Published online Sep 6, 2023. doi: 10.12998/wjcc.v11.i25.6000
Peer-review started: June 26, 2023
First decision: July 4, 2023
Revised: July 7, 2023
Accepted: August 8, 2023
Article in press: August 8, 2023
Published online: September 6, 2023
Processing time: 67 Days and 8.4 Hours
The mixed lineage leukemia (MLL)-eleven-nineteen lysine-rich leukemia (ELL) fusion gene is a rare occurrence among the various MLL fusion genes. We present the first case in which myeloid sarcoma (MS) was the only manifestation of adult MLL-ELL-positive acute myeloid leukemia (AML).
We report a case of a 33-year-old male patient who was admitted in June 2022 with a right occipital area mass measuring approximately 7 cm × 8 cm. Blood work was normal. The patient underwent right occipital giant subscalp mass excision and incisional flap grafting. Immunohistochemistry was positive for myeloperoxidase, CD43 and CD45 and negative for CD3, CD20, CD34, and CD56. The bone marrow aspirate showed hypercellularity with 20% myeloblasts. Flow cytometry showed that myeloblasts accounted for 27.21% of the nucleated cells, which expressed CD33, CD38, and CD117. The karyotype was 46, XY, t (11, 19) (q23; p13.1), -12, + mar/46, XY. Next-generation sequencing showed a fusion of MLL exon 7 to exon 2 of ELL. A diagnosis of MLL-ELL-positive AML (M2 subtype) with subcutaneous MS was made.
MLL-ELL-positive AML with MS is a rare clinical entity. Additional research is needed to elucidate the molecular mechanisms of the pathogenesis of MS.
Core Tip: This study described myeloid sarcoma as the first and only manifestation in an adult patient with mixed lineage leukemia-eleven-nineteen lysine-rich leukemia-positive acute myeloid leukemia. Based on our findings and information from a few previous reports, we speculate that our patient had (1): Transformation of preleukemia cells in the marrow followed by spread to extramedullary sites; or (2) homing of preleukemia cells to extramedullary sites, followed by spreading back to bone marrow. The current study helps increase the awareness of this particular disease and reduce the clinical underdiagnosis rate.
- Citation: Tang SJ, Zhang QG. Myeloid sarcoma as the only manifestation in a rare mixed lineage leukemia-fusion-driven acute myeloid leukemia: A case report. World J Clin Cases 2023; 11(25): 6000-6004
- URL: https://www.wjgnet.com/2307-8960/full/v11/i25/6000.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i25.6000
Myeloid sarcoma (MS) is a rare disease characterized by an extramedullary tumor composed of immature myeloid cells. When differentiating any extramedullary lesion infiltrated by heterogeneous cells, clinicians should consider the possibility of MS[1]. Mixed lineage leukemia (MLL) gene rearrangements define a unique leukemia with distinctive pathophysiology and phenotype. The MLL gene encodes a large histone methyltransferase that directly binds and actively regulates gene transcription. In MLL rearrangement leukemia, menin acts as an oncogenic cofactor leading to leukemogenesis by mediating aberrant gene expression through the HOX gene and cofactor meis homeobox 1[2]. A chromosomal translocation t (11; 19) (q23; P13), with breakpoints mainly located within the 19p13.1 subband, generates the MLL-eleven-nineteen lysine-rich leukemia (ELL) fusion gene and is predominantly found in acute myeloid leukemia (AML)[3]. We present a rare case in which MS was the only manifestation of adult MLL-ELL-positive AML.
A 33-year-old male patient was admitted in June 2022 with a right occipital area mass measuring approximately 7 cm × 8 cm.
The patient had scalp swelling for 4 mo prior to this presentation.
The patient had no history of chronic conditions.
The patients’ personal and family histories were not significant.
A hard, immobile mass measuring approximately 7 cm × 8 cm was observed in the right occipital region of the skull. The local scalp color appeared dark brown with no abnormalities in the surrounding area.
Blood work was normal. No malignant cells were seen in the cerebrospinal fluid.
