Published online Sep 6, 2023. doi: 10.12998/wjcc.v11.i25.5982
Peer-review started: May 26, 2023
First decision: July 17, 2023
Revised: July 25, 2023
Accepted: August 9, 2023
Article in press: August 9, 2023
Published online: September 6, 2023
Processing time: 97 Days and 20.1 Hours
Prion diseases are a group of degenerative nerve diseases that are caused by infectious prion proteins or gene mutations. In humans, prion diseases result from mutations in the prion protein gene (PRNP). Only a limited number of cases involving a specific PRNP mutation at codon 196 (E196A) have been reported. The coexistence of Korsakoff syndrome in patients with Creutzfeldt-Jakob disease (CJD) caused by E196A mutation has not been documented in the existing literature.
A 61-year-old Chinese man initially presented with Korsakoff syndrome, followed by rapid-onset dementia, visual hallucinations, akinetic mutism, myoclonus, and hyperthermia. The patient had no significant personal or familial medical history. Magnetic resonance imaging of the brain revealed extensive hyperintense signals in the cortex, while positron emission tomography/computed tomography showed a diffuse reduction in cerebral cortex metabolism. Routine biochemical and microorganism testing of the cerebrospinal fluid (CSF) yielded normal results. Tests for thyroid function, human immunodeficiency virus, syphilis, vitamin B1 and B12 levels, and autoimmune rheumatic disorders were normal. Blood and CSF tests for autoimmune encephalitis and autoantibody-associated paraneoplastic syndrome yielded negative results. A test for 14-3-3 protein in the CSF yielded negative results. Whole-genome sequencing revealed a disease-causing mutation in PRNP. The patient succumbed to the illness 11 months after the initial symptom onset.
Korsakoff syndrome, typically associated with alcohol intoxication, also manifests in CJD patients. Individuals with CJD along with PRNP E196A mutation may present with Korsakoff syndrome.
Core Tip: The present case report describes a rare mutation in the prion protein gene at codon 196 causing Creutzfeldt-Jakob disease (CJD) with a clinical presentation of Korsakoff syndrome. This study emphasizes the importance of considering this mutation in CJD patients presenting with Korsakoff syndrome based on clinical, laboratory, and imaging findings.
- Citation: Zhang YK, Liu JR, Yin KL, Zong Y, Wang YZ, Cao YM. Creutzfeldt-Jakob disease presenting as Korsakoff syndrome caused by E196A mutation in PRNP gene: A case report. World J Clin Cases 2023; 11(25): 5982-5987
- URL: https://www.wjgnet.com/2307-8960/full/v11/i25/5982.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i25.5982
Prion diseases arise from an abnormal transformation of normal cellular prion proteins into abnormal forms. These diseases include Creutzfeldt-Jakob disease (CJD), Kuru disease, Gerstmann-Straussler syndrome and fatal familial insomnia[1]. Sporadic cases account for approximately 85% of the prion diseases, genetic cases account for approximately 10%-15%, and acquired cases account for less than 1%[2]. According to epidemiological studies, the global incidence of CJD ranges from approximately one to two per million per year[3]. The clinical manifestations of CJD involve diffuse or localized dysfunction of the central nervous system, characterized by rapidly progressive dementia, myoclonus, and extrapyramidal, cerebellar, and pyramidal tract signs[4]. Uncommon symptoms include visual abnormalities, cranial nerve palsies, and epileptic seizures[4].
Prion proteins are encoded by the prion protein gene (PRNP) located on the 20th human chromosome. Mutations in PRNP can lead to the production of pathogenic prion proteins, thereby causing CJD. More than 55 different types of PRNP mutations have been associated with CJD, each exhibiting distinct clinical manifestations and neuropathological characteristics[5]. Mutation at codon 196 (E196A) in PRNP is a rare mutation that has been reported exclusively in Chinese patients with CJD. No association of Korsakoff syndrome with this mutation has been reported. Korsakoff syndrome is typically characterized by symptoms such as memory impairment, confabulation or falsification, apathy, and disorientation in time and space[6]. While this syndrome is common in individuals with alcoholism, it is rare in CJD patients. The current report presents a case of CJD caused by an E196A mutation that initially presented as Korsakoff syndrome. This case report aimed to provide a detailed description of the clinical manifestations associated with this rare PRNP mutation in CJD patients.
