Published online Sep 6, 2023. doi: 10.12998/wjcc.v11.i25.5878
Peer-review started: July 12, 2023
First decision: August 2, 2023
Revised: August 7, 2023
Accepted: August 11, 2023
Article in press: August 11, 2023
Published online: September 6, 2023
Processing time: 51 Days and 5.4 Hours
Acute respiratory distress syndrome precipitates is widespread pulmonary injury in impacted individuals, the neonatal respiratory distress syndrome (NRDS), primarily observed in preterm infants, represents a prevalent critical condition in neonatal clinical settings.
To investigate the clinical efficacy of various ventilation strategies combined with pulmonary surfactant (PS) therapy in the treatment of NRDS.
A total of 20 neonates diagnosed with respiratory distress syndrome, admitted between May 2021 and June 2022, were randomly assigned to either a research group or a control group. Neonates in the research group received treatment involving high-frequency oscillatory ventilation (HFOV) in conjunction with PS. In contrast, neonates in the control group were administered either controlled mechanical ventilation or synchronous intermittent mandatory ventilation, combined with PS. Arterial blood samples from the neonates in both groups were collected before treatment, as well as 6 h, 12 h, 24 h, and 48 h post-treatment. These samples underwent blood gas analysis, with measurements taken for pH value, partial pressures of oxygen (O2) and carbon dioxide. Concurrently, data was collected on the duration of ventilator use, length of hospitalization time, O2 treatment time, treatment outcomes, and complications of the ventilator.
From 6-48 h post-treatment, both groups demonstrated significant improvements in arterial blood pH and oxygen partial pressure, along with a significant decrease in carbon dioxide partial pressure compared to pre-treatment values (P < 0.05). Although these changes progressed over time, there were no significant differences between the two groups (P > 0.05). However, the research group had significantly lower X-ray scores, shorter hospitalization time, and less time on O2 therapy compared to the control group (P < 0.05). Mortality rates were similar between the two groups (P > 0.05), but the research group had a significantly lower incidence of complications (P < 0.05).
The integration of HFOV combine with PS has proven to effectively expedite the treatment duration, decrease the occurrence of complications, and secure the therapeutic efficacy in managing NRDS.
Core Tip: We observed 20 children with respiratory distress syndrome who received different ventilation strategies combined with pulmonary surfactant (PS) therapy, we divided into two groups: The observation group received high-frequency oscillatory ventilation (HFOV) combined with PS, and the control group received controlled mechanical ventilation or synchronous intermittent mandatory ventilation combined with PS. The observed indicators included blood gas analysis pH value partial pressures of oxygen and carbon dioxide. Our research results showed that the integration of HFOV combine with PS has proven to effectively expedite the treatment duration, decrease the occurrence of complications, and secure the therapeutic efficacy in managing neonatal respiratory distress syndrome.
- Citation: Qing Q, Zha P, Dai LY, Wang Y. Effect of different ventilation methods combined with pulmonary surfactant on neonatal acute respiratory distress syndrome. World J Clin Cases 2023; 11(25): 5878-5886
- URL: https://www.wjgnet.com/2307-8960/full/v11/i25/5878.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i25.5878
Acute respiratory distress syndrome (ARDS) precipitates a widespread pulmonary injury in impacted individuals, which attributable to the damage inflicted on alveolar epithelial cells and capillaries during an excessive efflux of inflammatory mediators and cells[1-3], is starkly reflected in the loss of pulmonary surfactant (PS). Alarmingly, when ARDS strikes neonates, it carries a considerable risk of causing neonatal mortality and high disability rates[4]. Key risk factors encompass selective cesarean section hypoxia, asphyxia, and meconium aspiration. Neonatal respiratory distress syndrome (NRDS), primarily observed in preterm infants, represents a prevalent critical condition in neonatal clinical settings. Mechanical ventilation and PS administration emerge as the prevailing treatment strategies for NRDS.