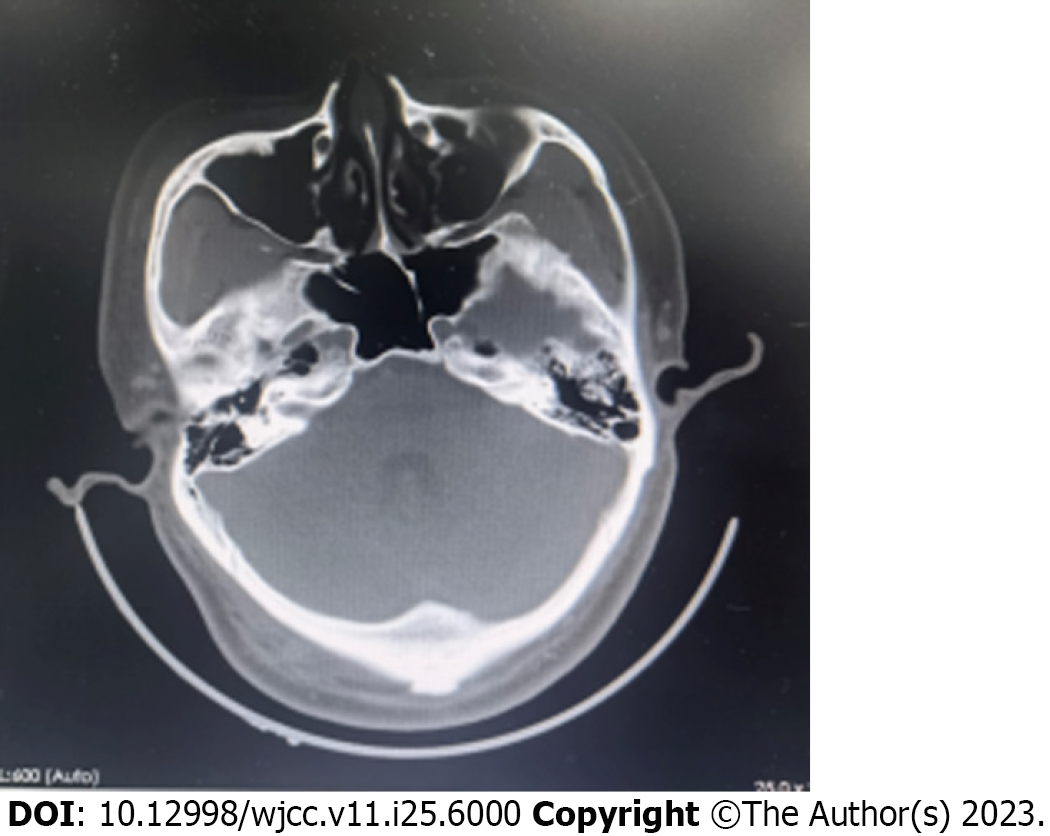
No bone erosion or abnormalities were detected in the brain parenchyma by computed tomography scan (Figure 1). The cranial magnetic resonance imaging also showed no significant abnormalities.
