Published online Oct 16, 2023. doi: 10.12998/wjcc.v11.i29.7127
Peer-review started: June 8, 2023
First decision: August 31, 2023
Revised: September 9, 2023
Accepted: September 25, 2023
Article in press: September 25, 2023
Published online: October 16, 2023
Processing time: 126 Days and 23.3 Hours
Digital subtraction angiography (DSA), the gold standard of cerebrovascular dis
Case 1: A 65-year-old man presented with a five-year history of bilateral lower limb weakness due to stroke. Physical examination showed decreased strength (5-/5) in both lower limbs. Carotid artery ultrasound, magnetic resonance angiogra
IVUS aids decision-making during CAS by accurately assessing carotid artery wall lesions and plaque nature pre
Core Tip: Intravascular ultrasonography (IVUS) improves carotid artery stenting (CAS) evaluations and outcomes vs those of the traditional gold-standard, digital subtraction angiography (DSA). We present two cases of cerebrovascular diseases. Case 1 involves a 65-year-old patient with stroke. IVUS-assisted CAS was performed after right proximal internal carotid artery stenosis was diagnosed via DSA. Preoperative IVUS imaging showed a low-risk plaque and accurately measured the ste
- Citation: Fu PC, Wang JY, Su Y, Liao YQ, Li SL, Xu GL, Huang YJ, Hu MH, Cao LM. Intravascular ultrasonography assisted carotid artery stenting for treatment of carotid stenosis: Two case reports. World J Clin Cases 2023; 11(29): 7127-7135
- URL: https://www.wjgnet.com/2307-8960/full/v11/i29/7127.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i29.7127
Intravascular ultrasonography (IVUS) is a novel method for diagnosing vascular diseases. It involves placing a minia
In recent years, neurologists have shown increasing interest in utilizing the advantages of IVUS for the treatment of cerebrovascular diseases. Further, by advancing research and development, the widespread application of IVUS has become increasingly urgent owing to the high incidence and disability rate of stroke[2,3]. IVUS not only provides high-resolution images of vessel walls and lumens but it can also distinguish various arterial plaque components and irregular structures in vessel walls based on differences in echo intensity[4]. IVUS has become a powerful tool for diagnosing and treating endovascular lesions (such as arterial dissection and arterial stenosis), specifically for preoperative evaluation and postoperative optimization in carotid artery stenting (CAS)[5-7]. IVUS is used to preoperatively evaluate the characteristics of carotid plaques and measure the length and diameter of diseased blood vessels to select the appropriate stent[4,8]. Postoperative examinations of plaque protrusions using IVUS have been shown to be more accurate than those of digital subtraction angiography (DSA)[9]. IVUS is used postoperatively for several reasons, including: (1) To determine whether or not the stent fully expands and tightly adheres to the wall; (2) To observe complications (e.g., plaque protru
Case 1: The patient, a 65-year-old male, presented with prolonged bilateral weakness in the lower limbs for five years due to sequelae of a stroke that occurred in July 2022.
Case 2: In July 2022, a 36-year-old male patient was admitted due to a right common carotid artery (CCA) dissection detected by a carotid ultrasound one day previously, which also revealed an occlusion in the left CCA.
Case 1: Computed tomography (CT) angiography (CTA) performed 10 d prior to hospital admission showed severe right posterior cerebral artery stenosis and possible basilar artery occlusion. CT perfusion (CTP) imaging showed decreased blood perfusion and blood volume in the right anterior and middle cerebral artery territory.
Case 2: The patient felt no discomfort and was not treated with medication at that time.
Case 1: The patient had a history of hypertension, diabetes mellitus, and smoking for more than 10 years, and had undergone percutaneous coronary intervention two years previously for coronary heart disease.
Case 2: The patient had no previous history of chronic diseases, smoking, or drinking.
Two patients’ personal and family histories were unremarkable.
Case 1: Physical examination at admission showed decreased muscle strength in the lower extremities (5-/5) but no other abnormal neurological signs.
