Published online Jan 26, 2023. doi: 10.12998/wjcc.v11.i3.514
Peer-review started: October 12, 2022
First decision: November 26, 2022
Revised: December 5, 2022
Accepted: January 10, 2023
Article in press: January 10, 2023
Published online: January 26, 2023
Processing time: 105 Days and 22.5 Hours
Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2, broke out in December 2019 in Wuhan city of China and spread rapidly worldwide. Therefore, by March 2020, the World Health Organization declared the disease a global pandemic. Apart from the respiratory system, various other organs of the human body are also seriously affected by the virus. Liver injury in patients with a severe form of COVID-19 is estimated to be 14.8%-53.0%. Elevated levels of total bilirubin, aspartate aminotransferase and alanine aminotransferase and low levels of serum albumin and prealbumin are the main laboratory findings. Patients with pre-existing chronic liver disease and cirrhosis are much more prone to develop severe liver injury. This literature review presented the recent scientific findings regarding the pathophysiological mechanisms responsible for liver injury in critically ill patients with COVID-19, the various interactions between drugs used to treat the disease and the function of the liver and the specific tests providing the possibility of early diagnosis of severe liver injury in these patients. Moreover, it highlighted the burden that COVID-19 put on health systems worldwide and its effect on transplant programs and the care provided to critically ill patients in general and particularly to those with chronic liver disease.
Core Tip: The liver follows the respiratory system with a lower but considerable frequency of affection by severe acute respiratory syndrome coronavirus 2. Coronavirus disease 2019 causes acute and acute-on-chronic liver injury. The pathophysiological mechanisms are complex. Certain biomarkers such as fibrosis-4 score and non-invasive point-of-care methods such as ultrasonography or transient elastography can be extremely helpful in the early diagnosis of liver injury and the assessment of its progression. Health systems, intensive care units, liver units and transplant programs were seriously affected by the pandemic. The clinician should recognize the symptoms and signs of liver injury early and take the appropriate measures to reverse it.
- Citation: Valsamaki A, Xanthoudaki M, Oikonomou KG, Vlachostergios PJ, Papadogoulas A, Katsiafylloudis P, Voulgaridi I, Skoura AL, Komnos A, Papamichalis P. Prevention, diagnostic evaluation, management and prognostic implications of liver disease in critically ill patients with COVID-19. World J Clin Cases 2023; 11(3): 514-527
- URL: https://www.wjgnet.com/2307-8960/full/v11/i3/514.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i3.514
In December 2019, an epidemic of pneumonia of unknown origin broke out in Wuhan city, in the Hubei province of China, causing global concern because of its ease of transmission and the significant rates of morbidity and mortality that accompanied it. To diagnose and control this highly infectious disease, patients were immediately isolated, and their clinical and epidemiological data were studied thoroughly. The immediate mobilization of the global scientific community rapidly identified the cause (severe acute respiratory syndrome coronavirus 2 [SARS-CoV-2]) of coronavirus disease 2019 (COVID-19)[1]. In the immediate aftermath, the disease spread very rapidly to all the regions of the world, forcing the World Health Organization to declare the COVID-19 outbreak as a “global pandemic” on March 11, 2020.
Over the next years, the pandemic greatly affected the health systems of all the countries of the world causing until September 16, 2022 more than 611550000 cases and more than 6525000 deaths[2,3]. SARS-CoV-2 has been associated with three primary modes of transmission, known as “contact,” “droplet” and “airborne” transmission[4].
Among the organs affected by COVID-19 is the liver, with several early scientific reports describing various degrees of liver dysfunction and injury[5]. The liver is responsible for the regulation of levels of many chemical substances and biomarkers in the human blood, carrying out crucial functions including but not limited to: the production and excretion of bile; the excretion of bilirubin, cholesterol, hormones and drugs; the metabolism of fats, proteins and carbohydrates; enzyme activation; the storage of glycogen, vitamins and minerals; synthesis of plasma proteins, such as albumin and clotting factors; the conversion of ammonia to urea; blood detoxification and purification; and the metabolism of hemoglobin for the use of its iron content. Chronic liver diseases are prevalent all over the world, imposing a significant burden on healthcare systems. According to Mohammed et al[6], patients with known chronic liver disease present a higher risk of complications from COVID-19 in comparison with the general population, with a mortality rate as high as 12%. Mortality from secondary liver injury in the intensive care unit (ICU) is significantly higher, ranging between 27%-48% for critically ill patients with cholestasis and between 40%-60% for critically ill patients with hypoxic liver injury[7].
This literature review presented the recent scientific findings regarding the pathophysiological mechanisms responsible for the induction of liver injury in critically ill patients with COVID-19, the various interactions between drugs used to treat the disease and the function of the liver, the tests providing the possibility of early diagnosis of severe liver injury in these patients, and the effect of the pandemic on health systems, transplant programs and critically ill patients with or without pre-existing chronic liver disease.
An advanced search strategy was made to identify studies published until August 2022 using the key words “COVID-19,” “Liver” and “Intensive Care Unit” in the PubMed electronic bibliographic database. Initially, 560 studies were identified. These studies were reviewed based on their title and abstract, thus excluding 301 studies. The full texts of the remaining 259 studies were assessed for eligibility based on their relevance to the subject of our review, particularly focusing on critical illness and liver disease. Most of these studies were excluded because they referred to patients with mild or moderate COVID-19. A total of 97 studies were finally included and analyzed for this systematic review. Overall, limited evidence exists regarding liver disease, critical illness and COVID-19.
SARS-CoV-2 just like its predecessors SARS-CoV (responsible for the SARS epidemic in 2003) and MERS-CoV (responsible for the Middle East respiratory syndrome epidemic in 2012) is a coronavirus and shares sequence homology and genome similarities with them[5]. The main symptoms caused by SARS-CoV-2, affecting men more severely than women, include fever, upper and lower respiratory symptomatology (cough, rhinorrhea, sore throat, flu-like symptoms and dyspnea), general muscle aches, anosmia, ageusia and increased likelihood of occurrence of vascular thrombosis.
Several reports regarding SARS-CoV and MERS-CoV reported that both of them caused liver injury in a significant number of patients. For example, Chau et al[8] reported 3 cases of hepatitis directly associated with SARS disease and revealed that various degrees of impairment of liver function had been reported in up to 60% of the patients suffering from SARS. Alsaad et al[9], after 14 years reported portal and lobular hepatitis at post-mortem histopathological findings in a 33-year-old male patient who died from MERS-CoV infection.
In May 2020, the results of a multicenter observational cohort study from 208 hospitals in the United Kingdom (20133 patients) were published. They investigated the outcome of patients with severe disease who were admitted to these hospitals[10]. Their median age was 73 years (range: 0–104 years), and 60% of them were men. The mortality rate in the cohort was 26%, whereas 41% of the patients were discharged alive, and the rest (34%) continued to be hospitalized at the end of the study. Liver disease was among the pathological conditions associated with increased in-hospital mortality, along with sex (male sex), age, obesity and chronic pulmonary, chronic kidney and chronic cardiac diseases.
Liver injury caused in patients severely affected by COVID-19 is estimated to be at levels of 14.8%- 53.0%[11]. The imaging findings in these patients include hepatomegaly, gall bladder thickness and prominence of the common bile duct in the ultrasonography, along with pericholecystic fat stranding and hypodensity of the liver in the computed tomography (CT) images[12].
According to Nardo et al[13], the most likely pathophysiological mechanisms involved in causing liver injury after severe infection from SARS-CoV-2 are as follows:
(1) Moderate hepatic steatosis: There is growing evidence that SARS-CoV-2 modifies the function and the activity of the mitochondria, downregulating nuclear-encoded mitochondrial genes that are associated with cellular respiration[14]. Another cause of steatosis seems to be the induction of endoplasmic reticulum stress by SARS-CoV-2, which in turn has been shown to cause lipogenesis in the hepatic cells[15]. Finally, another proposed possible mechanism is directly associated with the characteristic “cytokine storm”/cytokine release syndrome (CRS) observed in the severe forms of COVID-19. Interleukin (IL)-6 produced by the cytokine storm most probably causes hyperactivation of the mammalian target of rapamycin, which can induce lipogenesis inside the hepatic cell[16]. In conclusion, the above-mentioned process of excessive lipogenesis seems to be detrimental to the function of the hepatic cell and the liver as a whole, and on the other hand it enhances the potential of the virus, providing it with the necessary nutrient material to achieve its replication and exocytosis[13,17].
(2) Cholestasis and bile duct alterations: Apart from IL-6, during the cytokine storm a large number of other inflammatory cytokines are released, including IL-1 and tumor necrosis factor-alpha. These cytokines cause hepatocellular cholestasis, closely resembling cholestasis observed in severe cases of sepsis[18]. An additional pathophysiological lesion that has been observed in these patients comes from the so-called “triple hit” to the bile ducts, consisting of hypoxia due to respiratory failure, systemic inflammatory response syndrome resulting in inflammation and fibrosis of the bile ducts and direct infection of the cholangiocytes from the virus[19].
