Published online Sep 16, 2023. doi: 10.12998/wjcc.v11.i26.6223
Peer-review started: May 18, 2023
First decision: June 13, 2023
Revised: June 25, 2023
Accepted: August 11, 2023
Article in press: August 11, 2023
Published online: September 16, 2023
Processing time: 112 Days and 20.3 Hours
Zollinger–Ellison syndrome (ZES) results from hypersecretion of gastrin from pancreatic or duodenal neuroendocrine tumors, commonly referred to as gastri
A 72-year-old woman presented with the typical clinical manifestations of ZES, including upper abdominal pain, significant watery diarrhea, and acidic liquid vomitus. Surprisingly, however, she did not have an increased level of serum gastrin. In addition, there was no evidence of gastrinoma or any other ulcerogenic tumor. Esophagogastroduodenoscopy was conducted to examine the upper digestive tract. Revised diagnoses were considered, and an individualized treatment plan was developed. The patient responded to antacid medication while experiencing intermittent, recurring bouts of ZES. 18F-AlF-NOTA-octreotide positron emission tomography (18F-OC PET)/computed tomography (CT) helped locate the tumor. Postoperative pathology and immunohistochemistry results suggested that the tumor was a gastrinoma located at an unconventional site.
This present case study demonstrates the possibility of ZES-like manifestation in patients with absence of hypergastrinemia. 18F-OC PET/CT is a relatively new imaging technique that can be applied for diagnosing even tiny gastrinomas that are atypical in terms of location.
Core Tip: We recommend gastrinoma as one possible diagnosis for patients presenting Zollinger–Ellison syndrome-like clinical features, but without any typical tumor location or with normal serum gastrin levels. 18F-AlF-NOTA-octreotide positron emission tomography/computed tomography is an advanced imaging technique that can be used to locate and diagnose gastrinoma in such patients.
- Citation: Zhang JM, Zheng CW, Li XW, Fang ZY, Yu MX, Shen HY, Ji X. Typical Zollinger-Ellison syndrome-atypical location of gastrinoma and absence of hypergastrinemia: A case report and review of literature. World J Clin Cases 2023; 11(26): 6223-6230
- URL: https://www.wjgnet.com/2307-8960/full/v11/i26/6223.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i26.6223
Zollinger–Ellison syndrome (ZES) occurs due to increased gastrin secretion from pancreatic or duodenal, or stomach neuroendocrine tumors (NETs), which is commonly referred to as gastrinomas. Such high levels of gastrin-induce gastric acid overproduction is usually associated with peptic ulcer disease and severe watery diarrhea[1]. Although used interchangeably, gastrinoma refers to NETs that secrete gastrin, and ZES is the clinical manifestation of NET[2]. Severe cases of ZES may develop electrolyte imbalance, gastrointestinal perforation, and other complications. In addition, Some other rare causes are also responsible for substantial gastric acid hypersecretion, including idiopathic gastric acid hypersecretion, non-gastrin peptide secretion, and gastric acid hypersecretion tumors[3,4]. Here, we have presented a unique case of a ZES in a patient who is distinct from any previously mentioned groups of patients. The patient presented with ZES-like clinical features, including highly suspicious gastric acid-associated diarrhea and multiple ulcers in the second and third parts of the duodenum. However, the patient did not show any typical tumor location or elevated serum gastrin levels.
Watery diarrhea with upper abdominal pain and vomiting for the past 1 year.
A 72-year-old female presented to our clinic with watery diarrhea since the past 1 year, coupled with upper abdominal pain and vomiting. She suffered frequent vomiting with large-volume gastric secretion. She had been taking proton-pump inhibitors off and on for 1 year. After a series of laboratory, imaging, and esophagogastroduodenoscopy examinations, NET was suspected. A high-end 18F-AlF-NOTA-octreotide positron emission tomography (18F-OC PET)/computed tomography (CT) scan confirmed the location of the tumor in the posterior peritoneal region of the right para-aortic lymph node. Following surgical removal of the tumor, postoperative pathology confirmed NET. No diarrhea, abdominal pain, or other symptoms were observed during the postoperative follow-up.
No special history of past illnesses.
She and her family had no notable medical history.
A physical examination revealed that the patient was in a state of malnutrition with obvious dehydration. Epigastric tenderness with negative rebound tenderness was detected. Abdominal splashing sounds and vigorous borborygmus were also heard.