Case 2: Physical examination at admission showed no positive neurological signs.
Case 1: Laboratory analyses reported that syphilis and human immunodeficiency virus antibodies were negative, serum myoglobin, creatine phosphokinase, troponin I, B-type natriuretic peptide, and D-dimer levels were within normal limits, and thyroid and coagulation function indices showed no abnormalities. Abnormal laboratory findings are presented in Table 1.
| Parameter | Result | Reference range | Interpretation |
| Patient 1 | |||
| White blood cell count, × 109/L | 10.53 | 3.5-9.5 | Increased |
| Hemoglobin, g/L | 125.0 | 130-170 | Decreased |
| Urine glucose | 2 + | Negative | Increased |
| Urine protein | 1 + | Negative | Increased |
| C-reactive protein, mg/L | 26.12 | 0-5 | Increased |
| Total protein, g/L | 63.7 | 65.0-85.0 | Decreased |
| Serum glycocholic acid, mg/L | 3.74 | 0.00-2.70 | Increased |
| Serum creatinine, mmol/l | 126.1 | 57-111 | Decreased |
| Urea, mmol/L | 9.8 | 3.6-9.5 | Increased |
| Anticardiolipin antibody IgG, gplu/mL | 20.60 | < 12 | Increased |
| Anticardiolipin antibody IgA, aplu/mL | 26.40 | < 12 | Increased |
| Patient 2 | |||
| Urine protein | Weakly positive | Negative | Increased |
| Red blood cells in urine | 48.40/μL | 0-18/μL | Increased |