Commonly implemented modes of mechanical ventilation in neonates include[5,6]: Controlled Mechanical Ventilation (CMV), Synchronous Intermittent Mandatory Ventilation (SIMV), and Non-invasive High-Frequency Positive Pressure Ventilation (NHFPPV). While CMV and SIMV offer assured therapeutic outcomes, they are intricate, invasive, and associated with numerous adverse reactions. In contrast, NHFPPV, a non-physiological positive pressure ventilation approach, is less complex and non-invasive but carries the risk of amplifying lung injury[7]. High-Frequency Oscillatory Ventilation (HFOV) has been demonstrated to exert a lung-protective effect by leveraging a tidal volume equal to or smaller than the anatomical dead space for rapid gas exchange[8,9]. Furthermore, exogenous PS replacement therapy has been promoted and proven efficacious in mitigating NRDS. Presently, respiratory ailments such as NRDS, congenital diaphragmatic hernia, and meconium aspiration syndrome can be effectively managed with HFOV combined with PS (HFOV+PS) inhalation therapy in neonates and infants[10,11]. However, additional investigations are required to elucidate the impacts of the HFOV and PS combination on ARDS[12,13].
The aim of this study is to compare the effectiveness and safety of HFOV+PS with CMV and PS (CMV+PS) in treating ARDS, thereby offering a foundational guide for the clinical implementation of HFOV in ARDS therapy.
In this study, the research group comprised 35 patients, and the control group also had 35 patients. In the research group, there were 20 males and 15 females. The gestational ages ranged from 34 to 41 wk, with an average of 37.09 ± 1.12 wk. And the birth weights varied between 2000 g and 4317 g, with an average of 3456.09 ± 866.70 g. In the control group, there were 19 males and 16 females. Here, the gestational ages spanned from 35 to 40 wk, with an average of 37.67 ± 1.87 wk and the birth weights ranged from 2145 g to 4021 g, with an average of 3170.33 ± 767.18 g. Here, the guardians of the children participated voluntarily in this study.
This study represents a single-center, prospective, randomized controlled trial conducted at a provincial hospital in China spanning from May 2020 to June 2022. The investigation received approval from our institutional Ethics Committee and adhered to the principles outlined in the Helsinki Declaration (revised in 2013). Furthermore, written informed consent was obtained from the parents of all participating subjects. The study population comprised 70 neonates diagnosed with RDS, who were admitted to our hospital during the study period.
The inclusion criteria included: (1) Diagnosis conforming to the Montreux diagnostic criteria of 2017; and (2) clinical indications for mechanical ventilation treatment.
Exclusion criteria included congenital malformations of the respiratory tract, congenital heart disease, and intrauterine infectious pneumonia.
Utilizing the two independent sample r test sample size estimation module of PASS 15.0 software, with set parameters of α = 0.05 and β = 0.10, and assuming equal sample sizes, we referred to the expression levels of vascular endothelial growth factor, soluble receptor for advanced glycation endproducts, surfactant protein-D, and Ang-2 in research group and control group. Based on these calculations, the required sample size for this study was determined to be 30 subjects, n = 15 for each group. However, due to missed visits or incomplete data during the study, the final sample included 11 subjects in the research group and 9 in the control group.
PS (Calf Pulmonary Surfactant for Injection, CR Shuanghe Keli Su, China) was administered via intratracheal injection. All infants received a single dosage of 100 mg/kg within 6 h of ARDS diagnosis. Prior to administration, airway secretions were suctioned, and measures were taken to stabilize circulation and correct acid-base imbalances. Following PS injection, rapid ventilation under pressure was applied for 1 minute, after which the infants were connected to a ventilator to maintain either HFOV/CMV or SIMV ventilation.
In the research group, 35 patients received routine endotracheal intubation (Babylog, VN500; Drager, Germany) coupled with HFOV and PS (100 mg/kg). The PS was re-administered, up to 4 times, if hypoxemia persisted beyond 12 h. Initial ventilator settings were as follows.
Here, frequency set between 812 Hz and inhalation-exhalation ratio at 1:1. Mean airway pressure (MAP) initiated at 10-15 cm H2O, gradually increased by 1 cm H2O every 2 to 3 minutes until oxygenation ceased to improve. Thereafter, MAP was incrementally reduced by 1-2 cm H2O every 2 to 3 minutes until Transcutaneous Oxygen Saturation (TcSaO2) decreased, subsequently increasing MAP by 1-2 cm H2O from this point. The initial setting for amplitude was 30-40 cm H2O. The right diaphragm was maintained at the ninth rib level following an hour of mechanical ventilation, using chest radiographs to ascertain optimal lung function. Parameters were adjusted in accordance with the dynamic monitoring results of blood gas analysis. PS was injected into the endotracheal tube after at least 10 minutes of continuous ventilation. Throughout the treatment, variations in heart rate, respiration, blood pressure, and oxygen saturation (SaO2) were meticulously observed. If apnea, a drop in SaO2, or heart rate was observed, the PS injection was halted and oxygen was promptly administered until the infant's condition stabilized. Following PS administration, mechanical ventilation was continued with ventilator settings adjusted in line with SaO2 findings, blood gas analyses, and chest radiography. The infant was extubated when the following conditions were met: hemodynamic stability, MAP of 8 cm H2O, Fraction of Inspired Oxygen (FiO2) of 40%, and weaning from sedation. Before extubation, the infant was put on conventional ventilation. Post weaning, nasal continuous positive airway pressure (NCPAP) was administered.