Laboratory investigations revealed a high blood count (27.21 × 109/L), an elevated neutrophil differential count (93.1%), a high D-dimer level (> 4400 g/L), a low total protein (48.9 g/L), an low albumin (28.2 g/L), a normal hemoglobin level (112 g/L) in the blood, and a positive occult blood test, but no white cells in the stool. The stool cultures for bacterial, viral, and parasitic infections were negative. The vomit was expelled onto pH test paper, and upon development and comparison to the standard color card, it was determined to be acidic. The patient’s serum gastrin level was in the normal range (69.54 pg/mL, 13-115 pg/mL) in the first test. Although after two standard meal stimulations, the serum gastrin level increased to 182 pg/mL and 198 pg/mL respectively, it did not exceed 200 pg/mL.
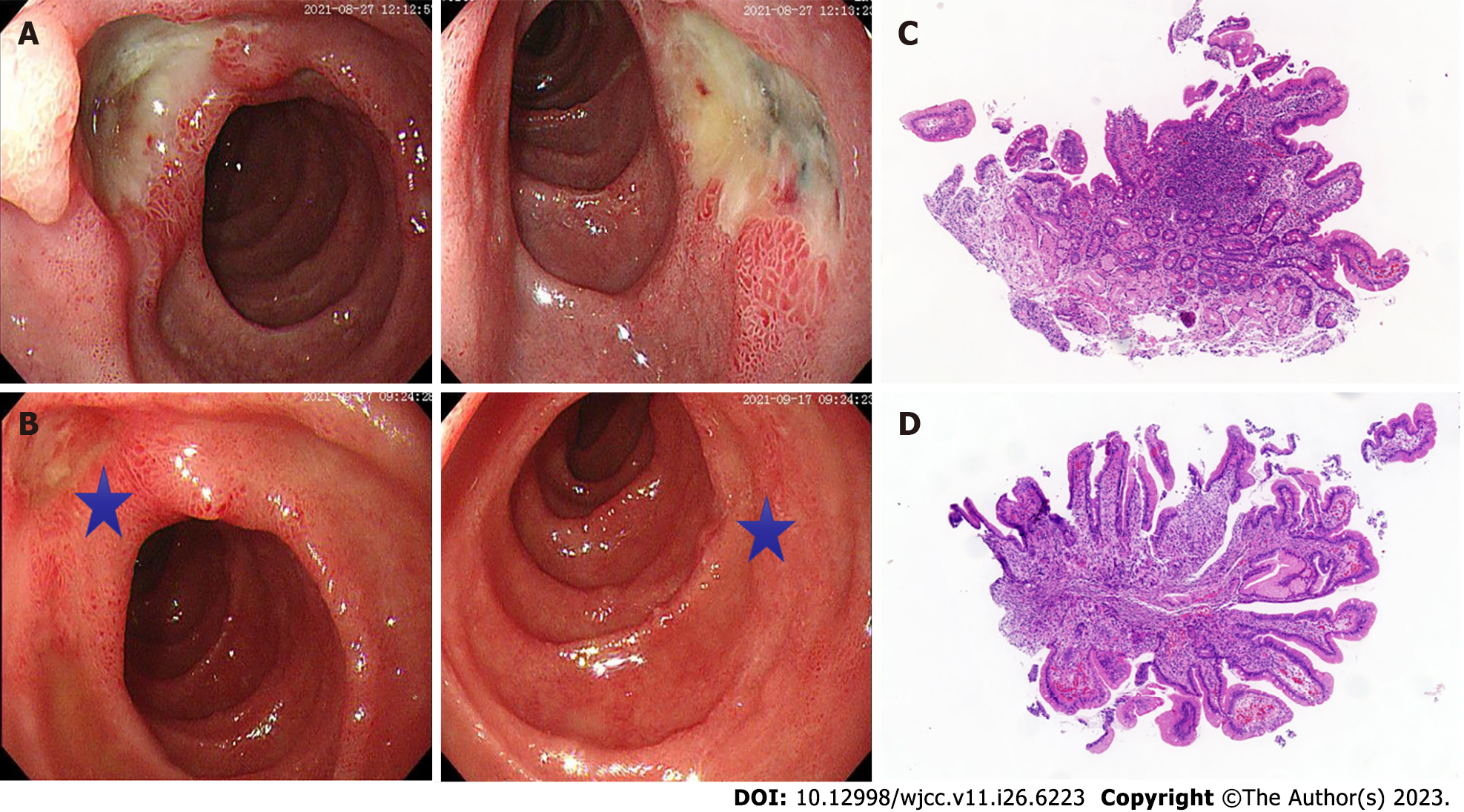
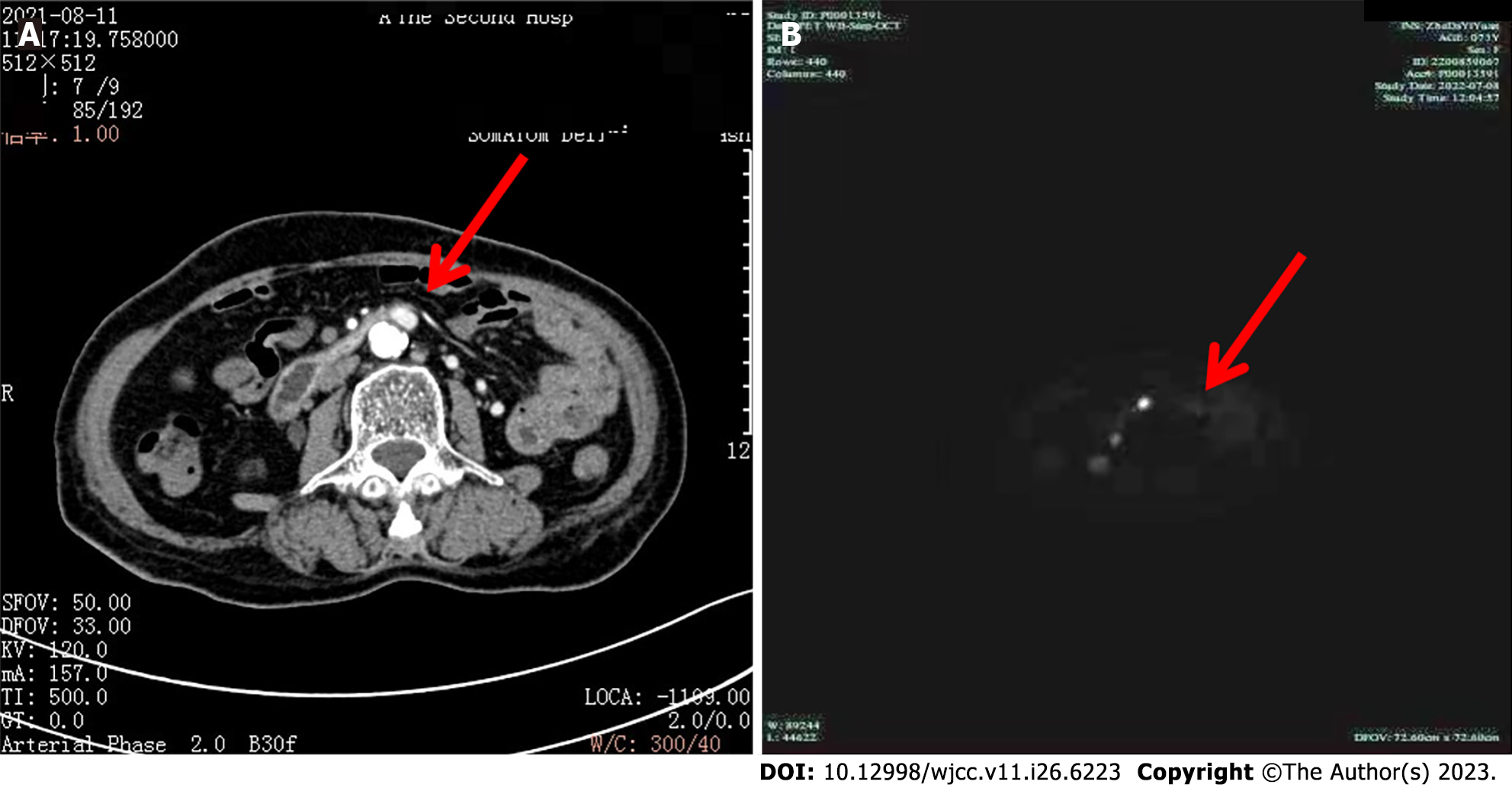
Esophagogastroduodenoscopy (Figure 1) revealed multiple deep and superficial ulcers in the second and third portions of the duodenum (Figure 1A). Conventional and contrast-enhanced abdominal CT displayed no gastrointestinal or pancreatic tumors. However, the enhancement of the posterior peritoneal para-aortic lymph node was detected in contrast-enhanced CT (Figure 2A). Next, we employed high-end 18F-OC PET/CT imaging to more accurately pinpoint the tumor’s site and evaluate any additional tumors that the patient may have. The results indicated that the lymph node of the posterior peritoneum was enlarged on the right side of the abdominal aorta (Figure 2B). Notably, an increased uptake of 18F-OC suggested high somatostatin expression and confirmed NET. 18F-OC PET/CT imaging in other parts of the body, including the brain, indicated no significant abnormal increase in the 18F-OC uptake.