| Fecal occult blood test | Weakly positive | Negative | Increased |
| Interleukin-6 at 3-mo follow-up, pg/mL | 13.56 | < 6.6 | Increased |
Case 2: Laboratory analysis showed normal levels of serum electrolytes, cholesterol, myoglobin, creatine phosphokinase, troponin I, B-type natriuretic peptide, glycosylated hemoglobin, D-dimer, antistreptolysin O, rheumatoid factor, C-reactive protein, anti-cyclic citrullinated peptide, anti-nuclear, extractable nuclear antigen, anti-ds-DNA, and antineutrophil cytoplasmic and anticardiolipin antibodies. Red and white blood cell counts and the erythrocyte sedimentation rate were within normal limits. Serum liver, kidney, thyroid, and coagulation function indices showed no abnormalities.
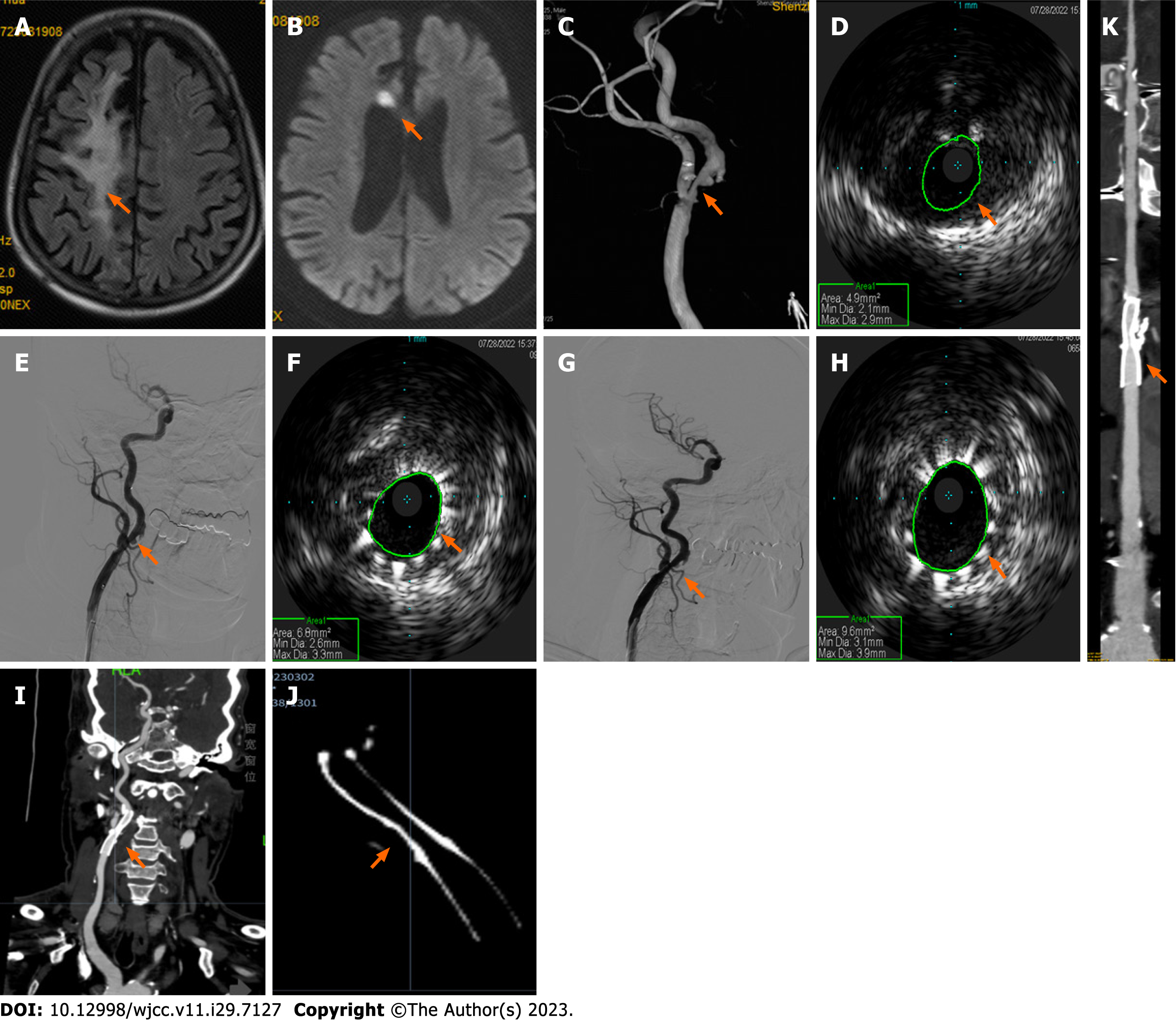
Case 1: Carotid artery ultrasound showed bilateral carotid intima-media thickening, plaque formation, and right proximal internal carotid artery (ICA) stenosis (70%-99%). Brain magnetic resonance imaging (MRI) revealed a paraven
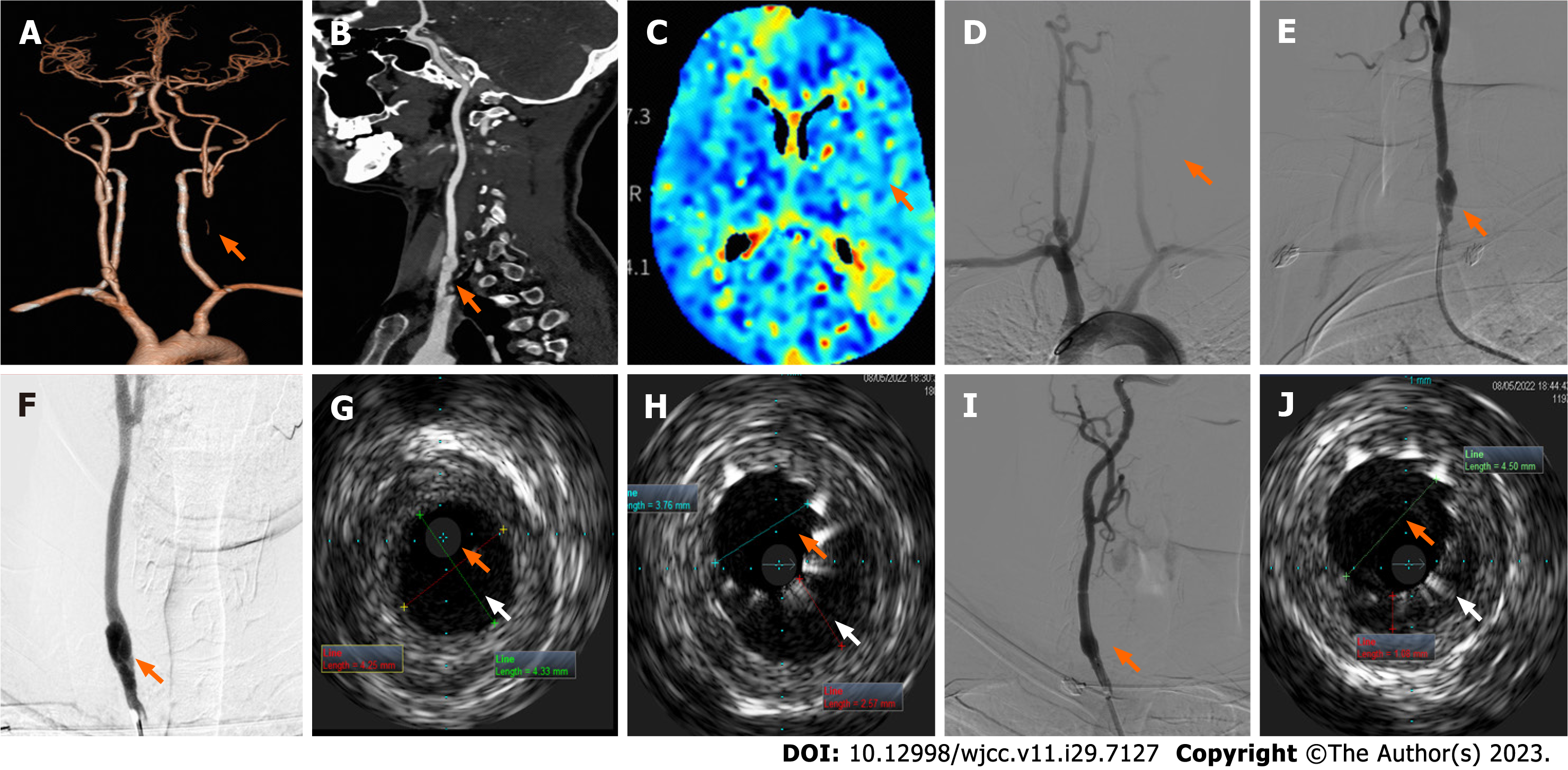
Case 2: Electrocardiogram, ambulatory blood pressure monitoring, echocardiogram, and ultrasound of the renal arteries and abdominal aorta results were unremarkable. Arterial ultrasounds of the lower extremities showed inhomogeneous thickening of the intima in artery segments on both sides and stenosis of the right common femoral artery and left superficial femoral artery (nearly occluded). Chest CT imaging reported scattered, patchy high-density lesions in both lungs (pneumonia was considered). Carotid ultrasound revealed a left CCA occlusion, right carotid artery stenosis, and local lumen dilation in the proximal CCA with an intima-like structure. Brain MRI showed no obvious abnormalities. Head and neck CTA showed left CCA occlusion (Figure 2A) and local bulging in the inferior segment of the right CCA with a strip-shaped low-density shadow (dissection or carotid web were considered, Figure 2B). Brain CTP images (Figure 2C) revealed that blood flow perfusion in the left frontal lobe, parietal lobe, right temporal lobe, and insula was lower than that on the contralateral side. Aortic arch angiography revealed an initial segment of the left CCA occlusion (Figure 2D). Angiography of the right CCA reported an initial segment of dissection (Figures 2E and F).
Case 1: Based on the patient’s medical history and laboratory and imaging findings, he was given a final diagnosis of stenosis of the right ICA, right posterior cerebral artery, CCA, and external carotid arteries.
Case 2: Combined with the patient’s medical history, the final diagnosis was CCA occlusion of the left and right initial segment.