In the control group, 35 patients received standard endotracheal intubation coupled with CMV using a Stephanie ventilator (Fritz Stephan GmbH, Gackenbach, Germany), and PS (100 mg/kg). If hypoxemia lasted beyond 12 h, PS was re-administered, up to 4 times. Initial parameters were set as follows.
FiO2: 30%-50%. Peak inspiratory pressure: 18-25cm H2O. Positive end-expiratory pressure: 4-6 cm H2O, I/E: 1:1.5. Respiratory Rate (RR): 30-40 breaths/min. The ventilator parameters were adjusted in line with SaO2 results, arterial blood gas (ABG) analysis, and chest radiographs. Post weaning, NCPAP was administered.
Peripheral venous blood was drawn from the children in both groups at the following intervals: before treatment, 6 h post-treatment, 12 h post-treatment, 24 h post-treatment, and 48 h post-treatment. Blood gas analysis was performed, and pH, blood oxygen partial pressure (PaO2), and PaCO2 were recorded. The duration of key treatment components, including ventilation, hospitalization time and O2 treatment time were noted. Moreover, treatment outcomes and complications were tracked and documented.
The data were analyzed using the 25.0 version of SPSS software for Windows (IBM SPSS Inc., Chicago, IL, United States). The measurement data are expressed as mean ± standard deviation, and the analysis of variance and t-test are used. The counting data was expressed in percentage (%), and perform χ2 test the difference was statistically significant with P < 0.05.
As is shown in Table 1, there was no statistically significant differences were observed in the baseline data between the two groups (P > 0.05), indicating that they were comparable.
| Item | Research group | Control group | P value |
| Gestational age (d) | 37.09 ± 1.12 | 37.67 ± 1.87 | 0.658 |
| Gender | |||
| Male | 20 (57.1) | 19 (54.3) | 0.810 |