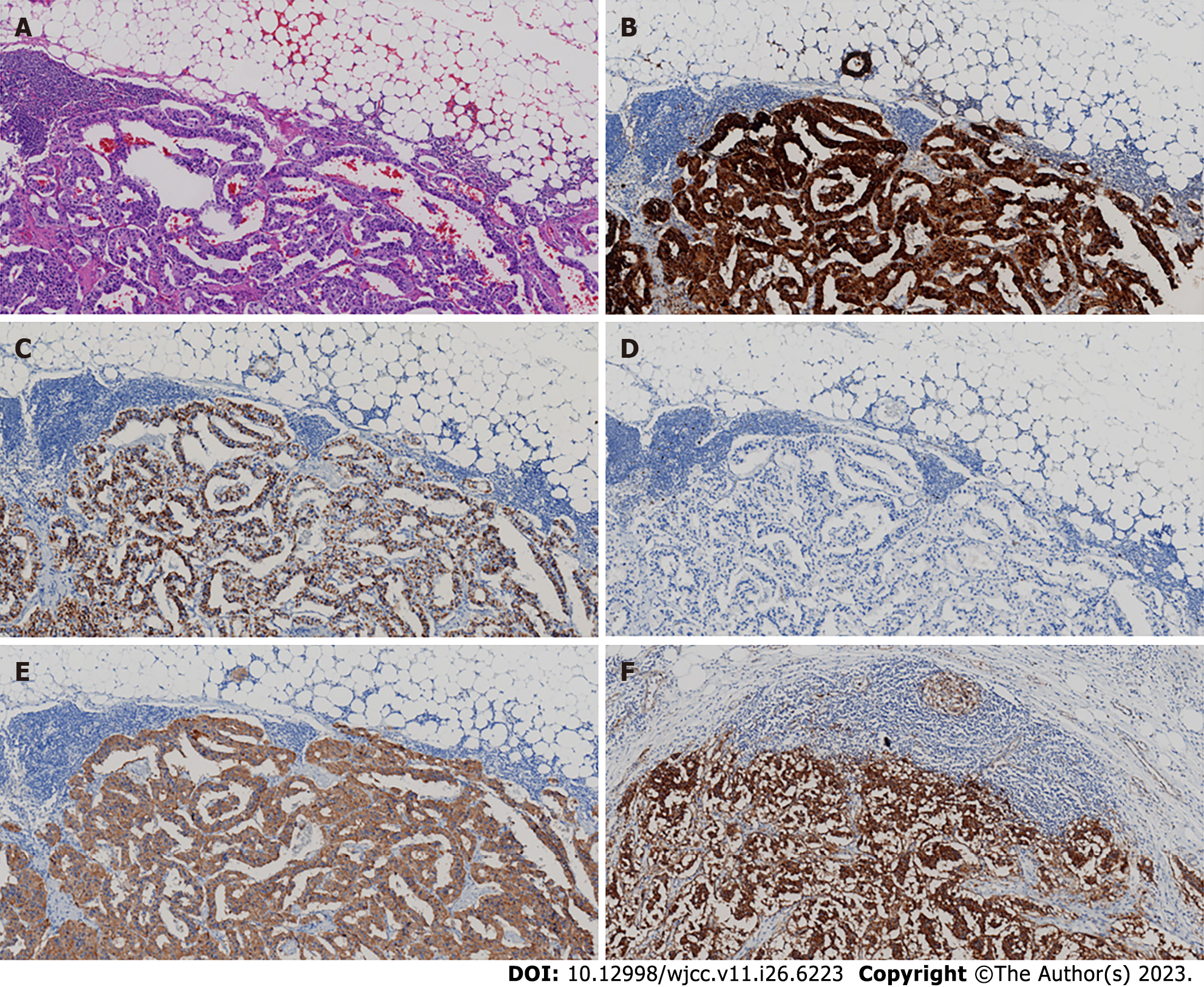
The outcome of the duodenal biopsy revealed chronic inflammation in the mucosa (Figure 1C and D). The pathological diagnosis of the specimen after retroperitoneal tumor resection therapy was NET. The immunohistochemistry (IHC) results were as follows: CK (pan) (+), CD56 (+),chromogranin A (CgA) (+), Ki-67 (+2), Syn (+), P53 (-), Gastrin (+), ATRX (+), and somatostatin receptor 2 (SSTR2) (+) (Figure 3).
The pathological diagnosis of the specimen after retroperitoneal tumor resection therapy was NET G1 (gastrinoma).
The patient was commenced on a proton pump inhibitor (PPI; esomeprazole, 80 mg/day), fluid infusion, and electrolyte supply treatment. After locating the tumor, surgical resection was performed.
No diarrhea, epigastric pain, or vomiting occurred at the 7-mo follow-up.