(3) Hypoxic hepatitis (HH): Pathophysiologically, the causes of HH during the course of severe COVID-19 are multifactorial, including acute respiratory failure, severe sepsis, heart failure, including right-sided heart failure, acute respiratory distress syndrome (ARDS), a hyper-coagulate state, deteriorating the congestion of the liver and the hemodynamic effects of positive-pressure ventilation[20].
(4) The gut–liver axis: Symptomatology from the gastrointestinal tract is common in patients with severe COVID-19, with relevant rates ranging from 4.9% to 74.0%. The most common symptoms are nausea, vomiting, diarrhea, loss of appetite and abdominal pain[21]. It is speculated that the damage caused by SARS-CoV-2 to the epithelial barrier of the small intestine may lead to the transmission of the virus into the hepatocytes through the portal vein, aggravating the lesions of the liver parenchyma. In addition, alterations in gut microbiota caused either by drugs for COVID-19 or by the virus itself may play a significant role through the gut-liver axis.
(5) Injury induced by treating medications: As SARS-CoV-2 is novel to the scientific community and no specific therapy for COVID-19 has been found, numerous different drugs have been used in several cases outside their officially approved indications. Typical examples are the antimalarial drug hydroxychloroquine, antibiotics (mainly from the family of macrolides), antiviral agents such as lopinavir, ritonavir and remdesivir, immunomodulating medications such as tocilizumab and dexamethasone and even anti-inflammatory and antipyretics in high doses[22]. Many of them presented already-known hepatotoxic side effects. For example, corticosteroids have been implicated as a cause of glycogenosis or steatosis[23], whereas tocilizumab is reported to cause drug-induced liver injury (DILI) in critically ill patients with COVID-19[24]. Specific reference should be made to paracetamol, the most commonly used analgesic and antipyretic medication in the elderly, prescribed in many cases in high doses and for a long time. According to Mian et al[25], old age and frailty decrease the clearance of paracetamol at percentages of 29.0%-45.7%, varying between 0.20-0.38 L/h/kg in older patients in comparison with values between 0.28-0.7 L/h/kg in younger patients. Another important influence of aging is on the volume of distribution of paracetamol, which decreases in older patients because of its incomplete distribution into body fat, with a consequent increase in the plasma concentration of paracetamol in the elderly.
In the United States of America, severe DILI presents the leading cause of acute liver failure (ALF), ahead of all the other causes even viral hepatitis. More than 1000 pharmaceutical agents have been identified as causes of serious liver disease, a figure that will increase significantly in the near future, as the pharmaceutical industry is constantly developing new drugs for use by the general population and patients. There are two main types of adverse reactions induced by drugs[26,27]: (1) Type A (intrinsic adverse reactions) are dose dependent and produce predictable toxicities; and (2) Type B (idiosyncratic adverse reactions) are difficult to be explained by their pharmacologic response or their dose and are associated with patient, drug or environmental risk factors, making them difficult to be predicted.
In order to predict the occurrence of a severe DILI early and take the appropriate preventive measures, various methods have been proposed. Hy’s Law is one of the most commonly used, named after Dr. Hyman Zimmerman. It is based on the observation that patients with elevated serum total bilirubin who have received a medication causing hepatocellular (not hepatobiliary) injury, with the absence of other possible causes that could explain these disorders, are at high risk for fatal or requiring transplantation DILI, with mortality ranging between 10%-50%[28]. Another valuable tool is the LiverTox free online database, which allows clinicians to be informed about the latest data of the hepatotoxicity of various pharmaceutical agents, while at the same time they are assisted in the diagnosis and treatment of DILI[29].
Another important area of scientific research is the way by which liver dysfunction of any etiology has the potential to affect the accumulation and the toxicity of various drugs. According to Bosilkovska et al[30], the physiologic changes that accompany any hepatic impairment alter the disposition of most of the drugs. Portosystemic shunting decreases the initial metabolism, increasing the oral bioavailability of highly extracted drugs, whereas a coexisting disorder in the production of drug-binding proteins can change the distribution of the drug. In addition, both the amount and the function of enzymes that are produced by the liver and are responsible for the metabolism of drugs are affected by hepatic damage. The final result is the reduction of drug clearance, along with increased plasma drug concentration, which are both difficult to be predicted. Thus, the pharmacologic properties of most of the drugs are altered during severe liver disease.
In conclusion, the mechanism of liver injury during COVID-19 is twofold[31]. Either SARS-CoV-2 directly attacks the hepatic cells and the cholangiocytes, or it causes damage to the liver parenchyma by activating (and dysregulating) the patient’s immune system, probably in a similar way to the severe lung injury caused by the cytokine storm process. In several cases, the damage is caused by a combination of the above two mechanisms. In other cases, the liver is affected by the medication used against COVID-19.
The histopathological features that have been described in critically ill patients with COVID-19 and concurrent hepatic involvement are various and, in most cases, nonspecific. Characteristic and specific for the disease is the detection of SARS-CoV-2 RNA in liver tissue in up to 55% of patients with severe liver injury[32]. Lagana et al[32] in a series of 40 critically ill patients who died from complications of COVID-19 reported that the most common hepatic histopathological findings were: (1) Macrovesicular steatosis (75% of the patients); (2) Lobular and portal necroinflammation (50% of the patients); and (3) Vascular pathology (primarily sinusoidal microthrombi) in a significantly smaller number of patients (15%). Finally, in another post-mortem report, the commonest findings in 22 critically ill patients who died from the disease were liver parenchymal congestion along with sinusoidal congestion and congestion of the small hepatic veins, extravasation of red cells into the Disse’s space, necrosis of a large number of hepatic cells and macro- and micro- vacuolar steatosis[33]. Nevertheless, all the above-mentioned findings seemed to be because of the combination of the organism’s systemic response to inflammation and its comorbidities, rather than the direct action of SARS-CoV-2 on the liver[34].
Studies on the evolution of liver injury from SARS-CoV-2 and on factors that can predict the outcome are relatively few. Various outcome measures have been studied[35] including liver function tests (LFTs). A broad spectrum of abnormal LFTs has been described in patients with COVID-19. Aminotransferases (aspartate aminotransferase [AST] and alanine aminotransferase [ALT]), alkaline phosphatase, gamma-glutamyl transpeptidase (GGT) and bilirubin have been the most extensively studied markers of liver function in patients with COVID-19. Various studies have demonstrated a correlation between liver injury and disease severity, albeit most of these data are not strictly limited to critically ill patients[36-39]. In several studies including severe and non-severe patients with COVID-19 strong association was found between LFT (particularly AST) abnormalities and disease severity and mortality[40-44]. Lok et al[45], reporting similar results, suggested that immune system dysregulation may be a plausible contributing factor to the former association.
The role of microRNAs, which are considered to alter the immune response, is notable. To the best of our knowledge, only one study has found an association between liver-derived miR-122 and patient mortality in a cohort including patients with severe COVID-19[46]. Whereas the role of microRNAs in the inflammatory process is well documented, their specific role in COVID-19 is yet to be clarified.
Focusing on critically ill patients, a wide range of abnormal LFTs has been reported, whereas the vast majority of published data deliberate on aminotransferases. The prevalence of abnormal LFTs seems to be higher in ICU patients than in ward patients with COVID-19, according to a large meta-analysis including 31 studies from various countries[47]. In a study including 166 patients requiring mechanical ventilation, AST and ALT elevation served as predictive factors for the requirement of invasive mechanical ventilation[48]. Similar results were reported by Yip et al[49]. An independent association was found between aminotransferases elevation and ICU admission, mechanical ventilation and/or death. In addition, an association was found between aminotransferase elevation and lopinavir/rito
Nevertheless, Roman et al[50], in a study including exclusively critically ill patients with laboratory-proven liver damage, failed to demonstrate a correlation between liver injury severity and mortality. Azad Allarakia et al[51] examined plausible associations between routine laboratory tests and disease severity. No difference was found regarding LFTs between ICU and ward patients; however, confounding factors were not adjusted. Similarly, in a study conducted early during the pandemic era, no association was found between disease severity and LFTs[52].
Regarding mortality, in a large cohort including 3812 patients with COVID-19, an association between elevated ALT, AST, GGT levels and ICU admission was reported, and AST elevation was associated with the risk of death after adjusting for confounding factors such as age, obesity and previous liver disease[41]. Following the aforementioned study, in the study of Salik et al[53], which included exclusively critically ill patients, liver dysfunction and liver injury were associated with higher 7-d and 28-d mortality in comparison with patients with COVID-19 without liver biochemistry abnormalities. Interestingly, in the study of Kasapoglu et al[54], although ICU patients had higher values of AST and GGT, only GGT among LFTs was found to be predictive of mortality in ICU patients.