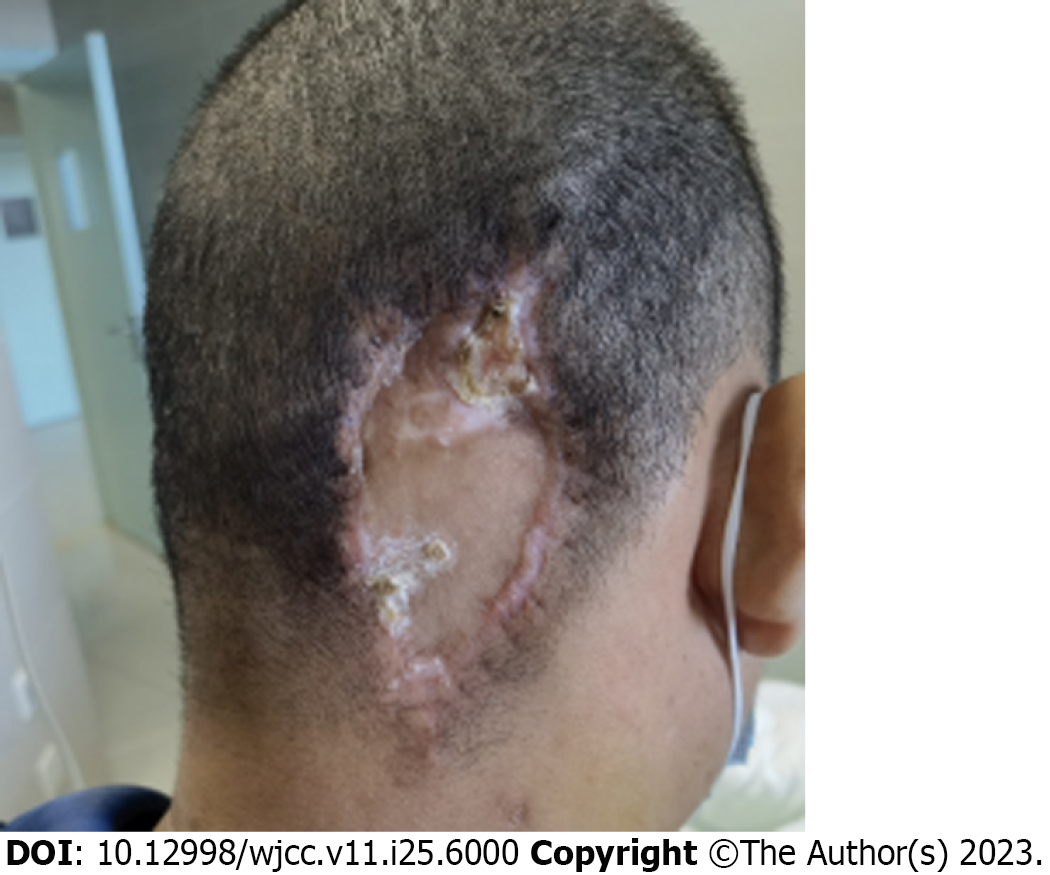
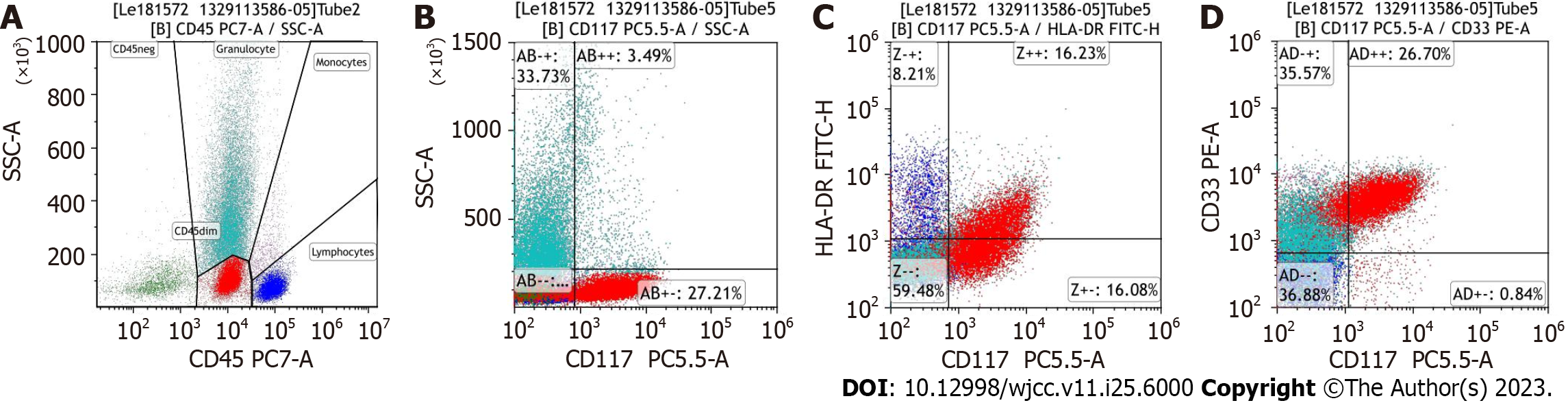
The patient underwent right occipital giant subscalp mass excision and incisional flap grafting (Figure 2). Resection tissue staining showed a small round-cell tumor, which was suspected to be lymphoma. Immunohistochemistry was positive for myeloperoxidase, CD43 and CD45 and negative for CD3, CD20, CD34, and CD56. The bone marrow (BM) aspirate showed hypercellularity with 20% myeloblasts. Flow cytometry showed that myeloblasts accounted for 27.21% of the nucleated cells, which expressed CD33, CD38, and CD117 (Figure 3). The karyotype was 46, XY, t (11, 19) (q23; p13.1), -12, + mar[4]/46, XY[5]. Next-generation sequencing showed a fusion of MLL exon 12 to exon 1 of ELL.