ZES is a clinical manifestation of gastrinoma caused by hypersecretion of gastrin. It has been reported in the literature that a limited proportion of individuals have an ulcerogenic tumor syndrome, which is defined by islet-cell tumors that do not contain or produce gastrin and stomach acid hypersecretion[3,4]. These individuals' symptoms of diarrhea and/or peptic ulcers are similar to those of ZES patients. These tumors have been proven to contain a yet-to-be-identified peptide that is separate from gastrin and that stimulates the production of gastric acid. Patients with idiopathic acid hypersecretion have markedly increased gastric acid output and are often likely to have ulcer disease[3]. They rarely had significant abdominal pain and/or diarrhea compared with those of ZES. There are no hypergastrin-producing cells or tumors in these diseases, but we found gastrinoma.
Gastrinomas are widely distributed. The prime site of their location is the the duodenum (70%) and pancreas (25%), with 5% of cases occurring in the stomach, liver, ovary, and lung. The most common clinical symptoms of ZES include abdominal pain and diarrhea. Around 80% of gastrinoma discovered in ZES patients were sporadic and solitary[5]. The remaining non-sporadic gastrinomas were linked to multiple endocrine neoplasia type 1 (MEN-I)[6]. The serum gastrin level is measured to diagnose ZES directly[1,2]. Similarly, the specificity and sensitivity of serum CgA in the diagnosis of NET can reach 60%-90%, in which chromogranin B is only slightly affected by PPI[7]. The serum gastrin levels of > 500 pg/mL, coupled with substantially elevated gastric-acid secretion, support the diagnosis of ZES. In most cases, the diagnosis of ZES may be eliminated if the fasting serum gastrin level is < 100 pg/mL. Stimulation tests are conducted when the fasting serum gastrin level is normal or slightly elevated. A rise in serum-stimulated gastrin level of ≥ 120 pg/mL, induced by intravenous calcium test or by the standard meal test, may support the diagnosis of ZES[8]. Another crucial diagnostic step for ZES is the localization of gastrinoma. Whereas gastrinomas mostly appear in the gastric antrum (Type I) or pancreas and other locations (Type II), they may be associated with MEN-I[9].
Although imaging techniques such as endoscopy and endoscopic ultrasound are efficient for detecting NETs, they are not always helpful because of the small size of the lesion that is also frequently multiple. Contrast-enhanced CT scans help locate primary tumors > 1 cm in size, pancreatic tumors, and liver metastases, but their sensitivity declines for tumors < 1 cm in size and those located outside of the pancreas[10,11].
We have reported here the case of a 72-year-old woman with typical clinical manifestations of ZES, including recurrent symptoms of upper abdominal pain, significant watery diarrhea, and acidic liquid vomitus. The endoscopy revealed multiple ulcer diseases in the second and third portions of the duodenum. Based on the examination results, we excluded the common diseases that could cause abdominal pain, diarrhea, and vomiting. These findings strongly suggested a diagnosis of gastrinoma causing the ZES. Surprisingly, the fasting serum gastrin levels were normal, which is a rare observation. Even after two standard meal stimulations, the serum gastrin level did not reach up to the diagnostic criterion. Patients with gastrinoma-produced ZES who had normal fasting serum gastrin levels have been described in the literature[12-15]. Possible explanations for this patient’s condition include the gastrinoma we found was small and atypical in location, the gastrin does not stimulate gastric acid secretion by parietal cells through blood flow. The abdominal contrast-enhanced CT scan detected an enhanced lymph node on the right side of the para-aortic area of the retroperitoneal abdominal region. The duodenal ulcer dramatically improved after 2 wk of PPI therapy (Figure 1B). Together, these observations further endorsed the diagnosis of ZES caused by atypical gastrinoma.
Nuclear medicine has become a method of choice for diagnosing NETs, especially due to the imaging of SSTRs. Whereas, the overexpression of SSTR on the surface of tumor cells is a unique feature of NETs, as it facilitate diagnostic molecular imaging using radionuclide-coupled somatostatin analogs[16]. SSTR-PET methods have demonstrated tremendous potential for enhancing the localization of gastrinomas and other NETs[17-19]. According to the European guidelines, SSTR PET is preferred over octreoscan for the diagnostic workup of NETs[20,21]. 18F is the most widely employed radionuclide in PET owing to its chemical, physical, and nuclear properties, which render it ideal for peptide-based imaging[22]. Despite these advantages, until recently, only a few radiotracers for SSTR labeled with 18F have been described[23,24]. 18F-AlF-NOTA-octreotide (18F-OC) is a peptide-imaging tracer that can be quickly synthesized and which exhibits a good tumor uptake in vivo. Another commonly used PET tracer 18F-FDG is utilized for assessing the glycolytic metabolism in aggressive, poorly differentiated NETs.