In addition to the aforementioned biomarkers, interest has been drawn to the role of albumin and prealbumin as prognostic markers of COVID-19 outcome. Hypoalbuminemia is common among critically ill patients with COVID-19. There are various mechanisms not directly related to hepatocellular damage that lead to hypoalbuminemia in these patients, including malnutrition, extravasation due to capillary leakage and a decreased rate of synthesis. Furthermore, measurements of serum albumin in hospitalized patients are often affected by exogenous albumin administration. Prealbumin is a precursor of albumin with a shorter half-life and can be used to assess protein status during a shorter time-frame. Low prealbumin levels were associated with disease severity and may be of prognostic value as they were identified as independent predictors of mortality in critically ill patients with COVID-19[55,56].
Various non-invasive fibrosis estimators include the fibrosis-4 (FIB-4) score, the FORNS index for liver fibrosis, the AST to platelet ratio index (APRI) score, the nonalcoholic fatty liver disease (NAFLD) fibrosis score (NFS) and the AST to ALT ratio.
Of the above-mentioned biomarkers and scores, the FIB-4 score attracted interest and applicability. It is a scoring system that uses four simple parameters, readily available in all in-patients: the age, the platelet count, and the values of AST and ALT. A score of < 1.45 has a negative predictive value of more than 90% for advanced fibrosis of the liver, whereas a score of > 3.25 has a positive predictive value of 65%, with 97% specificity[57].
Crisan et al[58] published the results of a retrospective cohort study (370 consecutive patients with COVID-19, from whom 289 presented with abnormal liver biomarkers at admission) to evaluate the predictive value of the various liver tests and estimators. They concluded that an elevated FIB-4 score (values > 3.25) and elevated AST were the only two tests that were independently associated with higher mortality in these patients. The FIB-4 score is a valuable tool that can help clinicians identify existing undiagnosed liver disease or the possibility of rapid deterioration of liver function during COVID-19, so that patients with abnormal values receive priority in their inpatient management[58].
Findings in association with the value of the FIB-4 score were also verified in the systematic review and meta-analysis of Liu et al[59], who concluded that along with the FIB-4 score, the APRI score, the NFS and the FORNS index could also serve as indicators for identifying patients at high risk of developing severe COVID-19 with worse outcomes. More specifically, one unit elevation of the APRI score increases the risk of death by 178%, higher NFS (≥ -1.5) increases the risk of developing severe COVID-19 by ten-fold, and an increase of the FORNS index by one point increases the risk of death by 41%.
Acute liver injury (ALI) has been reported in approximately 19% of patients with COVID-19; however, the percentage increases dramatically, up to 89.2%, in ICU patients[60,61]. The spectrum of ALI in critically ill patients with COVID-19 is wide, varying from simple elevations of LFTs to ALF, the need for advanced support and even the need for transplantation{62-64]. For patients with severe liver injury (approximately 6.4% of all patients with distorted liver biochemistry), a severe disease course is expected[65]. The correlation of impaired liver function with sudden death in patients with COVID-19 is another outstanding association[66]. Most studies reported a predominance of the hepatocellular pattern[67]. However, other distributions of liver injury pattern have been reported as well[62].
HH as a clinical presentation of COVID-19 is observed in approximately 5.9% of ICU patients with COVID-19 and has a significant effect on patient survival[68]. The diagnosis is made when the following criteria are met: (1) A massive but transiently elevated ALT level (more than 20-fold the upper limit of normal); (2) The presence of respiratory, cardiac or circulatory failure; and (3) Exclusion of other causes of liver injury[69]. The close monitoring of cardiac and respiratory function and early etiologic management of hemodynamic instability/shock is crucial for patient survival when HH is suspected[68].
Secondary sclerosing cholangitis is another devastating form of liver disease in COVID-19, which is associated with considerable morbidity and mortality. Contributing pathophysiological mechanisms include bile duct ischemia and toxic bile formation[70]. The underlying histopathological findings consist of ischemic damage to the perihilar bile ducts[71]. Ursodeoxycholic acid (UDCA) has been reported to give promising results; however, for a proportion of these patients, transplantation is required[72]. Rare but devastating clinical presentations include liver abscess with necrosis[73] and vascular thrombotic events in abdominal vessels such as portal and mesenteric vein thrombosis[74].
ALF is a life-threatening condition characterized by hepatic encephalopathy and coagulopathy in patients without pre-existing liver disease[75]. During the pandemic, an increased incidence of hepatitis of unknown etiology in the pediatric population has been reported with subsequent liver failure and the need for liver transplantation (LT) for a proportion of these children. This raised great concern about the possible vulnerability of children to this extremely severe complication. Although adenovirus is the main etiological agent suspected to be responsible, the association with COVID-19 and the role of other contributing factors remain to be clarified[76]. In adults, there are reports on other viruses as causative factors of ALF, such as the infection from or the reactivation of herpes simplex virus-1 following the immunosuppression that patients with COVID-19 receive for treating the CRS[77].
When assessing critically ill patients with COVID-19 and ALI, the diagnostic approach basically remains the same as for any patient who has ALI and is severely ill. However, some differences exist that must be pointed out.
Current guidelines recommend against unnecessary imaging [e.g., ultrasound (unless performed at the bedside), CT-magnetic resonance (MR) imaging/MR cholangiopancreatography][78]. The transport of these patients requires special knowledge, equipment and experience and should be kept for patients where the examination results may change the patient’s management.
Approaches that do not require patient transportation are preferred. An approach regarding the hemodynamic monitoring of these patients uses invasive cardiac monitors based on the thermodilution method. These methods are invasive, expensive and present septic and other catheter-related complications. Moreover, they have limitations in critically ill patients with liver failure such as the presence of ascites (extravascular third space fluid) or hepatic hydrothorax (extravascular lung water), which confuse the measurements and the lack of validation of these techniques on such patients. Remote point-of-care ultrasonography (POCUS) by a hepatologist or an ICU physician, with real-time interpretation by a cardiologist through telemedicine, is a trend that has been adopted in the COVID-19 era[79]. Information on the hemodynamic status and the cause of the hemodynamic compromise of these patients is safely and accurately collected. Basic diagnoses such as pulmonary embolism or myocardial infarction are made at the bedside. The evaluation of intravascular volume status helps to differentiate between prerenal acute kidney injury and hepatorenal syndrome or between transfusion-related acute lung injury and transfusion-associated circulatory overload. In addition, this powerful and non-invasive tool contributes to the prompt identification of liver-related pathologies, including portal vein or hepatic vein thrombosis, the presence of ascites, suspected pneumothorax and hemothorax. This approach has provided several solutions for liver units and ICUs during the pandemic.
Another non-invasive method that has been evaluated for the assessment of liver injury during COVID-19 is the vibration-controlled transient elastography/FibroScan. It may serve as a tool for identifying patients with elevated liver stiffness and thus at greater risk of developing ALI and progressing to severe COVID-19 with worse clinical outcomes, even when no history of pre-existing liver disease is present[80,81].
No special recommendations or measures exist which could prevent liver injury from COVID-19. The prophylaxis of the liver can only be achieved through measures that prevent infection from SARS-CoV-2. Thus, current guidelines suggest using personal protective equipment for healthcare personnel in the liver and other departments, cancelling all elective/nonurgent procedures and vaccinating with the approved vaccines the vulnerable population with or without pre-existing liver disease[78,82]. Another approach is the use of dietary supplements as prophylaxis for severe disease and liver involvement. Among the supplements used for the prevention of COVID-19, several pieces of evidence exist on the possible protective role of vitamins C and D in humans, whereas in animal models, xanthohumol has an anti-inflammatory action on liver injury[83].
Accordingly, no separate protocols exist for the treatment of liver injury from COVID-19. The implementation of the general therapeutic protocols for the disease is applicable[84], with special care for liver protection and early detection of liver injury in patients with COVID-19[85]. In cases of patients with progressive ALF, when the applied standard supportive care (hemodynamic, nutritional, respiratory support, avoidance of all unnecessary hepatotoxic factors) does not lead to the resolution of ALF, LT can be the final solution[75]. Removing hepatotoxic metabolites such as conjugated or unconjugated bilirubin, bile acids, phenols, fatty acids, cytokines, ammonia or amino acids with the use of extracorporeal blood purification techniques presents an interesting alternative approach, particularly when LT is not a feasible option or even as a bridging therapy toward transplantation[86,87]. These techniques eliminate not only hepatic metabolites but also inflammatory mediators responsible for the CRS, leading to the preservation of organ function and prevention of organ failure, while advanced support is offered in patients with COVID-19[86].
Acute-on-chronic liver failure has been reported in patients with pre-existing liver disease[40]. Particularly in patients with cirrhosis, the associated state of immunosuppression in conjunction with COVID-19 can lead to acute decompensation, most frequently manifested as worsening ascites with spontaneous bacterial peritonitis and to hepatic failure in patients with impaired and limited reserves[40,88]. Liver injury has been observed in 26.7% of patients with severe pneumonia[88]. Despite the lack of coagulation factors in decompensated liver disease, a hypercoagulable state may be present in COVID-19, and hepatic impairment may be associated with greater activation of the coagulation pathways[75,89].