Case 1: IVUS-assisted CAS utilizing an Eagle Eye Platinum IVUS diagnostic instrument (Volcano Corporation, United States) with a phase array probe (external diameter: 3.5 F; 18.6 MHz) was performed under local anesthesia on day six of hospitalization. First, we performed arteriography of the diseased carotid artery. A 6 F long sheath head end was placed in the right distal CCA, and the road map was redesigned. The end of the catheter reached the C2 segment of the right ICA and a distal protection device was deployed. Next, an IVUS imaging catheter was placed and the road map guided the catheter through the site of the right ICA stenosis. Finally, IVUS imaging confirmed severe right ICA stenosis with plaque formation and noticeable calcification (Figure 1D).
We inserted a 5 mm × 30 mm balloon through the narrowest part of the right ICA and ensured correct positioning. Subsequent angiography demonstrated improved right ICA stenosis. A 7 mm × 40 nm WallstentTM stent (Boston Scientific Corporation) was then positioned (Figure 1E). Subsequent IVUS confirmed unsatisfactory improvement of the narrowest part of the right ICA (Figure 1F). Therefore, post-stent balloon dilatation was performed.
Case 2: A long sheath was placed in the right distal CCA and a distal protection device was deployed. The IVUS probe was subsequently placed to check the dissection (Figure 2G), and a WallstentTM stent (Boston Scientific Corporation) was deployed at the site of dissection. IVUS images obtained immediately after stent placement showed insufficient dilation of the true lumen with a large intermural hematoma (Figure 2H) and post-balloon dilatation was performed.
Case 1: Postoperative angiography revealed the right ICA stenosis had noticeably improved (residual stenosis, 20%; Figure 1G), and IVUS confirmed good stent expansion and adherence (Figure 1H) without signs of plaque protrusions. CTA at six months postoperatively revealed mild in-stent stenosis in the right initial segment of ICA, with no discomfort reported by the patient (Figures 1I-K). The patient was satisfied with the treatment he received and his recovery.
Case 2: Final angiography showed a smooth intra-arterial lumen (Figure 2I), and postoperative IVUS images showed the true lumen increased in size with good stent expansion and adherence (Figure 2J). The patient experienced no postope
These cases demonstrate that IVUS can accurately determine the cross-sectional area of the narrowest point in the carotid artery and assess the area of the false lumen in arterial dissections, as well as plaque properties. This allows the operator to use IVUS to evaluate the impact of stent placement during the procedure. Specifically, IVUS can effectively detect unsatisfactory stent expansion and improve arterial stenosis by using post-balloon dilatation. The operator can effectively detect the postoperative residual false lumen using IVUS and accurately guide post-balloon dilatation. These advantageous characteristics of IVUS may help to improve patient prognosis.
IVUS enables a more accurate determination of stent size, expansion, and fit[13]. It also provides real-time intraluminal imaging, which is unavailable with DSA, making it a useful tool for endovascular treatment and pre- or postoperative assessments[14]. IVUS can also provide diagnostic information, specific intraluminal and transmural vessel data with high accuracy, aid the selection of appropriate angioplasty and intravascular devices, and assess intervention effects[12].
Using IVUS, we can precisely measure the cross-sectional area of the narrowest segment of the artery and false lumen and clarify the nature of the endovascular lesions. IVUS accurately reveals the cross-sectional morphology and changes after balloon dilatation[15]. IVUS plays an important role in preoperative balloon angioplasty and CAS, especially when mana
In the second case presented (case 2), intermural hematoma within the dissection was detected by IVUS after stent implantation, followed by post-balloon dilatation. Finally, IVUS indicated the stent was well placed and attached to the wall, noticeably reducing the false lumen. IVUS has unique advantages for the treatment of aortic dissection. IVUS-assisted CAS may be an effective treatment option to prevent intraoperative complications and further stroke recurrence for isolated spontaneous CCA dissection[19]. IVUS compensates for the insufficiency in the assessment of atherosclerotic plaque composition and intravascular arterial morphology, which are difficult to evaluate using DSA[9]. IVUS can also provide information on stent placement to facilitate the successful completion of surgery.