A 61-year-old Chinese man with hypertension presented with memory disorders, falsification, time and space disorientation, visual hallucinations, rapidly progressive dementia, akinetic mutism, and myoclonus.
Four months prior, the patient experienced memory disorders, falsification, and time and space disorientation without any identifiable triggers. In the second month after the symptom onset, the patient developed rapidly progressive dementia and visual hallucinations. By the third month, the patient experienced akinetic mutism combined with myoclonus. Hyperthermia occurred in the fourth month after the onset of symptoms.
The patient had a 10-year history of hypertension.
Vital signs were as follows: Body temperature, 36.5 °C; heart rate, 76 beats/min; blood pressure, 119/71 mmHg; and respiratory rate, 20 beats/min. The patient exhibited spontaneous eye opening but was unable to follow commands or exhibit eye tracking. Passive muscle tension, positive pathological reflexes, and myoclonic movements were also observed.
The initial electroencephalogram (EEG) test conducted in the fourth month after symptom onset showed basic rhythmic activity with moderate-potential 8-Hz a waves. Cerebrospinal fluid (CSF) was colorless and transparent without clots or flocculation. Perthes test and oligoclonal bands were negative. CSF analysis revealed normal white blood cell count and immunoglobulin (Ig) G, IgA, IgM, total protein, glucose, and chloride ion levels. Tests for herpes simplex virus, toxoplasmosis, rubella virus, cytomegalovirus, and Epstein-Barr virus in the CSF yielded negative results. Vitamin B1 and B12 levels and thyroid function test results were within normal ranges. Human immunodeficiency virus and syphilis antibody tests were negative. Antibodies associated with autoimmune encephalitis and paraneoplastic syndrome were not detected in the serum and CSF. Rheumatoid and autoimmune disease-related parameters, including erythrocyte sedimentation rate, rheumatoid factor, anti-cyclic citrullinated peptide antibodies, glucose phosphate isomerase, antinuclear antibodies, anti-cardiolipin antibodies, and anti-neutrophil antibodies, were within normal limits.
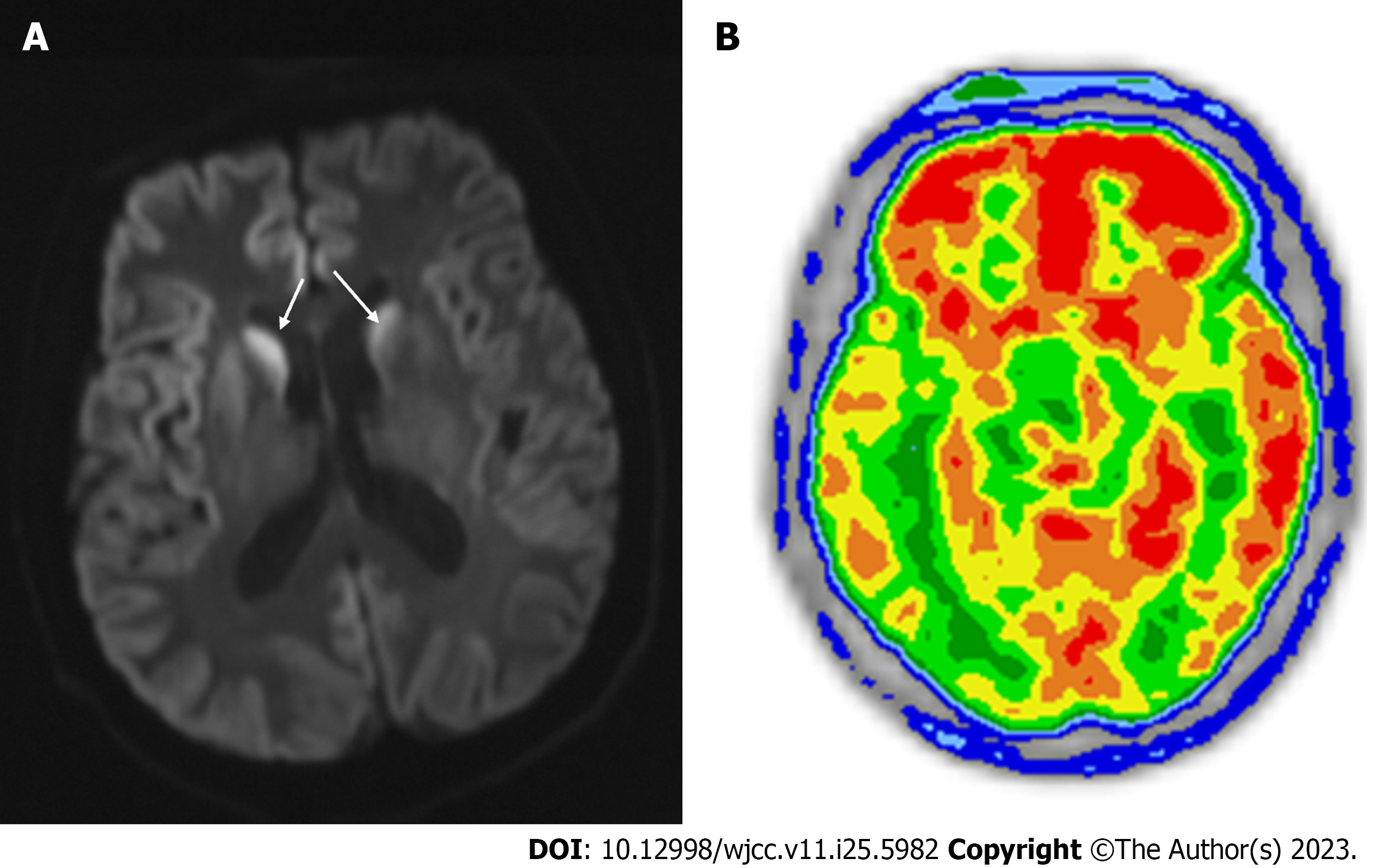
Magnetic resonance imaging (MRI) of the brain revealed hyperintense signals on diffusion-weighted imaging (DWI) sequences in the bilateral occipital, parietal, temporal, and frontal lobes, particularly in the right cortex. These hyperi
