Published online Oct 16, 2023. doi: 10.12998/wjcc.v11.i29.7156
Peer-review started: June 22, 2023
First decision: August 16, 2023
Revised: August 28, 2023
Accepted: September 6, 2023
Article in press: September 6, 2023
Published online: October 16, 2023
Processing time: 113 Days and 4.6 Hours
Platelet transfusion is of great significance in the treatment of thrombocytopenia caused by myelosuppression during intensive chemotherapy in patients with acute leukemia. In recent years, with platelet transfusion increasing, ineffective platelet transfusion has become increasingly prominent. Generally speaking, platelet antibodies can be produced after repeated transfusion, thus rendering subsequent platelet transfusion ineffective. We report a case of first platelet transfusion refractoriness (PTR) in a patient with acute myelocytic leukemia (AML). Due to the rarity of such cases in clinical practice, there have been no relevant case reports so far.
A 51-year-old female patient attended the hospital due to throat pain and abnormal blood cells for 4 d. Her diagnosis was acute myelocytic leukemia [M2 type Fms related receptor tyrosine kinase 3, Isocitrate Dehydrogenase 1, Nucleophosmin 1, Neuroblastoma RAS viral oncogene homolog (+) high-risk group]. She was treated with "IA" (IDA 10 mg day 1-3 and Ara-C 0.2 g day 1-5) chemotherapy. When her condition improved, the patient was discharged from the hospital, instructed to take medicine as prescribed by the doctor after discharge, and returned to the hospital for further chemotherapy on time.
We report a rare case of first platelet transfusion failure in a patient with AML during induction chemotherapy, which may be related to the production of platelet antibodies induced by antibiotics and excessive tumor load. This also suggests that we should consider the influence of antibiotics when the rare situation of first platelet transfusion failure occurs in patients with AML. When platelet antibodies are produced, immunoglobulins can be used to block antibodies, thereby reducing platelet destruction. For patients with PTR, both immune and non-immune factors need to be considered and combined in clinical practice along with individualized treatment to effectively solve the problem.
Core Tip: We report a rare case of first platelet transfusion failure in a patient with acute myelocytic leukemia (AML) during induction chemotherapy, which may be related to the production of platelet antibodies induced by antibiotics and excessive tumor load. This also suggests that we should consider the influence of antibiotics when the rare situation of first platelet transfusion failure occurs in patients with AML. When platelet antibodies are produced, immunoglobulins can be used to block antibodies, thereby reducing platelet destruction. For patients with platelet transfusion refractoriness, both immune and non-immune factors need to be considered and combined in clinical practice along with individualized treatment to effectively solve the problem.
- Citation: Tu SK, Fan HJ, Shi ZW, Li XL, Li M, Song K. First platelet transfusion refractoriness in a patient with acute myelocytic leukemia: A case report. World J Clin Cases 2023; 11(29): 7156-7161
- URL: https://www.wjgnet.com/2307-8960/full/v11/i29/7156.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i29.7156
Platelet transfusion refractoriness (PTR) occurs in patients who have received two or more consecutive and sufficient doses of ABO blood group from a random donor source with platelet consistency, and after infusion blood platelet counts do not effectively increase[1,2]. In this case report, the patient presented with a rare initial platelet transfusion that was ineffective. Due to the rarity of such cases in clinical practice, there have been no relevant case reports so far.
A 51-year-old female patient attended the hospital due to throat pain and abnormal blood cells for 4 d.
The patient stated that she had a sore throat accompanied by retching and diarrhea with no obvious cause for 4 d; however, the details were unclear. A routine blood examination at the local hospital showed elevated leukocytes and referral to another hospital for treatment was recommended.
Past medical history included intermittent ecchymosis of limbs for 20 years, low platelet count for 20 years, recurrent sore throat and hoarseness for more than 10 years, and surgery for a pharyngeal cyst.
No special personal or family history.