Although certain reports suggest an enhanced uptake of 18F-OC in some normal organs, such as the spleen, adrenal gland, renal parenchyma, pituitary gland, and liver, its uptake was significantly higher in NETs[25]. Therefore, the enhanced uptake of 18F-OC in NETs makes it a promising diagnostic tool for NET. When compared to the traditional diagnostic methods such as 68Ga-DOTATATE PET/CT, it is considered the current gold standard for SSTR imaging[26]. Indeed, 18F-OC detects lesions better, especially those in the liver[27-29]. This fact is substantiated by past studies conducted by Hou et al[26], who demonstrated that 18F-OC could detect relatively small peritoneal metastases (diameter < 5 mm) in a patient that were missed by 68Ga-DOTATATE[28]. This report can be attributed to the fact that 18F is a short-range positron emitter with better spatial resolution[30], which may be better suited for detecting small lesions. Relative to 18F-FDG, 18F-OC provides superior imaging of well-differentiated NETs[31].
Based on the results obtained by 18F-OC PET/CT imaging, we observed enlargement in the posterior peritoneal region of the right para-aortic lymph node (diameter 1.2 cm). Furthermore, the enhanced uptake of 18F-OC suggested a high expression of somatostatin and, subsequently, confirmed NET (gastrinoma). Given the patient's repeated symptoms of vomiting, diarrhea, and abdominal pain, retroperitoneal tumor resection was decided after excluding surgical contraindications. Postoperative pathology and IHC suggested that the tumor was gastrinoma. Although 18F-OC indicated neuroendocrine cell infiltration of lymph nodes, no primary lesion was detected. The possible causes include the presence of an undetected micro-gastrinoma with metastatic lymph node involvement, an unidentified primary tumor, or a rare primary lymph node gastrinoma, which have been well reported in other references[32-34]. Thus, a long-term follow-up is required to track postoperative changes in patients by using endoscopy and CT-enhanced scans of the whole abdomen once a year, with an extra 18F-OC PET/CT on detecting or suspecting other abnormalities.
Gastrinoma is a rare form of NET that causes overproduction of gastrin, which ultimately leads to the clinical manifestation referred to as ZES. Gastrinoma with low serum gastrin is relatively uncommon. If the symptoms linked with ZES are detected in a patient with no other associated disorders, gastrinoma should be taken into consideration initially. The diagnosis and classification of gastrinoma greatly depend on the results of the imaging examination. Regularly, abdominal enhanced CT should be improved, and when a positive result cannot be obtained, a PET/CT examination, particularly with 18F-OC or 68Ga-DOTATATE, should be opted for. Meanwhile, timely surgical removal of the tumor is necessary to treat patients and alleviate their symptoms in such cases.
The authors thank Shui-Liang Ruan, Ding-Tian Luo and Xin-Xing Xu for their very valuable comments on this case report.
| 1. | Epelboym I, Mazeh H. Zollinger-Ellison syndrome: classical considerations and current controversies. Oncologist. 2014;19:44-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 2. | De Angelis C, Cortegoso Valdivia P, Venezia L, Bruno M, Pellicano R. Diagnosis and management of Zollinger-Ellison syndrome in 2018. Minerva Endocrinol. 2018;43:212-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 3. | Collen MJ, Sheridan MJ. Definition for idiopathic gastric acid hypersecretion. A statistical and functional evaluation. Dig Dis Sci. 1991;36:1371-1376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 4. | Song Y, Chey WY, Chang TM, Lee KY. Mechanism of gastric acid hypersecretion in patients with islet cell tumor without hypergastrinemia: studies in rats. Gastroenterology. 1997;113:1129-1135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 5. | Guarnotta V, Martini C, Davì MV, Pizza G, Colao A, Faggiano A; NIKE group. The Zollinger-Ellison syndrome: is there a role for somatostatin analogues in the treatment of the gastrinoma? Endocrine. 2018;60:15-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 6. | Imamura M, Komoto I, Taki Y. How to treat gastrinomas in patients with multiple endocrine neoplasia type1: surgery or long-term proton pump inhibitors? Surg Today. 2022;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (1)] |