Postoperatively, IVUS helps to visualize stent placement, adherence, expansion, and complications such as stent edge dissection and determines whether the stent fully covers the lesion to optimize immediate results[20]. In this study, it was demonstrated that IVUS can be utilized to assess stent placement and perform balloon post-dilation to improve vascular stenosis.
IVUS is safe to perform during CAS and may not require mechanical protection for small vascular foci, as high-risk lesions can be safely assessed using IVUS under flow blockade[21]. In-stent protrusions (ISP) may occur following CAS, which increases the risk of postoperative embolization. ISP may be related to vulnerable plaques and fragments, and IVUS contributes to the assessment of stent protrusion during CAS[8]. IVUS performs better than DSA in detecting ISP, which is imperative to ensure the proper treatment of ISP which can reduce stroke complications[22].
A new type of virtual histology IVUS (VH-IVUS) was recently developed based on grayscale IVUS to detect atheroscle
IVUS was utilized preoperatively to evaluate the nature of plaques, measure lesions, reference vessels, and develop a pre
We would like to thank the “Double-First Class” Application Characteristic Discipline of Hunan Province (Pharmaceutical Science) for the support.
| 1. | Hassani S, Nogueira RG, Al-Bayati AR, Sachdeva R, McDaniel M, Haussen DC. Intravascular Ultrasound in Carotid Web. J Neurointerv Surg. 2020;12:531-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 2. | Escolar E, Weigold G, Fuisz A, Weissman NJ. New imaging techniques for diagnosing coronary artery disease. CMAJ. 2006;174:487-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 43] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 3. | Zacharatos H, Hassan AE, Qureshi AI. Intravascular ultrasound: principles and cerebrovascular applications. AJNR Am J Neuroradiol. 2010;31:586-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 37] [Article Influence: 2.3] [Reference Citation Analysis (1)] |
| 4. | Chen X. [A preliminary study of intravascular ultrasound in carotid arterial stenting]. Chin J Neuromed. 2017;16. [DOI] [Full Text] |
| 5. | Li S, Wan J, Ge J, Dai J, He B, Wang B, Han Z. [Application of intravascular ultrasound in intravascular stenting for carotid artery stenosis]. Acta Universitatis Medicinalis Secondae Shanghai. 2004;559-561. |
| 6. | Joan MM, Moya BG, Agustí FP, Vidal RG, Arjona YA, Alija MP, Paredero VM. Utility of intravascular ultrasound examination during carotid stenting. Ann Vasc Surg. 2009;23:606-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 7. | Hitchner E, Zayed MA, Lee G, Morrison D, Lane B, Zhou W. Intravascular ultrasound as a clinical adjunct for carotid plaque characterization. J Vasc Surg. 2014;59:774-780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 8. | Chiocchi M, Morosetti D, Chiaravalloti A, Loreni G, Gandini R, Simonetti G. Intravascular ultrasound assisted carotid artery stenting: randomized controlled trial. Preliminary results on 60 patients. J Cardiovasc Med (Hagerstown). 2019;20:248-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 9. | Shinozaki N, Ogata N, Ikari Y. Plaque protrusion detected by intravascular ultrasound during carotid artery stenting. J Stroke Cerebrovasc Dis. 2014;23:2622-2625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 10. | Morr S, Vakharia K, Fanous AA, Waqas M, Siddiqui AH. Utility of Intravascular Ultrasound During Carotid Angioplasty and Stenting with Proximal Protection. Cureus. 2019;11:e4935. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 11. | Wehman JC, Holmes DR Jr, Ecker RD, Sauvageau E, Fahrbach J, Hanel RA, Hopkins LN. Intravascular ultrasound identification of intraluminal embolic plaque material during carotid angioplasty with stenting. Catheter Cardiovasc Interv. 2006;68:853-857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 12. | Kan P, Binning MJ, Siddiqui AH. Intravascular ultrasound-guided thrombus retrieval with a multipurpose-angled catheter during carotid artery stenting. J Neuroimaging. 