In critically ill patients with COVID-19, pre-existing liver disease and evidence of liver impairment, LFTs must be frequently monitored[85]. Typically, no specific treatment is indicated, and emphasis should be placed on cause-directed therapy.
UDCA may be added as a treatment in patients with liver injury because of its anti-inflammatory and immunomodulatory properties[88]. In the ICU setting, treatment with vasopressors should be administered with caution in patients with cirrhosis and COVID-19, to avoid detrimental effects on cardiac output. Moreover, caution should be taken while administering immunosuppressive agents, such as tocilizumab and baricitinib, as they may cause the reactivation of chronic hepatitis B. In such cases, antiviral prophylaxis is indicated[88].
In terms of prognosis, it has been hypothesized that patients with chronic liver disease may be particularly vulnerable to developing severe COVID-19[90]. Higher mortality rates have been observed in patients with COVID-19, pre-existing chronic liver disease and cirrhosis caused by chronic hepatitis B and C[40]. Moreover, patients with NAFLD present a higher risk for progression to severe COVID-19[91]. Patients with cirrhosis having ARDS have a worse prognosis than patients without cirrhosis, and pre-existing liver fibrosis is independently associated with a significantly higher risk of death in patients with severe COVID-19 admitted to the ICU[92].
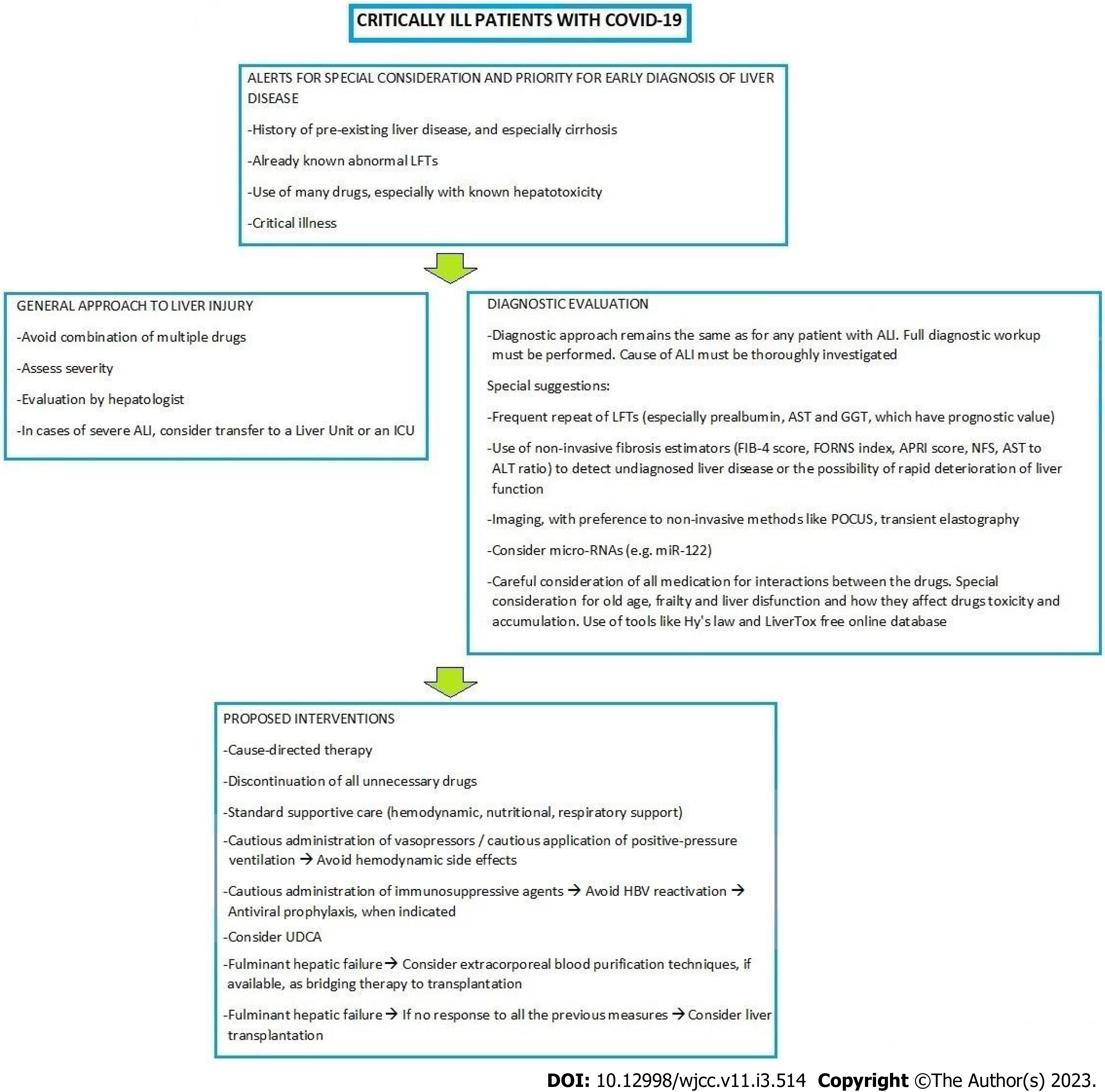
An approach to liver disease in critically ill patients with COVID-19 is proposed by the authors (Figure 1).
During the pandemic, health systems and ICUs were overburdened by critically ill patients. Higher mortality risk was observed and was associated with ICU patient load[4,93,94]. In line with this, patients with chronic liver disease had significantly high mortality during the pandemic, leading to suggestions regarding their primary and emergency care and their access to intensive care and high-dependency units[95]. In addition, the effect of the pandemic was significant on the treatment of complications of chronic liver disease such as hepatocellular carcinoma (HCC); surveillance for HCC and treatment of early-stage HCC were modified. Another significant change was the extensive use of telemedicine to minimize patients’ and healthcare workers’ exposure to COVID-19[96].
Transplant programs and care provided to LT recipients were also greatly affected. Living donor LT was suspended in some centers worldwide[96]. As a response to these issues, national protocols were specially prepared[97], and transplantation centers implemented special strategies to increase their successful transplantation rates[98]. Recommendations point out the need for the restoration of LT programs; however, prioritization of patients with poor short-term prognosis (with acute/acute-on-chronic liver failure, high Model for End-stage Liver Disease score and HCC at the upper limits of the Milan criteria) may be necessary in some cases[67].
In general, transplant recipients present higher rates of severe disease and higher mortality rates than nontransplant patients; thus, their exposure to COVID-19 should remain minimal[78]. Immunosuppression should be reduced only under special circumstances, e.g., symptomatic COVID-19, and with caution[78,99]. COVID-19 screening should be performed for both donors and recipients. Charts regarding LT organ offers are available to optimize the management of the procedures associated with LT in the COVID-19 era[78].
Apart from the upper and lower respiratory system, the liver is also greatly affected by COVID-19. The pathophysiological mechanisms include cholestasis, bile duct alterations, hepatic steatosis, involvement of the gut-liver axis, HH and hepatitis induced by the drugs that are used to treat the actual disease. The hepatocyte seems to be affected both directly, by SARS-CoV-2 itself, and by the disruption and dysregulation of the immune system. Not only patients with or without pre-existing liver disease individually but also health systems and transplant programs were greatly affected by the pandemic, and great effort has been made, which needs to be continued to minimize the consequences. Scientific research over the past 2 years has shown that certain biomarkers can be extremely useful in the early diagnosis of liver injury and the evaluation of its progression. Non-invasive assessment with transient elastography or POCUS is the trend for evaluating particularly patients in the ICU setting where biopsy is difficult to perform because of coagulation abnormalities and transport for CT or MR imaging is difficult and potentially dangerous. Although in most cases, liver involvement in COVID-19 is mild, the clinician should be able to recognize the symptoms and signs of liver dysfunction early and not focus exclusively on symptomatology from the respiratory system.