| 7. | Han X, Zhang C, Tang M, Xu X, Liu L, Ji Y, Pan B, Lou W. The value of serum chromogranin A as a predictor of tumor burden, therapeutic response, and nomogram-based survival in well-moderate nonfunctional pancreatic neuroendocrine tumors with liver metastases. Eur J Gastroenterol Hepatol. 2015;27:527-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 8. | Berna MJ, Hoffmann KM, Long SH, Serrano J, Gibril F, Jensen RT. Serum gastrin in Zollinger-Ellison syndrome: II. Prospective study of gastrin provocative testing in 293 patients from the National Institutes of Health and comparison with 537 cases from the literature. evaluation of diagnostic criteria, proposal of new criteria, and correlations with clinical and tumoral features. Medicine (Baltimore). 2006;85:331-364. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 149] [Cited by in RCA: 131] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 9. | Thakker RV, Newey PJ, Walls GV, Bilezikian J, Dralle H, Ebeling PR, Melmed S, Sakurai A, Tonelli F, Brandi ML; Endocrine Society. Clinical practice guidelines for multiple endocrine neoplasia type 1 (MEN1). J Clin Endocrinol Metab. 2012;97:2990-3011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 879] [Cited by in RCA: 917] [Article Influence: 65.5] [Reference Citation Analysis (0)] |
| 10. | Krampitz GW, Norton JA. Current management of the Zollinger-Ellison syndrome. Adv Surg. 2013;47:59-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 11. | Thoeni RF, Mueller-Lisse UG, Chan R, Do NK, Shyn PB. Detection of small, functional islet cell tumors in the pancreas: selection of MR imaging sequences for optimal sensitivity. Radiology. 2000;214:483-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 142] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 12. | Deveney CW, Deveney KS, Jaffe BM, Jones RS, Way LW. Use of calcium and secretin in the diagnosis of gastrinoma (Zollinger-Ellison syndrome). Ann Intern Med. 1977;87:680-686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 84] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 13. | Mee AS, Ismail S, Bornman PC, Marks IN. Changing concepts in the presentation, diagnosis and management of the Zollinger-Ellison syndrome. Q J Med. 1983;52:256-267. [PubMed] |
| 14. | Wolfe MM, Jain DK, Edgerton JR. Zollinger-Ellison syndrome associated with persistently normal fasting serum gastrin concentrations. Ann Intern Med. 1985;103:215-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 15. | Desai AA, McGuigan JE, Draganov P. Zollinger-Ellison phenotype in the absence of hypergastrinemia and islet-cell tumor. Int J Gastrointest Cancer. 2005;35:157-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 16. | Bozkurt MF, Virgolini I, Balogova S, Beheshti M, Rubello D, Decristoforo C, Ambrosini V, Kjaer A, Delgado-Bolton R, Kunikowska J, Oyen WJG, Chiti A, Giammarile F, Sundin A, Fanti S. Guideline for PET/CT imaging of neuroendocrine neoplasms with (68)Ga-DOTA-conjugated somatostatin receptor targeting peptides and (18)F-DOPA. Eur J Nucl Med Mol Imaging. 2017;44:1588-1601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 332] [Article Influence: 36.9] [Reference Citation Analysis (0)] |
| 17. | Fortunati E, Argalia G, Zanoni L, Fanti S, Ambrosini V. New PET Radiotracers for the Imaging of Neuroendocrine Neoplasms. Curr Treat Options Oncol. 2022;23:703-720. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 45] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 18. | Sharma P, Arora S, Mukherjee A, Pal S, Sahni P, Garg P, Khadgawat R, Thulkar S, Bal C, Kumar R. Predictive value of 68Ga-DOTANOC PET/CT in patients with suspicion of neuroendocrine tumors: is its routine use justified? Clin Nucl Med. 2014;39:37-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (1)] |
| 19. | Skoura E, Michopoulou S, Mohmaduvesh M, Panagiotidis E, Al Harbi M, Toumpanakis C, Almukhailed O, Kayani I, Syed R, Navalkissoor S, Ell PJ, Caplin ME, Bomanji J. The Impact of 68Ga-DOTATATE PET/CT Imaging on Management of Patients with Neuroendocrine Tumors: Experience from a National Referral Center in the United Kingdom. J Nucl Med. 2016;57:34-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 112] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 20. | Sundin A, Arnold R, Baudin E, Cwikla JB, Eriksson B, Fanti S, Fazio N, Giammarile F, Hicks RJ, Kjaer A, Krenning E, Kwekkeboom D, Lombard-Bohas C, O'Connor JM, O'Toole D, Rockall A, Wiedenmann B, Valle JW, Vullierme MP; Antibes Consensus Conference participants. ENETS Consensus Guidelines for the Standards of Care in Neuroendocrine Tumors: Radiological, Nuclear Medicine & Hybrid Imaging. Neuroendocrinology. 2017;105:212-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 325] [Article Influence: 36.1] [Reference Citation Analysis (0)] |