A diagnosis of MLL-ELL-positive AML (M2 subtype) with subcutaneous MS was made.
Complete remission (CR) was achieved after the first course of standard 7 + 3 (idarubicin and cytarabine) induction chemotherapy. He then received 2 cycles of high-dose cytarabine-based consolidation therapy followed by a myeloablative, allogeneic, matched, unrelated-donor hematopoietic stem cell transplant.
When last seen in May 2023, he was still in CR.
To our knowledge, we present the first case in which MS was the only manifestation of adult MLL-ELL-positive AML. The molecular mechanisms underlying extramedullary involvement remain unknown, but 70% of MS cases have concurrent AML. “extramedullary AML tumor” may be a more accurate term for MS[6].
The MLL-ELL fusion gene is a rare occurrence among the various MLL fusion genes. It is associated with the genesis of AML in a mouse model[7]. MLL-ELL fusions are present in 12% of adult AML patients, 7% of pediatric AML patients and 15% of infant AML patients[8]. No concurrent MLL-ELL-positive MS cases have been mentioned in the literature, although two cases of MLL-ELL-positive MS with no evidence of AML have been reported[9,10]. Based on our findings and information from a few previous reports, we speculate that our patient had (1): Transformation of preleukemia cells in the marrow followed by spread to extramedullary sites; or and (2) homing of preleukemia cells to extramedullary sites, followed by spreading back to BM. This hypothesis has been proven in the development of blastic plasmacytoid dendritic cell neoplasm[4].
MLL-ELL-positive AML is associated with high response rates to conventional chemotherapy, but relapse is common, and some patients may benefit from allogeneic hematopoietic stem cell transplantation (allo-HSCT)[11]. Our patient achieved BM minimal residual disease-negative CR, and concurrently, 10% hematogones[5] existed in his BM, which may imply longer overall survival and a lower rate of acute graft-versus-host disease after HSCT.
In conclusion, MLL-ELL-positive AML with MS is a rare clinical entity. Additional research is needed to elucidate the molecular mechanisms of the pathogenesis of MS.