This paper is dedicated to the memory of the exceptional doctor Tilemachos Zafeiridis (1974-2021), 1 year after his premature death, to remind us how much we miss him.
| 1. | Ciotti M, Ciccozzi M, Terrinoni A, Jiang WC, Wang CB, Bernardini S. The COVID-19 pandemic. Crit Rev Clin Lab Sci. 2020;57:365-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1007] [Cited by in RCA: 530] [Article Influence: 88.3] [Reference Citation Analysis (0)] |
| 2. | Cucinotta D, Vanelli M. WHO Declares COVID-19 a Pandemic. Acta Biomed. 2020;91:157-160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2704] [Reference Citation Analysis (1)] |
| 3. | COVID-19 Map [cited Sep 17, 2022]. Johns Hopkins Coronavirus Resource Center. [Internet]. Available from: https://gisanddata.maps.arcgis.com/apps/dashboards/bda7594740fd40299423467b48e9ecf6. |
| 4. | Tusabe F, Tahir IM, Akpa CI, Mtaki V, Baryamujura J, Kamau B, Lidoroh S, Kobugabe PL, Maaga NO, Bongomin F. Lessons Learned from the Ebola Virus Disease and COVID-19 Preparedness to Respond to the Human Monkeypox Virus Outbreak in Low- and Middle-Income Countries. Infect Drug Resist. 2022;15:6279-6286. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 5. | Cheng ZJ, Shan J. 2019 Novel coronavirus: where we are and what we know. Infection. 2020;48:155-163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 339] [Cited by in RCA: 310] [Article Influence: 51.7] [Reference Citation Analysis (0)] |
| 6. | Mohammed A, Paranji N, Chen PH, Niu B. COVID-19 in Chronic Liver Disease and Liver Transplantation: A Clinical Review. J Clin Gastroenterol. 2021;55:187-194. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 7. | Perez Ruiz de Garibay A, Kortgen A, Leonhardt J, Zipprich A, Bauer M. Critical care hepatology: definitions, incidence, prognosis and role of liver failure in critically ill patients. Crit Care. 2022;26:289. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 54] [Reference Citation Analysis (0)] |
| 8. | Chau TN, Lee KC, Yao H, Tsang TY, Chow TC, Yeung YC, Choi KW, Tso YK, Lau T, Lai ST, Lai CL. SARS-associated viral hepatitis caused by a novel coronavirus: report of three cases. Hepatology. 2004;39:302-310. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 307] [Cited by in RCA: 305] [Article Influence: 13.9] [Reference Citation Analysis (1)] |
| 9. | Alsaad KO, Hajeer AH, Al Balwi M, Al Moaiqel M, Al Oudah N, Al Ajlan A, AlJohani S, Alsolamy S, Gmati GE, Balkhy H, Al-Jahdali HH, Baharoon SA, Arabi YM. Histopathology of Middle East respiratory syndrome coronovirus (MERS-CoV) infection - clinicopathological and ultrastructural study. Histopathology. 2018;72:516-524. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 204] [Cited by in RCA: 224] [Article Influence: 24.9] [Reference Citation Analysis (1)] |
| 10. | Docherty AB, Harrison EM, Green CA, Hardwick HE, Pius R, Norman L, Holden KA, Read JM, Dondelinger F, Carson G, Merson L, Lee J, Plotkin D, Sigfrid L, Halpin S, Jackson C, Gamble C, Horby PW, Nguyen-Van-Tam JS, Ho A, Russell CD, Dunning J, Openshaw PJ, Baillie JK, Semple MG; ISARIC4C investigators. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ. 2020;369:m1985. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2061] [Cited by in RCA: 2091] [Article Influence: 348.5] [Reference Citation Analysis (0)] |
| 11. | Velarde-Ruiz Velasco JA, García-Jiménez ES, Remes-Troche JM. Hepatic manifestations and impact of COVID-19 on the cirrhotic patient. Rev Gastroenterol Mex (Engl Ed). 2020;85:303-311. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 12. | Lei P, Zhang L, Han P, Zheng C, Tong Q, Shang H, Yang F, Hu Y, Li X, Song Y. Liver injury in patients with COVID-19: clinical profiles, CT findings, the correlation of the severity with liver injury. Hepatol Int. 2020;14:733-742. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 50] [Article Influence: 8.3] [Reference Citation Analysis (1)] |
| 13. | Nardo AD, Schneeweiss-Gleixner M, Bakail M, Dixon ED, Lax SF, Trauner M. Pathophysiological mechanisms of liver injury in COVID-19. Liver Int. 2021;41:20-32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 281] [Cited by in RCA: 276] [Article Influence: 55.2] [Reference Citation Analysis (2)] |
| 14. | Miller B, Silverstein A, Flores M, Xiang W, Cao K, Kumagai H, Mehta HH, Yen K, Kim SJ, Cohen P. SARS-CoV-2 induces a unique mitochondrial transcriptome signature. 2020 Preprint. Available from: Research Square. [DOI] [Full Text] |
| 15. | Malhi H, Kaufman RJ. Endoplasmic reticulum stress in liver disease. J Hepatol. 2011;54:795-809. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 980] [Cited by in RCA: 977] [Article Influence: 65.1] [Reference Citation Analysis (0)] |
| 16. | Peterson TR, Sengupta SS, Harris TE, Carmack AE, Kang SA, Balderas E, Guertin DA, Madden KL, Carpenter AE, Finck BN, Sabatini DM. mTOR complex 1 regulates lipin 1 localization to control the SREBP pathway. Cell. 2011;146:408-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 795] [Cited by in RCA: 983] [Article Influence: 65.5] [Reference Citation Analysis (0)] |
| 17. | Campos-Varela I, Villagrasa A, Simon-Talero M, Riveiro-Barciela M, Ventura-Cots M, Aguilera-Castro L, Alvarez-Lopez P, Nordahl EA, Anton A, Bañares J, Barber C, Barreira-Diaz A, Biagetti B, Camps-Relats L, Ciudin A, Cocera R, Dopazo C, Fernandez A, Jimenez C, Jimenez MM, Jofra M, Gil C, Gomez-Gavara C, Guanozzi D, Guevara JA, Lobo B, Malagelada C, Martinez-Camprecios J, Mayorga L, Miret E, Pando E, Pérez-Lopez A, Pigrau M, Prio A, Rivera-Esteban JM, Romero A, Tasayco S, Vidal-Gonzalez J, Vidal L, Minguez B, Augustin S, Genesca J. The role of liver steatosis as measured with transient elastography and transaminases on hard clinical outcomes in patients with COVID-19. Therap Adv Gastroenterol. 2021;14:17562848211016567. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 18. | Cheung A, Flamm S. Hepatobiliary Complications in Critically Ill Patients. Clin Liver Dis. 2019;23:221-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 19. | Méndez-Sánchez N, Valencia-Rodríguez A, Qi X, Yoshida EM, Romero-Gómez M, George J, Eslam M, Abenavoli L, Xie W, Teschke R, Carrion AF, Keaveny AP. What Has the COVID-19 Pandemic Taught Us so Far? J Clin Transl Hepatol. 2020;8:0024. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 20. | Kariyawasam JC, Jayarajah U, Abeysuriya V, Riza R, Seneviratne SL. Involvement of the Liver in COVID-19: A Systematic Review. Am J Trop Med Hyg. 2022;106:1026-1041. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 21. | Oikonomou KG, Papamichalis P, Zafeiridis T, Xanthoudaki M, Papapostolou E, Valsamaki A, Bouliaris K, Papamichalis M, Karvouniaris M, Vlachostergios PJ, Skoura AL, Komnos A. Gastroenterology and liver disease during COVID-19 and in anticipation of post-COVID-19 era: Current practice and future directions. World J Clin Cases. 2021;9:4918-4938. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 3] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (1)] |
| 22. | Provenzani A, Polidori P. Covid-19 and drug therapy, what we learned. Int J Clin Pharm. 2020;42:833-836. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 23. | Garcia-Cortes M, Robles-Diaz M, Stephens C, Ortega-Alonso A, Lucena MI, Andrade RJ. Drug induced liver injury: an update. Arch Toxicol. 2020;94:3381-3407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 176] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 24. | Muhović D, Bojović J, Bulatović A, Vukčević B, Ratković M, Lazović R, Smolović B. First case of drug-induced liver injury associated with the use of tocilizumab in a patient with COVID-19. Liver Int. 2020;40:1901-1905. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 95] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 25. | Mian P, Allegaert K, Spriet I, Tibboel D, Petrovic M. Paracetamol in Older People: Towards Evidence-Based Dosing? Drugs Aging. 2018;35:603-624. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 26. | Iasella CJ, Johnson HJ, Dunn MA. Adverse Drug Reactions: Type A (Intrinsic) or Type B (Idiosyncratic). Clin Liver Dis. 2017;21:73-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 27. | Egan LJ. Mechanisms of drug toxicity or intolerance. Dig Dis. 2011;29:172-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 28. | Robles-Diaz M, Lucena MI, Kaplowitz N, Stephens C, Medina-Cáliz I, González-Jimenez A, Ulzurrun E, Gonzalez AF, Fernandez MC, Romero-Gómez M, Jimenez-Perez M, Bruguera M, Prieto M, Bessone F, Hernandez N, Arrese M, Andrade RJ; Spanish DILI Registry; SLatinDILI Network; Safer and Faster Evidence-based Translation Consortium. Use of Hy's law and a new composite algorithm to predict acute liver failure in patients with drug-induced liver injury. Gastroenterology. 2014;147:109-118.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 256] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 29. | National Institute of Diabetes and Digestive and Kidney Diseases. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. (2012). [cited Sep 17, 2022]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK547852/. |