Considering rapid cognitive decline, diffuse brain damage, and hyperthermia, a probable diagnosis of CJD was made. Viral encephalitis, which can be treated, could not be ruled out. Antiviral therapy was attempted by intravenous administration of gamma globulin (300 mg/day) for five days. Physical cooling and benzodiazepines were used to manage the fever and myoclonus.
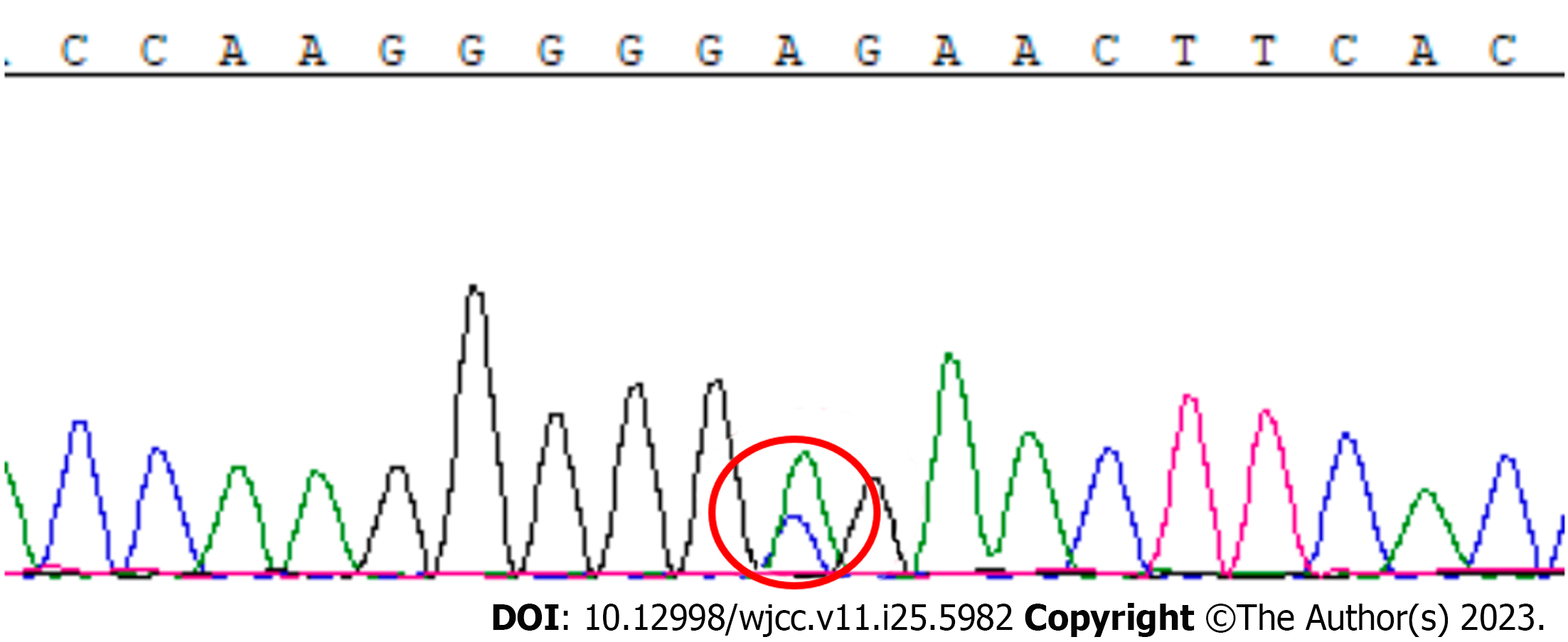
Despite gamma globulin treatment, the patient's condition did not improve. EEG performed in the fifth month after symptom onset showed bilateral persistent 4-5 Hz q waves, 2-3 Hz d waves, and sharp slow waves. In the sixth month after symptom onset, the EEG showed bilaterally symmetrical low-to-moderate-potential 8-Hz a waves with a large number of moderate-to-high potential 4–6 Hz q waves. Repeated examinations of serum and CSF for autoimmune encephalitis-related antibodies yielded negative results. Other potential diagnoses were ruled out, and CJD remained a potential diagnosis. Although the 14-3-3 protein was not detected in the CSF, the combination of the patient's clinical manifestations and imaging findings further supported the suspicion of CJD. Sequencing of PRNP revealed a heterozygous c.A587C base change in the second exon, resulting in a glutamate-to-alanine substitution at E196A that indicated a potential pathogenic mutation (Figure 2).
According to diagnostic criteria for CJD[7,8], the final diagnosis in this case was probably CJD caused by an E196A mutation in PRNP.
Physical cooling and antipyretic drugs were administered to manage patient’s hyperthermia. Considering the possibility of viral encephalitis, gamma globulin was initially administered. After the diagnosis of CJD, the patient's family opted for palliative care.
The patient succumbed to illness within 11 mo of onset. The family declined an autopsy.
Mutations in PRNP, which is located on the 20th human chromosome, can lead to the development of prion diseases. One specific mutation, E196A, has been reported in cases of CJD. Between 2014 and 2019, four cases of CJD with E196A mutation were individually documented[9-11]. In 2021, the Chinese National CJD Surveillance System reported 16 Chinese CJD patients with the E196A mutation, including four previously reported cases[12]. All 