2012;22:394-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 13. | Clark DJ, Lessio S, O'Donoghue M, Schainfeld R, Rosenfield K. Safety and utility of intravascular ultrasound-guided carotid artery stenting. Catheter Cardiovasc Interv. 2004;63:355-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 53] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 14. | Hachinohe D, Mitomo S, Candilio L, Latib A. A Practical Approach to Assessing Stent Results with IVUS or OCT. Methodist Debakey Cardiovasc J. 2018;14:32-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 15. | Tobis JM, Mahon DJ, Moriuchi M, Honye J, McRae M. Intravascular ultrasound imaging following balloon angioplasty. Int J Card Imaging. 1991;6:191-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 16. | Volkov SV, Mytsyk SA, Naumov SM, Korobkov AO, Gontarenko VN. Intravascular ultrasound-guided internal carotid artery stenting. Angiol Sosud Khir. 2019;25:41-52. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 17. | American College of Cardiology Clinical Expert Consensus Document on Standards for Acquisition, Measurement and Reporting of Intravascular Ultrasound Studies (IVUS). A report of the American College of Cardiology Task Force on Clinical Expert Consensus Documents developed in collaboration with the European Society of Cardiology endorsed by the Society of Cardiac Angiography and Interventions. Eur J Echocardiogr. 2001;2:299-313. [PubMed] |
| 18. | Hussain AS, Hussain NS. Intravascular Ultrasound for Intracranial and Extracranial Carotid Artery Stent Placement. Cureus. 2016;8:e732. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 19. | Sanada T, Wada H, Sato H, Shirai W, Kinoshita M, Tokumitsu N. Carotid artery stenting assisted with intravascular ultrasonography for isolated spontaneous common carotid artery dissection. J Surg Case Rep. 2021;2021:rjab232. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 20. | Liu X, Tsujita K, Maehara A, Mintz GS, Weisz G, Dangas GD, Lansky AJ, Kreps EM, Rabbani LE, Collins M, Stone GW, Moses JW, Mehran R, Leon MB. Intravascular ultrasound assessment of the incidence and predictors of edge dissections after drug-eluting stent implantation. JACC Cardiovasc Interv. 2009;2:997-1004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 63] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 21. | Musialek P, Pieniazek P, Tracz W, Tekieli L, Przewlocki T, Kablak-Ziembicka A, Motyl R, Moczulski Z, Stepniewski J, Trystula M, Zajdel W, Roslawiecka A, Zmudka K, Podolec P. Safety of embolic protection device-assisted and unprotected intravascular ultrasound in evaluating carotid artery atherosclerotic lesions. Med Sci Monit. 2012;18:MT7-M18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 22. | Okazaki T, Sakamoto S, Shinagawa K, Ichinose N, Ishii D, Matsushige T, Kiura Y, Kurisu K. Detection of in-stent protrusion (ISP) by intravascular ultrasound during carotid stenting: Usefulness of stent-in-stent placement for ISP. Eur Radiol. 2019;29:77-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 23. | Nasu K, Tsuchikane E, Katoh O, Vince DG, Virmani R, Surmely JF, Murata A, Takeda Y, Ito T, Ehara M, Matsubara T, Terashima M, Suzuki T. Accuracy of in vivo coronary plaque morphology assessment: a validation study of in vivo virtual histology compared with in vitro histopathology. J Am Coll Cardiol. 2006;47:2405-2412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 400] [Cited by in RCA: 376] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 24. | Peng J, Qin Z, Diao X, Jin C, Wang T, Chen S. [Progress in the research on key technologies for medical ultrasound, part one: ultrasonic transducer and ultrasonic coded excitation]. Shanghai of Biomedical Engineering. 2013;34:21-27. |
| 25. | Dong QL. [Current status and research progress of intravascular ultrasound probes]. Cardiovascular Dis Elect J Integrated Tradit Chin Western Med. 2016;4. [DOI] [Full Text] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chow WK, Taiwan S-Editor: Wang JJ L-Editor: A P-Editor: Wang JJ