transfer cycles: a retrospective cohort study. Fertil Steril. 2014;101:162-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 23. | Nargund G, Fauser BC, Macklon NS, Ombelet W, Nygren K, Frydman R; Rotterdam ISMAAR Consensus Group on Terminology for Ovarian Stimulation for IVF. The ISMAAR proposal on terminology for ovarian stimulation for IVF. Hum Reprod. 2007;22:2801-2804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 110] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 24. | Nargund G, Datta AK, Fauser BCJM. Mild stimulation for in vitro fertilization. Fertil Steril. 2017;108:558-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 50] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 25. | Santos-Ribeiro S, Tournaye H, Polyzos NP. Trends in ectopic pregnancy rates following assisted reproductive technologies in the UK: a 12-year nationwide analysis including 160 000 pregnancies. Hum Reprod. 2016;31:393-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 26. | Tan Y, Bu ZQ, Shi H, Song H, Zhang YL. Risk Factors of Recurrent Ectopic Pregnancy in Patients Treated With in vitro Fertilization Cycles: A Matched Case-Control Study. Front Endocrinol (Lausanne). 2020;11:552117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 27. | Pierzynski P. Oxytocin and vasopressin V(1A) receptors as new therapeutic targets in assisted reproduction. Reprod Biomed Online. 2011;22:9-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 38] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 28. | Marconi N, Raja EA, Bhattacharya S, Maheshwari A. Perinatal outcomes in singleton live births after fresh blastocyst-stage embryo transfer: a retrospective analysis of 67 147 IVF/ICSI cycles. Hum Reprod. 2019;34:1716-1725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Reproductive biology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ait Addi R, Morocco; Didziokaite G, Lithuania S-Editor: Liu JH L-Editor: A P-Editor: Liu JH