| 30. | Bosilkovska M, Walder B, Besson M, Daali Y, Desmeules J. Analgesics in patients with hepatic impairment: pharmacology and clinical implications. Drugs. 2012;72:1645-1669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 120] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 31. | Su YJ, Chang CW, Chen MJ, Lai YC. Impact of COVID-19 on liver. World J Clin Cases. 2021;9:7998-8007. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 12] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (1)] |
| 32. | Lagana SM, Kudose S, Iuga AC, Lee MJ, Fazlollahi L, Remotti HE, Del Portillo A, De Michele S, de Gonzalez AK, Saqi A, Khairallah P, Chong AM, Park H, Uhlemann AC, Lefkowitch JH, Verna EC. Hepatic pathology in patients dying of COVID-19: a series of 40 cases including clinical, histologic, and virologic data. Mod Pathol. 2020;33:2147-2155. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 130] [Cited by in RCA: 184] [Article Influence: 30.7] [Reference Citation Analysis (1)] |
| 33. | Falasca L, Nardacci R, Colombo D, Lalle E, Di Caro A, Nicastri E, Antinori A, Petrosillo N, Marchioni L, Biava G, D'Offizi G, Palmieri F, Goletti D, Zumla A, Ippolito G, Piacentini M, Del Nonno F. Postmortem Findings in Italian Patients With COVID-19: A Descriptive Full Autopsy Study of Cases With and Without Comorbidities. J Infect Dis. 2020;222:1807-1815. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 156] [Cited by in RCA: 148] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 34. | Tarik Aslan A, Yasemin Balaban H. An overview of SARS-COV-2-related hepatic injury. Hepatol Forum. 2021;2:122-127. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 35. | Jothimani D, Venugopal R, Abedin MF, Kaliamoorthy I, Rela M. COVID-19 and the liver. J Hepatol. 2020;73:1231-1240. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 364] [Cited by in RCA: 364] [Article Influence: 60.7] [Reference Citation Analysis (4)] |
| 36. | Henry BM, de Oliveira MHS, Benoit S, Plebani M, Lippi G. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): a meta-analysis. Clin Chem Lab Med. 2020;58:1021-1028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 980] [Cited by in RCA: 1184] [Article Influence: 197.3] [Reference Citation Analysis (0)] |
| 37. | Lei F, Liu YM, Zhou F, Qin JJ, Zhang P, Zhu L, Zhang XJ, Cai J, Lin L, Ouyang S, Wang X, Yang C, Cheng X, Liu W, Li H, Xie J, Wu B, Luo H, Xiao F, Chen J, Tao L, Cheng G, She ZG, Zhou J, Wang H, Lin J, Luo P, Fu S, Ye P, Xiao B, Mao W, Liu L, Yan Y, Chen G, Huang X, Zhang BH, Yuan Y. Longitudinal Association Between Markers of Liver Injury and Mortality in COVID-19 in China. Hepatology. 2020;72:389-398. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 318] [Cited by in RCA: 313] [Article Influence: 52.2] [Reference Citation Analysis (0)] |
| 38. | Sharma A, Jaiswal P, Kerakhan Y, Saravanan L, Murtaza Z, Zergham A, Honganur NS, Akbar A, Deol A, Francis B, Patel S, Mehta D, Jaiswal R, Singh J, Patel U, Malik P. Liver disease and outcomes among COVID-19 hospitalized patients - A systematic review and meta-analysis. Ann Hepatol. 2021;21:100273. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 82] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 39. | Mendizabal M, Piñero F, Ridruejo E, Anders M, Silveyra MD, Torre A, Montes P, Urzúa A, Pages J, Toro LG, Díaz J, Gonzalez Ballerga E, Miranda-Zazueta G, Peralta M, Gutiérrez I, Michelato D, Venturelli MG, Varón A, Vera-Pozo E, Tagle M, García M, Tassara A, Brutti J, Ruiz García S, Bustios C, Escajadillo N, Macias Y, Higuera-de la Tijera F, Gómez AJ, Dominguez A, Castillo-Barradas M, Contreras F, Scarpin A, Schinoni MI, Toledo C, Girala M, Mainardi V, Sanchez A, Bessone F, Rubinstein F, Silva MO. Prospective Latin American cohort evaluating outcomes of patients with COVID-19 and abnormal liver tests on admission. Ann Hepatol. 2021;21:100298. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 40. | Garrido M, Pereira Guedes T, Alves Silva J, Falcão D, Novo I, Archer S, Rocha M, Maia L, Sarmento-Castro R, Pedroto I. Impact of Liver Test Abnormalities and Chronic Liver Disease on the Clinical Outcomes of Patients Hospitalized with COVID-19. GE Port J Gastroenterol. 2021;158:1-12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 41. | Paštrovic F, Lucijanic M, Atic A, Stojic J, Barisic Jaman M, Tjesic Drinkovic I, Zelenika M, Milosevic M, Medic B, Loncar J, Mijic M, Filipec Kanizaj T, Kralj D, Lerotic I, Virovic Jukic L, Ljubicic N, Luetic K, Grgic D, Majerovic M, Ostojic R, Krznaric Z, Luksic I, Piskac Zivkovic N, Keres T, Grabovac V, Persec J, Barsic B, Grgurevic I. Prevalence and Prognostic Impact of Deranged Liver Blood Tests in COVID-19: Experience from the Regional COVID-19 Center over the Cohort of 3812 Hospitalized Patients. J Clin Med. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 42. | Ampuero J, Sánchez Y, García-Lozano MR, Maya-Miles D, Romero Gómez M. Impact of liver injury on the severity of COVID-19: a systematic review with meta-analysis. Rev Esp Enferm Dig. 2021;113:125-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 43. | Balderramo D, Mattos AZ, Mulqui V, Chiesa T, Plácido-Damián Z, Abarca J, Bolomo A, Carlino Y, Bombassaro IZ, Wiltgen D, Castillo LT, Díaz K, Acuña J, Manero E, Prieto J, Carrera E, Díaz-Ferrer J, Debes JD. Abnormal Liver Tests during Hospitalization Predict Mortality in Patients with COVID-19: A Multicenter Study from South America. Can J Gastroenterol Hepatol. 2021;2021:1622533. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 44. | Chaibi S, Boussier J, Hajj WE, Abitbol Y, Taieb S, Horaist C, Jouannaud V, Wang P, Piquet J, Maurer C, Lahmek P, Nahon S. Liver function test abnormalities are associated with a poorer prognosis in Covid-19 patients: Results of a French cohort. Clin Res Hepatol Gastroenterol. 2021;45:101556. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 45. | Lok J, Gess M. Liver dysfunction in COVID-19: a useful prognostic marker of severe disease? Frontline Gastroenterol. 2021;12:293-298. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 46. | Gutmann C, Khamina K, Theofilatos K, Diendorfer AB, Burnap SA, Nabeebaccus A, Fish M, McPhail MJW, O'Gallagher K, Schmidt LE, Cassel C, Auzinger G, Napoli S, Mujib SF, Trovato F, Sanderson B, Merrick B, Roy R, Edgeworth JD, Shah AM, Hayday AC, Traby L, Hackl M, Eichinger S, Shankar-Hari M, Mayr M. Association of cardiometabolic microRNAs with COVID-19 severity and mortality. Cardiovasc Res. 2022;118:461-474. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 53] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 47. | Dong ZY, Xiang BJ, Jiang M, Sun MJ, Dai C. The Prevalence of Gastrointestinal Symptoms, Abnormal Liver Function, Digestive System Disease and Liver Disease in COVID-19 Infection: A Systematic Review and Meta-Analysis. J Clin Gastroenterol. 2021;55:67-76. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 52] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 48. | Higuera-de la Tijera F, Servín-Caamaño A, Reyes-Herrera D, Flores-López A, Robiou-Vivero EJA, Martínez-Rivera F, Galindo-Hernández V, Chapa-Azuela O, Chávez-Morales A, Rosales-Salyano VH. Impact of liver enzymes on SARS-CoV-2 infection and the severity of clinical course of COVID-19. Liver Res. 2021;5:21-27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 49. | Yip TC, Lui GC, Wong VW, Chow VC, Ho TH, Li TC, Tse YK, Hui DS, Chan HL, Wong GL. Liver injury is independently associated with adverse clinical outcomes in patients with COVID-19. Gut. 2021;70:733-742. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 119] [Cited by in RCA: 124] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 50. | Roman A, Moldovan S, Santini A, Stoian M, Dobru D. Impact of the Severity of Liver Injury in COVID-19 Patients Admitted to an Intensive Care Unit During the SARS-CoV2 Pandemic Outbreak. J Crit Care Med (Targu Mures). 2021;7:211-216. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 51. | Azad Allarakia BM, Gattan HS, Abdeen RH, Al-Ahmadi BM, Shater AF, Bazaid MB, Althomali OW, Bazaid AS. Predicting Intensive Care Unit Admission for COVID-19 Patients from Laboratory Results. Dis Markers. 2022;2022:4623901. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 52. | Zhao Y, Zhou J, Pan L, Zhang Y, Wang H, Wu W, He J, Chen J, Huang H. Detection and analysis of clinical features of patients with different types of coronavirus disease 2019. J Med Virol. 2021;93:401-408. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 53. | Salık F, Uzundere O, Bıçak M, Akelma H, Akgündüz M, Korhan Z, Kandemir D, Kaçar CK. Liver function as a predictor of mortality in COVID-19: A retrospective study. Ann Hepatol. 2021;26:100553. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 54. | Kasapoglu B, Yozgat A, Tanoglu A, Can G, Sakin YS, Kekilli M. Gamma-glutamyl-transferase may predict COVID-19 outcomes in hospitalised patients. Int J Clin Pract. 2021;75:e14933. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 55. | Fan H, Cai J, Tian A, Li Y, Yuan H, Jiang Z, Yu Y, Ruan L, Hu P, Yue M, Chen N, Li J, Zhu C. Comparison of Liver Biomarkers in 288 COVID-19 Patients: A Mono-Centric Study in the Early Phase of Pandemic. Front Med (Lausanne). 2020;7:584888. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 56. | Li T, Guo Y, Zhuang X, Huang L, Zhang X, Wei F, Yang B. Abnormal liver-related biomarkers in COVID-19 patients and the role of prealbumin. Saudi J Gastroenterol. 2020;26:272-278. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 57. | McPherson S, Stewart SF, Henderson E, Burt AD, Day CP. Simple non-invasive fibrosis scoring systems can reliably exclude advanced fibrosis in patients with non-alcoholic fatty liver disease. Gut. 2010;59:1265-1269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 572] [Cited by in RCA: 699] [Article Influence: 43.7] [Reference Citation Analysis (0)] |
| 58. | Crisan D, Avram L, Grapa C, Dragan A, Radulescu D, Crisan S, Grosu A, Militaru V, Buzdugan E, Stoicescu L, Radulescu L, Ciovicescu F, Jivanescu DB, Mocan O, Micu B, Donca V, Marinescu L, Macarie A, Rosu M, Nemes A, Craciun R. Liver Injury and Elevated FIB-4 Define a High-Risk Group in Patients with COVID-19. J Clin Med. 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 59. | Liu M, Mei K, Tan Z, Huang S, Liu F, Deng C, Ma J, Yu P, Liu X. Liver Fibrosis Scores and Hospitalization, Mechanical Ventilation, Severity, and Death in Patients with COVID-19: A Systematic Review and Dose-Response Meta-Analysis. Can J Gastroenterol Hepatol. 2022;2022:7235860. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 60. | Çelik I, Öztürk R. From asymptomatic to critical illness: decoding various clinical stages of COVID-19. Turk J Med Sci. 2021;51:3284-3300. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 61. | Qian SZ, Hong WD, Lingjie-Mao, Chenfeng-Lin, Zhendong-Fang, Pan JY. Clinical Characteristics and Outcomes of Severe and Critical Patients With 2019 Novel Coronavirus Disease (COVID-19) in Wenzhou: A Retrospective Study. Front Med (Lausanne). 2020;7:552002. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 62. | Bender JM, Worman HJ. Coronavirus Disease 2019 and Liver Injury: A Retrospective Analysis of Hospitalized Patients in New York City. J Clin Transl Hepatol. 2021;9:551-558. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 63. | Shehab M, Alrashed F, Shuaibi S, Alajmi D, Barkun A. Gastroenterological and hepatic manifestations of patients with COVID-19, prevalence, mortality by country, and intensive care admission rate: systematic review and meta-analysis. BMJ Open Gastroenterol. 2021;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 49] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 64. | Sikkema BJB, Sint Nicolaas JJ, van Wijngaarden PP. No association between COVID-19 related liver injury and the course of disease: a retrospective study. Scand J Gastroenterol. 2021;56:68-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 65. | Phipps MM, Barraza LH, LaSota ED, Sobieszczyk ME, Pereira MR, Zheng EX, Fox AN, Zucker J, Verna EC. Acute Liver Injury in COVID-19: Prevalence and Association with Clinical Outcomes in a Large U.S. Cohort. Hepatology. 2020;72:807-817. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 201] [Cited by in RCA: 279] [Article Influence: 46.5] [Reference Citation Analysis (3)] |
| 66. | Yang N, Tian K, Jin M, Zhang X, Zhang F, Shi X, Wang X, Niu S, Shi J, Hu K, Liu K, Peng P, Wang Y, Zhang H, Tian J. Sudden death of COVID-19 patients in Wuhan, China: A retrospective cohort study. J Glob Health. 2021;11:05006. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 67. | Boettler T, Marjot T, Newsome PN, Mondelli MU, Maticic M, Cordero E, Jalan R, Moreau R, Cornberg M, Berg T. Impact of COVID-19 on the care of patients with liver disease: EASL-ESCMID position paper after 6 months of the pandemic. JHEP Rep. 2020;2:100169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 121] [Article Influence: 20.2] [Reference Citation Analysis (1)] |
| 68. | Huang H, Li H, Chen S, Zhou X, Dai X, Wu J, Zhang J, Shao L, Yan R, Wang M, Wang J, Tu Y, Ge M. Prevalence and Characteristics of Hypoxic Hepatitis in COVID-19 Patients in the Intensive Care Unit: A First Retrospective Study. Front Med (Lausanne). 2020;7:607206. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 69. | Zhang Y, Liu J, Yu L, Zhou N, Ding W, Zheng S, Shi D, Li L. Prevalence and characteristics of hypoxic hepatitis in the largest single-centre cohort of avian influenza A(H7N9) virus-infected patients with severe liver impairment in the intensive care unit. Emerg Microbes Infect. 2016;5:e1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 70. | Edwards K, Allison M, Ghuman S. Secondary sclerosing cholangitis in critically ill patients: a rare disease precipitated by severe SARS-CoV-2 infection. BMJ Case Rep. 2020;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 71. | Bütikofer S, Lenggenhager D, Wendel Garcia PD, Maggio EM, Haberecker M, Reiner CS, Brüllmann G, Buehler PK, Gubler C, Müllhaupt B, Jüngst C, Morell B. Secondary sclerosing cholangitis as cause of persistent jaundice in patients with severe COVID-19. Liver Int. 2021;41:2404-2417. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 58] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 72. | Hunyady P, Streller L, Rüther DF, Groba SR, Bettinger D, Fitting D, Hamesch K, Marquardt JU, Mücke VT, Finkelmeier F, Sekandarzad A, Wengenmayer T, Bounidane A, Weiss F, Peiffer KH, Schlevogt B, Zeuzem S, Waidmann O, Hollenbach M, Kirstein MM, Kluwe J, Kütting F, Mücke MM. Secondary sclerosing cholangitis following COVID-19 disease: a multicenter retrospective study. Clin Infect Dis. 2022;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 73. | Liemarto AK, Budiono BP, Chionardes MA, Oliviera I, Rahmasiwi A. Liver abscess with necrosis in post COVID-19: A case report. Ann Med Surg (Lond). 2021;72:103107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 74. | Harouachi A, Bouhout T, Hadj Kacem H, Serji B, Berkhli H, Madani H, El Harroudi T. Acute hepatitis with portal and mesenteric vein thrombosis revealing SARS-CoV-2 infection: Case report and literature review. Ann Med Surg (Lond). 2022;77:103706. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 75. | Haji Esmaeil Memar E, Mamishi S, Sharifzadeh Ekbatani M, Alimadadi H, Yaghmaei B, Chegini V, Janani S, Mahmoudi S. Fulminant hepatic failure: A rare and devastating manifestation of Coronavirus disease 2019 in an 11-year-old boy. Arch Pediatr. 2020;27:502-505. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (1)] |
| 76. | Romaní Vidal A, Vaughan A, Innocenti F, Colombe S, Nerlander L, Rachwal N, Ciancio BC, Mougkou A, Carvalho C, Delgado E, Mook P, de Muylder G, Peeters M, Tenev T, Golkocheva-Markova E, Vorobieva Solholm Jensen V, Koch A, Figoni J, Brouard C, Nikolopoulou G, Zisouli A, Murphy N, Broderick A, Goldberg L, Rich R, Hecht Sagie L, Tosti ME, Suligoi B, Joosten R, Pijnacker R, Fjeldheim I, Heen E, Stępień M, Polański P, Tato Marinho R, Vieira Martins J, Varela C, Avellón A, Andersson E, Jansson Mörk M, Mandal S, Watson C, Coughlan L, Chand M, Neill C, Bradley DT, Li K, O'Leary M, McInnes N, Williams CJ, Moore C, Gjini A, Duffell E, Pebody R. Hepatitis of unknown aetiology in children - epidemiological overview of cases reported in Europe, 1 January to 16 June 2022. Euro Surveill. 2022;27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 77. | Busani S, Bedini A, Biagioni E, Serio L, Tonelli R, Meschiari M, Franceschini E, Guaraldi G, Cossarizza A, Clini E, Maiorana A, Gennari W, De Maria N, Luppi M, Mussini C, Girardis M; Modena Covid-19 Working Group (MoCo19). Two Fatal Cases of Acute Liver Failure Due to HSV-1 Infection in COVID-19 Patients Following Immunomodulatory Therapies. Clin Infect Dis. 2021;73:e252-e255. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 78. | Fix OK, Hameed B, Fontana RJ, Kwok RM, McGuire BM, Mulligan DC, Pratt DS, Russo MW, Schilsky ML, Verna EC, Loomba R, Cohen DE, Bezerra JA, Reddy KR, Chung RT. Clinical Best Practice Advice for Hepatology and Liver Transplant Providers During the COVID-19 Pandemic: AASLD Expert Panel Consensus Statement. Hepatology. 2020;72:287-304. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 338] [Cited by in RCA: 425] [Article Influence: 70.8] [Reference Citation Analysis (0)] |