| Female | 15 (42.9) | 16 (45.7) | |
| Birth weight (g) | 3456.09 ± 866.70 | 3170.33 ± 767.18 | 0.453 |
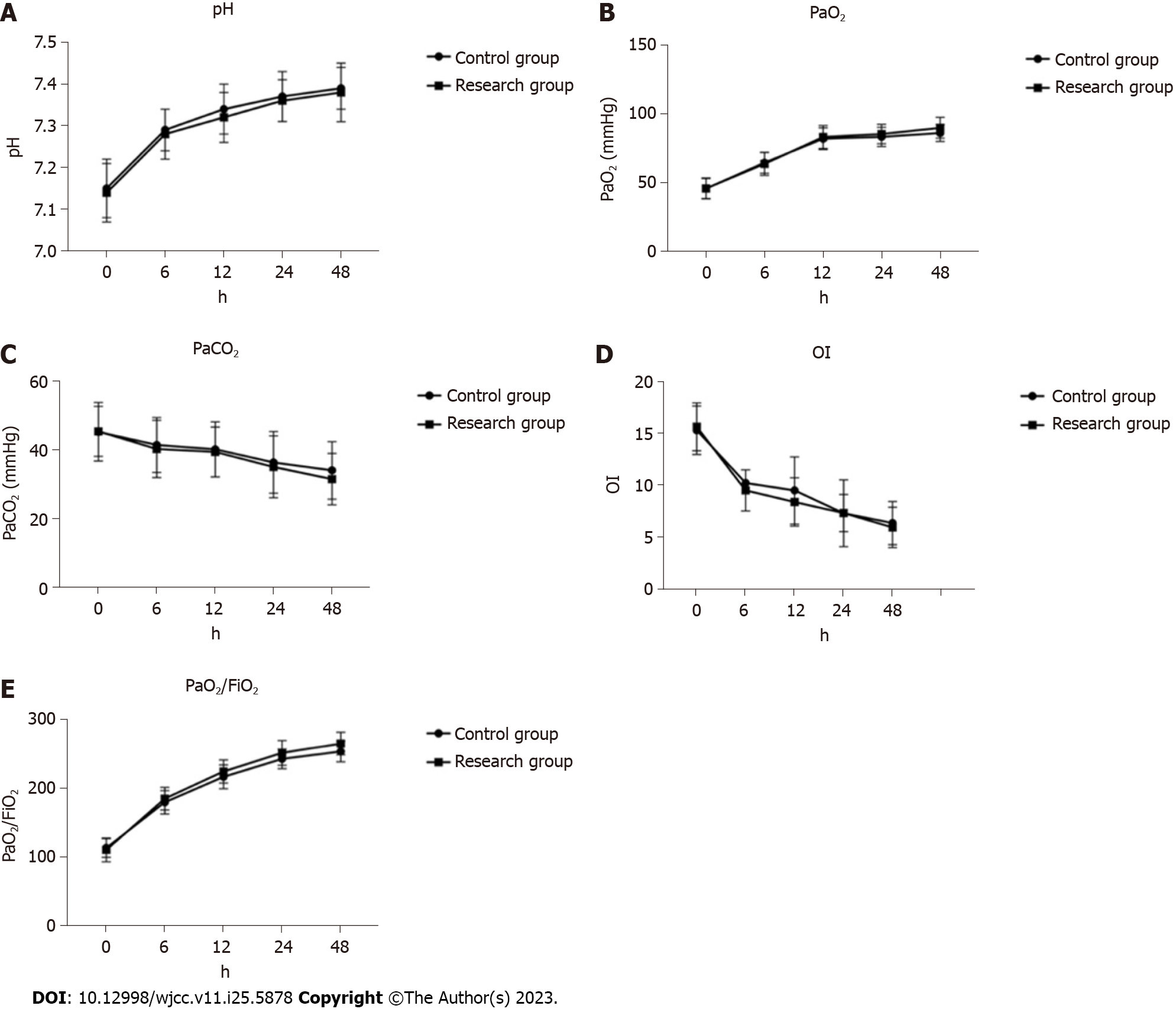
In this study, there was no significant difference was observed between the two groups before treatment (P > 0.05). As is shown in Figure 1, following treatment, both the arterial blood pH and blood PaO2 significantly increased compared to their pre-treatment levels, while the PaCO2 significantly decreased. The oxygen deficiency index (OI) was lower post-treatment, and the oxygenation index (PaO2/FiO2) was notably higher than the pre-treatment values (P < 0.05). As treatment duration increased, the arterial blood pH, PaO2, and PaO2/FiO2 in both groups showed significant increases, while PaCO2 and OI demonstrated significant decreases. However, no significant differences were noted between the two groups in terms of arterial blood pH, blood PaO2, and PaCO2 at different post-treatment time points (P > 0.05).
The duration of ventilator use, hospitalization and O2 therapy in the research group were significantly shorter than those in the control group (P < 0.05), as shown in Table 2.
The cure rate of children in the research group was significantly higher than that in the control group (P < 0.05). Moreover, there was no significant difference in mortality between the two groups (P > 0.05) (Table 3).
| Group | Cure rate | Improvement rate | Case fatality rate |
| Control group | 3 (8.6) | 27 (77.1) | 5 (14.3) |
| Research group | 14 (40.0) | 19 (54.3) | 2 (5.7) |
| χ2 | 9.401 | 4.058 | 1.429 |
| P value | 0.002 | 0.044 | 0.232 |
Before treatment, there was no statistically significant difference in chest X-ray score and Apgar score between the two groups of patients (P > 0.05). After treatment, the research group had higher chest X-ray score and Apgar score than the control group (P < 0.05) (Table 4).