| 1. | Almond LM, Charalampakis M, Ford SJ, Gourevitch D, Desai A. Myeloid Sarcoma: Presentation, Diagnosis, and Treatment. Clin Lymphoma Myeloma Leuk. 2017;17:263-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 170] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 2. | Krivtsov AV, Evans K, Gadrey JY, Eschle BK, Hatton C, Uckelmann HJ, Ross KN, Perner F, Olsen SN, Pritchard T, McDermott L, Jones CD, Jing D, Braytee A, Chacon D, Earley E, McKeever BM, Claremon D, Gifford AJ, Lee HJ, Teicher BA, Pimanda JE, Beck D, Perry JA, Smith MA, McGeehan GM, Lock RB, Armstrong SA. A Menin-MLL Inhibitor Induces Specific Chromatin Changes and Eradicates Disease in Models of MLL-Rearranged Leukemia. Cancer Cell. 2019;36:660-673.e11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 332] [Article Influence: 47.4] [Reference Citation Analysis (1)] |
| 3. | Panagopoulos I, Gorunova L, Kerndrup G, Spetalen S, Tierens A, Osnes LT, Andersen K, Müller LS, Hellebostad M, Zeller B, Heim S. Rare MLL-ELL fusion transcripts in childhood acute myeloid leukemia-association with young age and myeloid sarcomas? Exp Hematol Oncol. 2015;5:8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 4. | Griffin GK, Booth CAG, Togami K, Chung SS, Ssozi D, Verga JA, Bouyssou JM, Lee YS, Shanmugam V, Hornick JL, LeBoeuf NR, Morgan EA, Bernstein BE, Hovestadt V, van Galen P, Lane AA. Ultraviolet radiation shapes dendritic cell leukaemia transformation in the skin. Nature. 2023;618:834-841. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 44] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 5. | Chantepie SP, Cornet E, Salaün V, Reman O. Hematogones: an overview. Leuk Res. 2013;37:1404-1411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 6. | Shallis RM, Gale RP, Lazarus HM, Roberts KB, Xu ML, Seropian SE, Gore SD, Podoltsev NA. Myeloid sarcoma, chloroma, or extramedullary acute myeloid leukemia tumor: A tale of misnomers, controversy and the unresolved. Blood Rev. 2021;47:100773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 87] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 7. | Lavau C, Luo RT, Du C, Thirman MJ. Retrovirus-mediated gene transfer of MLL-ELL transforms primary myeloid progenitors and causes acute myeloid leukemias in mice. Proc Natl Acad Sci U S A. 2000;97:10984-10989. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 118] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 8. | Meyer C, Burmeister T, Gröger D, Tsaur G, Fechina L, Renneville A, Sutton R, Venn NC, Emerenciano M, Pombo-de-Oliveira MS, Barbieri Blunck C, Almeida Lopes B, Zuna J, Trka J, Ballerini P, Lapillonne H, De Braekeleer M, Cazzaniga G, Corral Abascal L, van der Velden VHJ, Delabesse E, Park TS, Oh SH, Silva MLM, Lund-Aho T, Juvonen V, Moore AS, Heidenreich O, Vormoor J, Zerkalenkova E, Olshanskaya Y, Bueno C, Menendez P, Teigler-Schlegel A, Zur Stadt U, Lentes J, Göhring G, Kustanovich A, Aleinikova O, Schäfer BW, Kubetzko S, Madsen HO, Gruhn B, Duarte X, Gameiro P, Lippert E, Bidet A, Cayuela JM, Clappier E, Alonso CN, Zwaan CM, van den Heuvel-Eibrink MM, Izraeli S, Trakhtenbrot L, Archer P, Hancock J, Möricke A, Alten J, Schrappe M, Stanulla M, Strehl S, Attarbaschi A, Dworzak M, Haas OA, Panzer-Grümayer R, Sedék L, Szczepański T, Caye A, Suarez L, Cavé H, Marschalek R. The MLL recombinome of acute leukemias in 2017. Leukemia. 2018;32:273-284. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 366] [Cited by in RCA: 531] [Article Influence: 59.0] [Reference Citation Analysis (1)] |
| 9. | Ouansafi I, Arabadjief M, Mathew S, Srivastara S, Orazi A. Myeloid sarcoma with t(11;19)(q23;p13.3) (MLL-ELL) in the uterine cervix. Br J Haematol. 2011;153:679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Bao H, Gao J, Chen YH, Altman JK, Frankfurt O, Wilson AL, Sukhanova M, Chen Q, Lu X. Rare myeloid sarcoma with KMT2A (MLL)-ELL fusion presenting as a vaginal wall mass. Diagn Pathol. 2019;14:26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 11. | Muto T, Takeuchi M, Yamazaki A, Sugita Y, Tsukamoto S, Sakai S, Takeda Y, Mimura N, Ohwada C, Sakaida E, Aotsuka N, Iseki T, Nakaseko C. Efficacy of myeloablative allogeneic hematopoietic stem cell transplantation in adult patients with MLL-ELL-positive acute myeloid leukemia. Int J Hematol. 2015;102:86-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Akbulut S, Turkey; Salvadori M, Italy S-Editor: Qu XL L-Editor: A P-Editor: Zhao S