| 79. | Premkumar M, Kajal K, Kulkarni AV, Gupta A, Divyaveer S. Point-of-Care Echocardiography and Hemodynamic Monitoring in Cirrhosis and Acute-on-Chronic Liver Failure in the COVID-19 Era. J Intensive Care Med. 2021;36:511-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 80. | Effenberger M, Grander C, Fritsche G, Bellmann-Weiler R, Hartig F, Wildner S, Seiwald S, Adolph TE, Zoller H, Weiss G, Tilg H. Liver stiffness by transient elastography accompanies illness severity in COVID-19. BMJ Open Gastroenterol. 2020;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 81. | Demirtas CO, Keklikkiran C, Ergenc I, Erturk Sengel B, Eskidemir G, Cinel I, Odabasi Z, Korten V, Yilmaz Y. Liver stiffness is associated with disease severity and worse clinical scenarios in coronavirus disease 2019: A prospective transient elastography study. Int J Clin Pract. 2021;75:e14363. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 82. | Cornberg M, Buti M, Eberhardt CS, Grossi PA, Shouval D. EASL position paper on the use of COVID-19 vaccines in patients with chronic liver diseases, hepatobiliary cancer and liver transplant recipients. J Hepatol. 2021;74:944-951. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 183] [Cited by in RCA: 172] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 83. | Rossi RE, Chen J, Caplin ME. The Role of Diet and Supplements in the Prevention and Progression of COVID-19: Current Knowledge and Open Issues. Prev Nutr Food Sci. 2022;27:137-149. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 84. | Dalekos GN, Stefos A, Georgiadou S, Lygoura V, Michail A, Ntaios G, Samakidou A, Giannoulis G, Gabeta S, Vlychou M, Petinaki E, Leventogiannis K, Giamarellos-Bourboulis EJ, Gatselis NK. Lessons from pathophysiology: Use of individualized combination treatments with immune interventional agents to tackle severe respiratory failure in patients with COVID-19. Eur J Intern Med. 2021;88:52-62. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 85. | Mameli S, Marcialis MA, Bassareo PP, Fanos V. COVID-19 and hepatic damage: what we know? Panminerva Med. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 86. | Ronco C, Bagshaw SM, Bellomo R, Clark WR, Husain-Syed F, Kellum JA, Ricci Z, Rimmelé T, Reis T, Ostermann M. Extracorporeal Blood Purification and Organ Support in the Critically Ill Patient during COVID-19 Pandemic: Expert Review and Recommendation. Blood Purif. 2021;50:17-27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 88] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 87. | Tampe D, Korsten P, Bremer SCB, Winkler MS, Tampe B. Kinetics of Bilirubin and Ammonia Elimination during Hemadsorption Therapy in Secondary Sclerosing Cholangitis Following ECMO Therapy and Severe COVID-19. Biomedicines. 2021;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 88. | Ozkurt Z, Çınar Tanrıverdi E. COVID-19: Gastrointestinal manifestations, liver injury and recommendations. World J Clin Cases. 2022;10:1140-1163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 29] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (7)] |
| 89. | Váncsa S, Hegyi PJ, Zádori N, Szakó L, Vörhendi N, Ocskay K, Földi M, Dembrovszky F, Dömötör ZR, Jánosi K, Rakonczay Z Jr, Hartmann P, Horváth T, Erőss B, Kiss S, Szakács Z, Németh D, Hegyi P, Pár G. Pre-existing Liver Diseases and On-Admission Liver-Related Laboratory Tests in COVID-19: A Prognostic Accuracy Meta-Analysis With Systematic Review. Front Med (Lausanne). 2020;7:572115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 90. | Simon TG, Hagström H, Sharma R, Söderling J, Roelstraete B, Larsson E, Ludvigsson JF. Risk of severe COVID-19 and mortality in patients with established chronic liver disease: a nationwide matched cohort study. BMC Gastroenterol. 2021;21:439. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 91. | Sharma P, Kumar A, Anikhindi S, Bansal N, Singla V, Shivam K, Arora A. Effect of COVID-19 on Pre-existing Liver disease: What Hepatologist Should Know? J Clin Exp Hepatol. 2021;11:484-493. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 92. | Romero-Cristóbal M, Clemente-Sánchez A, Piñeiro P, Cedeño J, Rayón L, Del Río J, Ramos C, Hernández DA, Cova M, Caballero A, Garutti I, García-Olivares P, Hortal J, Guerrero JE, García R, Bañares R, Rincón D. Possible unrecognised liver injury is associated with mortality in critically ill COVID-19 patients. Therap Adv Gastroenterol. 2021;14:17562848211023410. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 93. | Wilde H, Mellan T, Hawryluk I, Dennis JM, Denaxas S, Pagel C, Duncan A, Bhatt S, Flaxman S, Mateen BA, Vollmer SJ. The association between mechanical ventilator compatible bed occupancy and mortality risk in intensive care patients with COVID-19: a national retrospective cohort study. BMC Med. 2021;19:213. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 94. | Bravata DM, Perkins AJ, Myers LJ, Arling G, Zhang Y, Zillich AJ, Reese L, Dysangco A, Agarwal R, Myers J, Austin C, Sexson A, Leonard SJ, Dev S, Keyhani S. Association of Intensive Care Unit Patient Load and Demand With Mortality Rates in US Department of Veterans Affairs Hospitals During the COVID-19 Pandemic. JAMA Netw Open. 2021;4:e2034266. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 221] [Cited by in RCA: 213] [Article Influence: 42.6] [Reference Citation Analysis (0)] |
| 95. | Williams R, Alessi C, Alexander G, Allison M, Aspinall R, Batterham RL, Bhala N, Day N, Dhawan A, Drummond C, Ferguson J, Foster G, Gilmore I, Goldacre R, Gordon H, Henn C, Kelly D, MacGilchrist A, McCorry R, McDougall N, Mirza Z, Moriarty K, Newsome P, Pinder R, Roberts S, Rutter H, Ryder S, Samyn M, Severi K, Sheron N, Thorburn D, Verne J, Williams J, Yeoman A. New dimensions for hospital services and early detection of disease: a Review from the Lancet Commission into liver disease in the UK. Lancet. 2021;397:1770-1780. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 96. | Akbulut S, Garzali IU, Hargura AS, Aloun A, Yilmaz S. Screening, Surveillance, and Management of Hepatocellular Carcinoma During the COVID-19 Pandemic: a Narrative Review. J Gastrointest Cancer. 2022;1-12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 97. | Abd Elbaset HS, Sultan AM, Montasser IF, Soliman HEM, Elayashy M, Makhlouf NA; Scientific Committee of Ministry of Health (MOH) National Project of Waiting Lists, Egypt. Egyptian protocol for living donor liver transplantation (LDLT) during SARS-CoV-2 pandemic. Egypt Liver J. 2021;11:14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 98. | Muller X, Tilmans G, Chenevas-Paule Q, Lebossé F, Antonini T, Poinsot D, Rode A, Guichon C, Schmitt Z, Ducerf C, Mohkam K, Lesurtel M, Mabrut JY. Strategies for liver transplantation during the SARS-CoV-2 outbreak: Preliminary experience from a single center in France. Am J Transplant. 2020;20:2989-2996. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 99. | Pereira MR, Mohan S, Cohen DJ, Husain SA, Dube GK, Ratner LE, Arcasoy S, Aversa MM, Benvenuto LJ, Dadhania DM, Kapur S, Dove LM, Brown RS Jr, Rosenblatt RE, Samstein B, Uriel N, Farr MA, Satlin M, Small CB, Walsh TJ, Kodiyanplakkal RP, Miko BA, Aaron JG, Tsapepas DS, Emond JC, Verna EC. COVID-19 in solid organ transplant recipients: Initial report from the US epicenter. Am J Transplant. 2020;20:1800-1808. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 541] [Cited by in RCA: 668] [Article Influence: 111.3] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Critical care medicine
Country/Territory of origin: Greece
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Dasuqi SA, Saudi Arabia; Tusabe F S-Editor: Chang KL L-Editor: Filipodia P-Editor: Chang KL