As is shown in Table 5, the total incidence of complications such as air leakage in the control group was 37.1%, and that in the research group was 11.4%. The incidence of complications in the research group was significantly lower than that in the control group (P < 0.05).
| Group | Emphysema | Ventilator-associated pneumonia | Intraventricular hemorrhage | Patent ductus arteriosus | Total complications |
| Control group | 4 | 2 | 3 | 3 | 13 (37.1) |
| Research group | 2 | 1 | 1 | 4 (11.4) | |
| χ2 | 6.293 | ||||
| P value | 0.012 |
Due to immature lung development and surfactant deficiency, ARDS is characterized by alveolar collapse, decreased lung compliance, and diminished ventilation-perfusion ratio. The primary manifestations include refractory hypoxemia and significant enhancement of alveolar permeability. Hypoxia and acidosis can induce injury to alveolar epithelial cells and pulmonary vascular endothelial cells. Moreover, ARDS-related fluid leakage and heightened vascular permeability not only exacerbate acidosis and hypoxia but also diminish the production and secretion of PS, establishing a self-perpetuating cycle. Increased pulmonary microvascular permeability can lead to alveolar and interstitial edema, injury to alveolar type II cells, and decreased generation or release and activity of endogenous PS. This, in turn, decreases lung compliance, escalates alveolar surface tension, compromises lung function, and reduces SaO2 levels[14,15]. Acute hemodynamic alterations induced by hypoxia and acidosis could exacerbate the state of decreased cardiac output, potentially culminating in multiple organ failure.
PS can enhance the clinical prognosis of neonates with RDS, promoting the regeneration of pulmonary epithelial cells and the release of endogenous surfactant. Beyond preserving functional residual capacity, stabilizing alveolar pressure, and minimizing fluid leakage from capillaries to alveoli, PS replacement therapy can also reduce alveolar surface tension, preventing alveolar collapse at the end of the expiratory phase. Moreover, it can decrease ventilator parameters while concurrently improving lung compliance and ventilation function[16,17]. Early administration of PS in neonates with ARDS has demonstrated a reduction in the necessity for mechanical ventilation and an enhancement in oxygenation[18,19]. Clinical observations suggest that mechanical ventilation should be employed in conjunction with PS therapy, as this therapy alone exhibits limited efficacy in addressing NRDS. Mechanical ventilation, which has been shown to significantly improve the oxygenation capacity in children with respiratory dysfunction, is currently the most favored treatment approach for neonates with acute and severe respiratory difficulties.
Study has linked the repetitive opening and closing of collapsed alveoli, mainly induced by high airway pressure, large lung capacity, and recurrent alveolar collapse, to the release of inflammatory mediators and subsequent multi-organ dysfunction[20]. Consequently, the focus of mechanical ventilation lies in lung-protective strategies. The two primary goals are to prevent the recurrent closure of end-expiratory alveoli and to limit excessive alveolar expansion to mitigate lung injury and enhance disease recovery. HFOV is characterized by low-cycle pressure changes, biphasic pressure changes, tidal volume at or below the level of the anatomical dead space, and supra-physiological respiratory frequency oscillations. This facilitates an effective gas exchange ventilation mode at the alveolar level, rapidly achieving even alveolar expansion, enhanced gas exchange, and increased lung compliance. HFOV supports the processes of inhalation and exhalation during ventilation, improving oxygenation and carbon dioxide removal while minimizing barotrauma. Its hypoventilation approach can protect the lungs during mechanical ventilation, thus improving survival rates[21,22]. The research conducted by Poddutoor et al[23] studied 675 neonates who were administered CMV. They found that of those, 97 neonates had to be switched to HFOV due to failure of CMV treatment. Impressively, there was a significant improvement in lung oxygenation and ABG parameters within 2 h of switching to HFOV. Our study aligns with these findings. We observed that the ABG parameters in the HFOV+PS group improved significantly after treatment. The CMV+PS group also showed improvements in their ABG parameters, however, the effect was not as pronounced as in the HFOV+PS group.
Moreover, infants treated with the HFOV+PS method experienced significantly reduced hospitalization and boarding durations compared to those in the CMV+PS group. The duration of hospital-stay and the need for mechanical ventilation were markedly less for the HFOV+PS group, demonstrating its effectiveness over the CMV+PS approach. This can be attributed to the fact that, in comparison to CMV, the rapid airflow created by HFOV can swiftly disperse PS across the alveolar surface, reducing surface tension. This effect further expands the alveoli, ensuring their stability, enhancing alveolar ventilation and respiration function, and alleviating symptoms of hypoxia and acidosis. Furthermore, when combined with mechanical ventilation, PS can notably augment the PaO2/FiO2 ratio, decrease OI, and significantly improve oxygenation capabilities in children with ARDS[21,24]. This study also discovered that the duration of mechanical ventilation and the need for oxygen therapy was notably reduced in the HFOV+PS group as compared to the CMV+PS group. This might be attributed to the ability of HFOV, which can clear obstructions in the small airways and alveoli. The distinct mode of gas exchange and rapid ventilation frequency foster a uniform distribution of PS along the alveolar wall. Additionally, surfactants exert immunomodulatory, physicochemical, and antibacterial effects which contribute to the stabilization of alveoli, reduction in alveolar capillary edema, and ultimately an enhancement in lung function[24]. The combination of PS with HFOV significantly optimizes respiration and diminishes the time needed for ventilation.