16 patients[12] and the patient mentioned in this case study were of Han Chinese ethnicity and had no relevant family history or known exposure to prion diseases. Patients with the E196A mutation exhibit nonspecific symptoms at onset, and disease progression demonstrated typical features of sporadic prion diseases. The disease duration varied between 2 and 28 mo in the reported Chinese cases[12]. No brain tissue biopsy or autopsy was conducted in any of the 17 patients, leading to inconclusive evidence regarding the relationship between the E196A mutation in PRNP and occurrence of CJD.
Based on the clinical manifestations and imaging features, the patient was diagnosed with probable CJD. A recent study have found that the criterion of displaying at least one positive brain region on DWI to diagnose CJD has a sensitivity and specificity higher than 90%[13]. Other possible diagnoses were ruled out during examination. PRNP sequencing results firmly established the diagnosis of CJD. CJD patients harboring the E196A mutation present with various clinical manifestations, including mental problems, dementia, cerebellar disorders, visual disturbances, pyramidal dysfunction, and extrapyramidal dysfunction[12,14]. None of the reported patients with the E196A mutation mentioned Korsakoff syndrome. Korsakoff syndrome is a mental disorder caused by vitamin B1 deficiency in the brain, and is characterized by memory impairment, confabulation or falsification, apathy, and time and space disorientation. Although cases of CJD presenting as Korsakoff syndrome have been reported[15], imaging examinations in previous cases, including the present case, did not reveal typical lesions associated with vitamin B1 deficiency. Given the rapidly progressive dementia following Korsakoff syndrome and the normal serum level of vitamin B1, CJD is considered a more likely diagnosis than Wernicke encephalopathy, despite the presence of Korsakoff syndrome in early stages of the disease[16,17]. Current study is the first report of a CJD patient associated with the E196A mutation who displayed Korsakoff syndrome at an early stage. Based on the findings of MRI of the brain, the thalamic region is speculated to be involved in the occurrence of Korsakoff syndrome in this CJD patient. The survival time for CJD patients presenting with Korsakoff syndrome ranges from 2 to 18 mo[18].