Upon examination, the patient’s vital signs were stable. Physical examination revealed an enlarged lymph node with a diameter of approximately 1 cm in the right axillary fossa and two ecchymoses with a diameter of 0.5 cm in the right lower limb. Moreover, there was tenderness in the sternum, tenderness beneath the xiphoid process, and percussion pain in the liver area. The lips were slightly pale and the pharynx was not red.
Auxiliary examinations (August 12, 2021) in the emergency department revealed the following: Leukocytes 148.22 × 109/L, erythrocytes 3.28 × 1012/L, hemoglobin concentration 105 g/L, and platelet count 97 × 109/L. The results of a bone marrow biopsy after admission indicated possible AML (Figure 1) and the findings of a marrow smear are shown in Figure 2. Common fusion gene screening for leukemia was negative.
Acute myelocytic leukemia (AML) [M2 type Fms related receptor tyrosine kinase 3 (FLT3), Isocitrate Dehydrogenase 1 (IDH1), Nucleophosmin 1 (NPM1), Neuroblastoma RAS viral oncogene homolog (NRAS) (+) high-risk group].
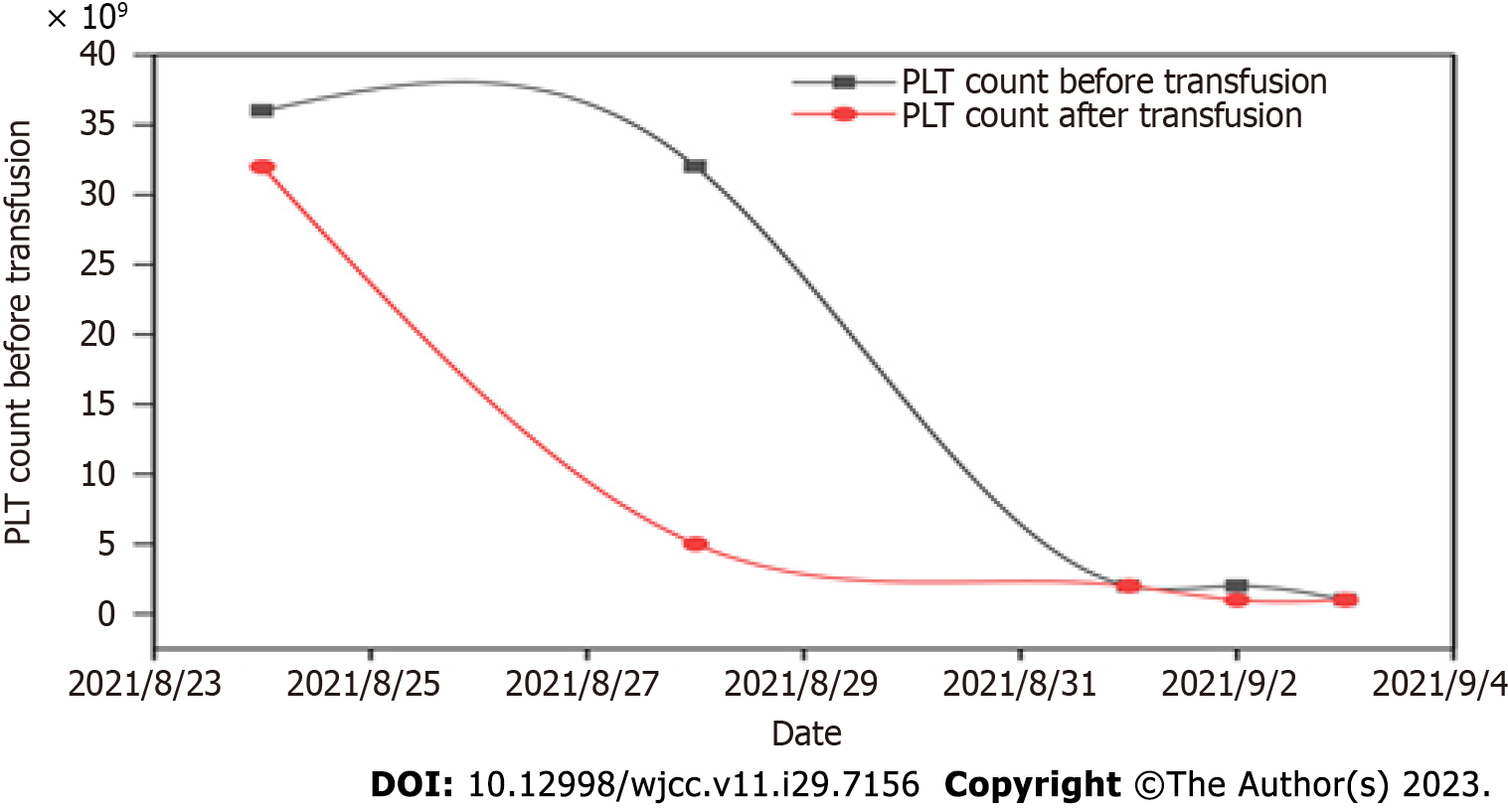
On August 22, 2021, "IA" (IDA 10 mg day 1-3 and Ara-C 0.2 g day 1-5) chemotherapy was administered. During chemotherapy, the patient developed severe myelosuppression, and severe agranulocytosis with extremely low platelets. The following drugs were given successively: Sulperazon, teicoplanin, and meropenem to resist bacteria, voriconazole to prevent fungal infection, recombinant human