| 21. | Kwekkeboom DJ, Krenning EP, Lebtahi R, Komminoth P, Kos-Kudła B, de Herder WW, Plöckinger U; Mallorca Consensus Conference participants; European Neuroendocrine Tumor Society. ENETS Consensus Guidelines for the Standards of Care in Neuroendocrine Tumors: peptide receptor radionuclide therapy with radiolabeled somatostatin analogs. Neuroendocrinology. 2009;90:220-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 130] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 22. | Ambrosini V, Zanoni L, Filice A, Lamberti G, Argalia G, Fortunati E, Campana D, Versari A, Fanti S. Radiolabeled Somatostatin Analogues for Diagnosis and Treatment of Neuroendocrine Tumors. Cancers (Basel). 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 23. | Waldmann CM, Stuparu AD, van Dam RM, Slavik R. The Search for an Alternative to [(68)Ga]Ga-DOTA-TATE in Neuroendocrine Tumor Theranostics: Current State of (18)F-labeled Somatostatin Analog Development. Theranostics. 2019;9:1336-1347. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 24. | Niedermoser S, Chin J, Wängler C, Kostikov A, Bernard-Gauthier V, Vogler N, Soucy JP, McEwan AJ, Schirrmacher R, Wängler B. In Vivo Evaluation of ¹⁸F-SiFAlin-Modified TATE: A Potential Challenge for ⁶⁸Ga-DOTATATE, the Clinical Gold Standard for Somatostatin Receptor Imaging with PET. J Nucl Med. 2015;56:1100-1105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 88] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 25. | Hou J, Long T, Yang N, Chen D, Zeng S, Zheng K, Liao G, Hu S. Biodistribution of (18)F-AlF-NOTA-octreotide in Different Organs and Characterization of Uptake in Neuroendocrine Neoplasms. Mol Imaging Biol. 2021;23:827-835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 26. | Hope TA, Bergsland EK, Bozkurt MF, Graham M, Heaney AP, Herrmann K, Howe JR, Kulke MH, Kunz PL, Mailman J, May L, Metz DC, Millo C, O'Dorisio S, Reidy-Lagunes DL, Soulen MC, Strosberg JR. Appropriate Use Criteria for Somatostatin Receptor PET Imaging in Neuroendocrine Tumors. J Nucl Med. 2018;59:66-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 258] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 27. | Hou J, Long T, He Z, Zhou M, Yang N, Chen D, Zeng S, Hu S. Evaluation of (18)F-AlF-NOTA-octreotide for imaging neuroendocrine neoplasms: comparison with (68)Ga-DOTATATE PET/CT. EJNMMI Res. 2021;11:55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 28. | Pauwels E, Cleeren F, Tshibangu T, Koole M, Serdons K, Dekervel J, Van Cutsem E, Verslype C, Van Laere K, Bormans G, Deroose CM. [(18)F]AlF-NOTA-octreotide PET imaging: biodistribution, dosimetry and first comparison with [(68)Ga]Ga-DOTATATE in neuroendocrine tumour patients. Eur J Nucl Med Mol Imaging. 2020;47:3033-3046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 70] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 29. | Pauwels E, Cleeren F, Tshibangu T, Koole M, Serdons K, Boeckxstaens L, Dekervel J, Vandamme T, Lybaert W, den Broeck BV, Laenen A, Clement PM, Geboes K, Cutsem EV, Stroobants S, Verslype C, Bormans G, Deroose CM. (18)F-AlF-NOTA-Octreotide Outperforms (68)Ga-DOTATATE/NOC PET in Neuroendocrine Tumor Patients: Results from a Prospective, Multicenter Study. J Nucl Med. 2023;64:632-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 49] [Reference Citation Analysis (1)] |
| 30. | Conti M, Eriksson L. Physics of pure and non-pure positron emitters for PET: a review and a discussion. EJNMMI Phys. 2016;3:8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 167] [Cited by in RCA: 248] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 31. | Long T, Yang N, Zhou M, Chen D, Li Y, Li J, Tang Y, Liu Z, Li Z, Hu S. Clinical Application of 18F-AlF-NOTA-Octreotide PET/CT in Combination With 18F-FDG PET/CT for Imaging Neuroendocrine Neoplasms. Clin Nucl Med. 2019;44:452-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 56] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 32. | Singh D, Lal SB, Sood A, Gupta R, Kumar R, Vashishta RK, Mittal BR. Management of Primary Lymph Nodal Gastrinoma With Liver Metastases Resulting in Zollinger-Ellison Syndrome. Clin Nucl Med. 2019;44:e36-e39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 33. | Cavalcanti E, Stasi E, Coletta S, Lorusso D, Rinaldi CM, Armentano R. Primary lymph node gastrinoma: a case report and review of the literature. World J Surg Oncol. 2020;18:80. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 34. | Robin L, Sauvanet A, Walter T, Najah H, Falconi M, Pattou F, Gaujoux S; Gastrinoma Surgery Collaborative group. Recurrence after surgical resection of nonmetastatic sporadic gastrinoma: Which prognostic factors and surgical procedure? Surgery. 2023;173:1144-1152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Giacomelli L, Italy; Kim S, South Korea S-Editor: Fan JR L-Editor: A P-Editor: Yu HG