The 14-3-3 protein, a classic biomarker of CJD, was not found in the patient's CSF in this case. Similar results were observed in previous cases of CJD with E196A mutation[12]. This may be related to the fact that some patients are in the early stages of the disease or that the 14-3-3 protein has low sensitivity in certain CJD subtypes[19].
Another mutation at the same codon, E196K, was identified in a Caucasian population and confirmed to be associated with CJD[20]. Patients with CJD caused by the E196K mutation were positive for CSF 14-3-3 protein and had a shorter disease course, averaging 6.5 mo. Since the E196A mutation is located adjacent to E196K, it is possible that E196A may have a pathogenic role similar to that of E196K. However, the neuropathological characteristics associated with E196A mutation have not yet been clarified. The conclusions drawn from the present case report are based on the available information and current understanding of CJD and prion diseases. Further research, including brain tissue analyses and autopsy studies, is required to establish a definitive relationship between the E196A mutation in PRNP and CJD, and to elucidate the neuropathological characteristics associated with this mutation.
However, CJD is incurable. Some researchers have recently attempted to inhibit prion duplication to prolong the survival of CJD patients[19,21]. The present study’s findings offer new insights for the treatment of CJD.
Multiple factors, including clinical manifestations, laboratory test results, and imaging findings, should be considered when diagnosing CJD. The diagnosis of CJD can be challenging owing to its diverse clinical presentations and the need to exclude other possible causes. The rarity of E196A mutation in CJD highlights the complexity of this disease.
We thank the Department of Vascular Disease nurses who helped draw blood.
| 1. | Imran M, Mahmood S. An overview of human prion diseases. Virol J. 2011;8:559. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 118] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 2. | Prusiner SB. The prion diseases. Brain Pathol. 1998;8:499-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 258] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 3. | Uttley L, Carroll C, Wong R, Hilton DA, Stevenson M. Creutzfeldt-Jakob disease: a systematic review of global incidence, prevalence, infectivity, and incubation. Lancet Infect Dis. 2020;20:e2-e10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 146] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 4. | Shir D, Lazar EB, Graff-Radford J, Aksamit AJ, Cutsforth-Gregory JK, Jones DT, Botha H, Ramanan VK, Prusinski C, Porter A, Day GS. Analysis of Clinical Features, Diagnostic Tests, and Biomarkers in Patients With Suspected Creutzfeldt-Jakob Disease, 2014-2021. JAMA Netw Open. 2022;5:e2225098. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 5. | Jeong BH, Kim YS. Genetic studies in human prion diseases. J Korean Med Sci. 2014;29:623-632. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 80] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 6. | Oudman E, Wijnia JW, Oey MJ, van Dam M, Postma A. Wernicke-Korsakoff syndrome despite no alcohol abuse: A summary of systematic reports. J Neurol Sci. 2021;426:117482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 65] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 7. | Manix M, Kalakoti P, Henry M, Thakur J, Menger R, Guthikonda B, Nanda A. Creutzfeldt-Jakob disease: updated diagnostic criteria, treatment algorithm, and the utility of brain biopsy. Neurosurg Focus. 2015;39:E2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 104] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 8. | Hermann P, Appleby B, Brandel JP, Caughey B, Collins S, Geschwind MD, Green A, Haïk S, Kovacs GG, Ladogana A, Llorens F, Mead S, Nishida N, Pal S, Parchi P, Pocchiari M, Satoh K, Zanusso G, Zerr I. Biomarkers and diagnostic guidelines for sporadic Creutzfeldt-Jakob disease. Lancet Neurol. 2021;20:235-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 207] [Article Influence: 41.4] [Reference Citation Analysis (0)] |