granulocyte-stimulating factor to increase leukocytes, thrombopoietin (TPO) to promote platelet production, as well as the transfusion of platelets and suspended leukocyte-depleted red blood cells. During this period, ineffective platelet transfusion occurred (Figure 3). The five items of anti-platelet antibody testing were improved with positive anti-platelet membrane glycoprotein IIb and IIIa specific antibodies. Due to ineffective platelet transfusion, referral to another hospital for human leukocyte antigen matching and bone marrow transplantation was recommended. However, the patient's family refused due to economic reasons. Subsequently, administration of gamma globulin reduced platelet destruction, myelosuppression improved, and platelets returned to normal.
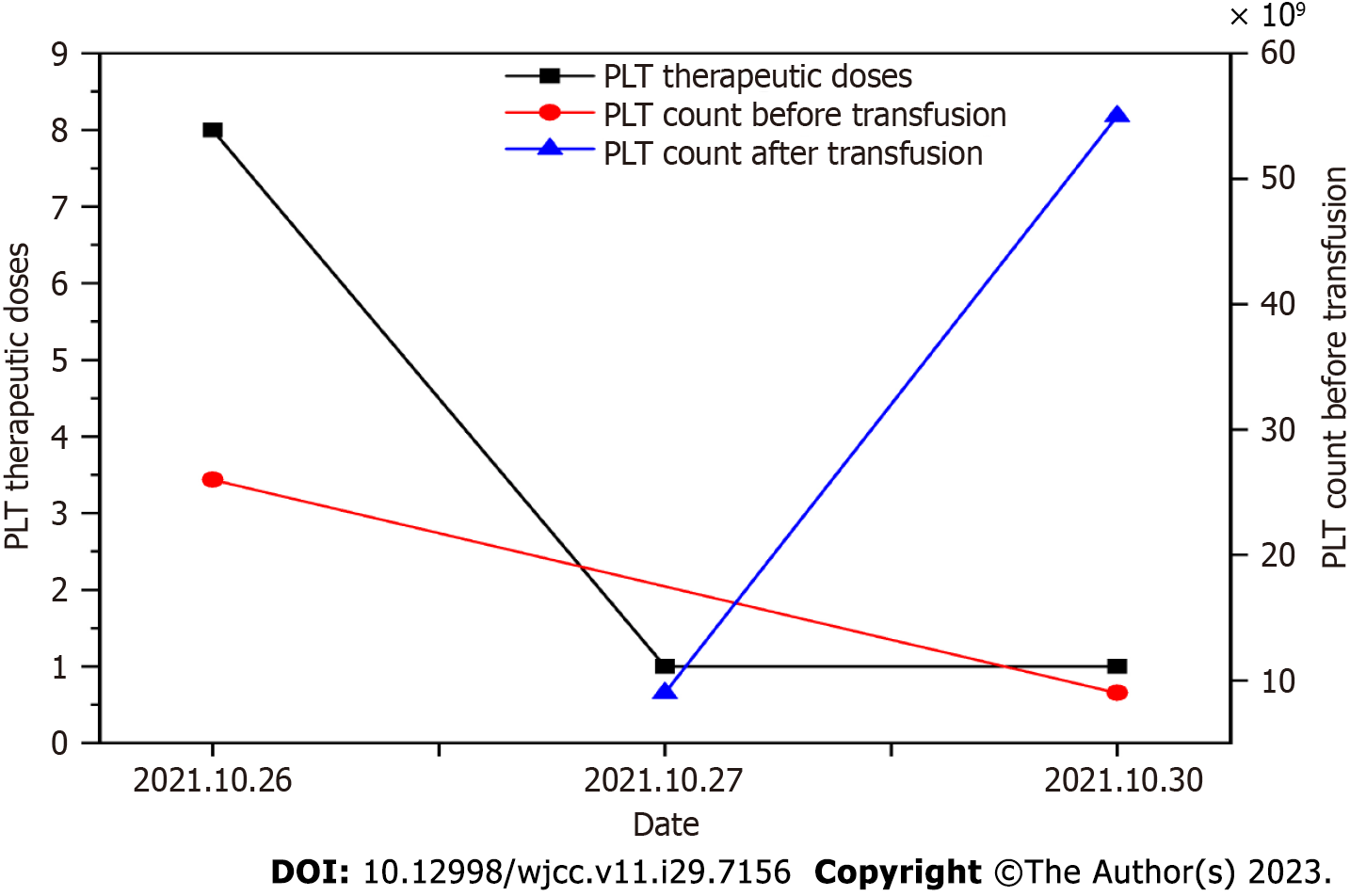
The patient was rehospitalized for the second cycle of chemotherapy on October 3, 2021. No discomfort was mentioned at the time of admission. The five items of antiplatelet antibody testing were improved. Bone marrow cell morphology showed a myelogram of trilineage hyperplasia and an increased erythroid ratio following AML treatment. Flow cytometry of bone marrow showed that no primitive/naive myeloid cells with obvious abnormal immunophenotypes were detected in samples. The result of bone marrow chromosome testing was Karyotype, 46, XX, and the analysis of 20 metaphase cells showed non-clonal structural and numerical aberrations. After admission, "IA" (IDA 10 mg day 1-3 Ara-C 0.2 g day 1-5) chemotherapy was administered on October 14, 2021. During chemotherapy, the patient also developed myelosuppression and was treated with platelet transfusion, resulting in a gradual increase of platelets (Figure 4).
The chemotherapy process went smoothly as did symptomatic and supportive treatments with increased leukocytes, anti-infection, and maintained water and electrolyte balance.
PTR occurs in a patient who has received two or more consecutive and sufficient doses of ABO blood group from a random donor source with platelet consistency, and after infusion blood platelet counts do not effectively increase[1,2]. Studies[3] have found that platelet transfusion rates can be ineffective in 25%–60% of cases. In this case report, the patient had a rare initial platelet transfusion that was ineffective.
PTR is a serious problem often encountered in clinical transfusion therapy, and is mainly observed in patients with blood system diseases requiring platelet transfusion. Existing studies[4,5] have shown that ineffective platelet transfusion is mainly caused by immune and non-immune factors (Table 1). Previous studies have found that PTR mainly occurs in patients with multiple or repeated blood transfusions but in this case report, the patient suffered from ineffective initial platelet transfusion, and the reason for this is worth exploring. At present, PTR is mainly considered to be caused by tumor load and the production of platelet antibodies induced by antibiotics.