| 9. | Zhang H, Wang M, Wu L, Zhang H, Jin T, Wu J, Sun L. Novel prion protein gene mutation at codon 196 (E196A) in a septuagenarian with Creutzfeldt-Jakob disease. J Clin Neurosci. 2014;21:175-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 10. | Shi Q, Zhou W, Chen C, Zhang BY, Xiao K, Wang Y, Dong XP. Rare E196A mutation in PRNP gene of 3 Chinese patients with Creutzfeldt-Jacob disease. Prion. 2016;10:331-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 11. | Dai Y, Lang Y, Ding M, Zhang B, Han X, Duan G, Cui L. Rare genetic Creutzfeldt-Jakob disease with E196A mutation: a case report. Prion. 2019;13:132-136. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 12. | Shi Q, Xiao K, Chen C, Zhou W, Gao LP, Wu YZ, Wang Y, Hu C, Gao C, Dong XP. Characteristics of Chinese patients with genetic CJD who have E196A or E196K mutation in PRNP: comparative analysis of patients identified in the Chinese National CJD Surveillance System. BMJ Open. 2021;11:e054551. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (1)] |
| 13. | Bizzi A, Pascuzzo R, Blevins J, Grisoli M, Lodi R, Moscatelli MEM, Castelli G, Cohen ML, Schonberger LB, Foutz A, Safar JG, Appleby BS, Gambetti P. Evaluation of a New Criterion for Detecting Prion Disease With Diffusion Magnetic Resonance Imaging. JAMA Neurol. 2020;77:1141-1149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 55] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 14. | Shi Q, Chen C, Xiao K, Zhou W, Gao LP, Chen DD, Wu YZ, Wang Y, Hu C, Gao C, Dong XP. Genetic Prion Disease: Insight from the Features and Experience of China National Surveillance for Creutzfeldt-Jakob Disease. Neurosci Bull. 2021;37:1570-1582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 15. | Pietrini V. Creutzfeldt-Jakob disease presenting as Wernicke-Korsakoff syndrome. J Neurol Sci. 1992;108:149-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 16. | Chen B, Zhang S, Xiao Y, Wu Y, Tang W, Yan L, Zhang Z, Qin S, Dai M, You Y. Genetic Creutzfeldt-Jakob disease shows fatal family insomnia phenotype. Prion. 2021;15:177-182. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 17. | Iwasaki Y, Hashimoto R, Saito Y, Aiba I, Inukai A, Akagi A, Mimuro M, Miyahara H, Kitamoto T, Yoshida M. An autopsied case of MM1-type sporadic Creutzfeldt-Jakob disease with pathology of Wernicke encephalopathy. Prion. 2019;13:13-20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 18. | Goossens K, van Bruchem-Visser RL. A patient with a 'typical presentation' of Wernicke encephalopathy was found to have sporadic Creutzfeldt-Jakob disease. Neth J Med. 2017;75:211-214. [PubMed] |
| 19. | Vallabh SM, Minikel EV, Schreiber SL, Lander ES. Towards a treatment for genetic prion disease: trials and biomarkers. Lancet Neurol. 2020;19:361-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 52] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 20. | Peoc'h K, Manivet P, Beaudry P, Attane F, Besson G, Hannequin D, Delasnerie-Lauprêtre N, Laplanche JL. Identification of three novel mutations (E196K, V203I, E211Q) in the prion protein gene (PRNP) in inherited prion diseases with Creutzfeldt-Jakob disease phenotype. Hum Mutat. 2000;15:482. [PubMed] [DOI] [Full Text] |
| 21. | Minikel EV, Zhao HT, Le J, O'Moore J, Pitstick R, Graffam S, Carlson GA, Kavanaugh MP, Kriz J, Kim JB, Ma J, Wille H, Aiken J, McKenzie D, Doh-Ura K, Beck M, O'Keefe R, Stathopoulos J, Caron T, Schreiber SL, Carroll JB, Kordasiewicz HB, Cabin DE, Vallabh SM. Prion protein lowering is a disease-modifying therapy across prion disease stages, strains and endpoints. Nucleic Acids Res. 2020;48:10615-10631. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 98] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Beran RG, Australia; Toledano A, Spain S-Editor: Fan JR L-Editor: A P-Editor: Cai YX