| Nonimmune causes | Immune-mediated causes |
| Fever, infection, or sepsis | Antibodies against HLA class I |
| Bleeding | ABO-mismatched platelets |
| Accelerated platelet consumption (DIC, microangiopathic hemolytic anemia) | Antibodies against human platelet antigens |
| Drugs (amphotericin B, vancomycin, ATG, interferons) | Antibodies against drug–platelet glycoprotein complex |
| Splenic sequestration | |
| Graft-versus-host disease | |
| Poor platelet quality or greater storage age |
In this case, severe myelopathic depression occurred during induction chemotherapy. To prevent bacterial and fungal infection, cefoperazone sulbactam sodium, teicoplanin, meropenem, and voriconazole were successively administered. Previous studies[6-8] have reported that cefoperazone sulbactam sodium can act as an immune mediator in vivo, causing immune responses and destroying platelets, and simultaneously binding to complete antigens as haptens and carriers in vivo to stimulate antibody production in the body. After drug sensitization, contact with the same drug to form an antigen-antibody complex and adsorption on the platelet membrane can activate complement and quickly clear the circulation of platelets. This may be one reason why the patient's first platelet infusion was ineffective. Li et al[9] found that the higher the white blood cell count in patients with leukemia, the more serious the destruction of blood vessel walls. Elevated white blood cells can induce the formation of a thrombus and platelet aggregation. Activation and consumption increase during the process of thrombus formation, thereby affecting platelet infusion. In the second cycle of chemotherapy, the patient also showed myelosuppression and platelet count decline and platelet infusion was effective. At the beginning of chemotherapy, the patient's white blood cells basically returned to the normal range and the tumor load decreased. Thus, we consider that high tumor load might also be one of the reasons why platelet transfusion was ineffective.
We report a rare case of first platelet transfusion failure in a patient with AML during induction chemotherapy, which may be related to the production of platelet antibodies induced by antibiotics and excessive tumor load. This also suggests that we should consider the influence of antibiotics when the rare situation of first platelet transfusion failure occurs in patients with AML. When platelet antibodies are produced, immunoglobulins can be used to block antibodies, thereby reducing platelet destruction. For patients with PTR, both immune and non-immune factors need to be considered and combined in clinical practice along with individualized treatment to effectively solve the problem.
| 1. | Forest SK, Hod EA. Management of the Platelet Refractory Patient. Hematol Oncol Clin North Am. 2016;30:665-677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 2. | Saris A, Pavenski K. Human Leukocyte Antigen Alloimmunization and Alloimmune Platelet Refractoriness. Transfus Med Rev. 2020;34:250-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 3. | Lieberman L, Liu Y, Portwine C, Barty RL, Heddle NM. An epidemiologic cohort study reviewing the practice of blood product transfusions among a population of pediatric oncology patients. Transfusion. 2014;54:2736-2744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 4. | Youk HJ, Hwang SH, Oh HB, Ko DH. Evaluation and management of platelet transfusion refractoriness. Blood Res. 2022;57:6-10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 5. | Cohn CS. Platelet transfusion refractoriness: how do I diagnose and manage? Hematology Am Soc Hematol Educ Program. 2020;2020:527-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 69] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 6. | Wong RS, Cheng G, Chan NP, Wong WS, NG MH. Use of cefoperazone still needs a caution for bleeding from induced vitamin K deficiency. Am J Hematol. 2006;81:76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | Vayne C, Guéry EA, Rollin J, Baglo T, Petermann R, Gruel Y. Pathophysiology and Diagnosis of Drug-Induced Immune Thrombocytopenia. J Clin Med. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 96] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 8. | Bakchoul T, Marini I. Drug-associated thrombocytopenia. Hematology Am Soc Hematol Educ Program. 2018;2018:576-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 86] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 9. | Li XY, Zhou YL, Zhang Y, Huang X, Li JH, Wang HP, Zhou D, Li L, Zhu LX, Xie MX, Huang XB, Xie WZ, Lou YJ, Meng HT, Yu WJ, Tong HY, Jin J, Ye XJ, Zhu HH. Coagulation profile in newly diagnosed T-cell acute lymphoblastic leukemia. Thromb Res. 2021;203:69-71. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Lin Q; Sultana N, Bangladesh S-Editor: Li L L-Editor: Webster JR P-Editor: Zhao S