Based on the findings of the study, post-treatment arterial blood pH value, blood PaO2, and blood PaCO2 of both groups were significantly higher than their pre-treatment levels. Meanwhile, the oxygenation index (PaO2/FiO2) was markedly lower post-treatment compared to its pre-treatment value. As the duration of treatment extended, the arterial blood pH value, PaO2, and PaO2/FiO2 of both groups significantly rose, while PaCO2 and OI saw a substantial decrease. To enhance oxygenation and pulmonary ventilation function in children with NRDS, this study suggests no significant difference between HFOV, CMV, or SIMV when used in conjunction with PS. Children in the research group spent notably less time on ventilators, had significantly shorter hospitalization durations, and required oxygen treatments for a significantly shorter period than those in the control group. Furthermore, the recovery rate among children in the research group was significantly higher than in the control group, whereas the mortality rate remained fairly consistent across both groups. However, the incidence of complications was lower in the research group compared to the control group, suggesting a positive impact of HFOV.
Our study has some limitations. Firstly, the sample size used in our research was small, with only 20 cases in total, 11 in the observation group and 9 in the control group, which could affect the persuasiveness of our research results. Additionally, we lacked long-term follow-up on patients after discharge, which means we were unable to provide evidence for the long-term therapeutic effects of HFOV combined with PS.
In conclusion, our findings suggest that for infants with ARDS, the utilization of HFOV in conjunction with PS demonstrates a significant advantage over the use of CMV with PS. Specifically, the HFOV+PS approach notably reduces both the duration of hospitalization and the necessity for prolonged mechanical ventilation. Importantly, these benefits are achieved without any attendant increase in the incidence of complications. This evidence provides a compelling case for the preferential use of HFOV+PS in the management of infant ARDS, to optimize patient outcomes and enhance the efficiency of care.
Acute respiratory distress syndrome (ARDS) precipitates is widespread pulmonary injury in impacted individuals, the neonatal respiratory distress syndrome (NRDS), primarily observed in preterm infants, represents a prevalent critical condition in neonatal clinical settings.
Presently, respiratory ailments such as NRDS, congenital diaphragmatic hernia, and meconium aspiration syndrome can be effectively managed with high-frequency oscillatory ventilation (HFOV) combined with pulmonary surfactant (PS) (HFOV+PS) inhalation therapy in neonates and infants. However, additional investigations are required to elucidate the impacts of the HFOV and PS combination on ARDS.
The aim of this study is to investigate the clinical efficacy of various ventilation strategies combined with PS therapy in the treatment of NRDS.
A total of 20 neonates diagnosed with RDS, admitted between May 2021 and June 2022, were randomly assigned to either a research group or a control group. Neonates in the research group received treatment involving HFOV in conjunction with PS. In contrast, neonates in the control group were administered either controlled mechanical ventilation (CMV) or synchronous intermittent mandatory ventilation, combined with PS.
From 6-48 h post-treatment, both groups demonstrated significant improvements in arterial blood pH and oxygen partial pressure, along with a significant decrease in carbon dioxide partial pressure compared to pre-treatment values (P < 0.05). Although these changes progressed over time, there were no significant differences between the two groups (P > 0.05). However, the research group had significantly lower X-ray scores, shorter hospitalization time, and less time on O2 therapy compared to the control group (P < 0.05). Mortality rates were similar between the two groups (P > 0.05), but the research group had a significantly lower incidence of complications (P < 0.05).
Our findings suggest that for infants with ARDS, the utilization of HFOV in conjunction with PS demonstrates a significant advantage over the use of CMV with PS. Specifically, the HFOV+PS approach notably reduces both the duration of hospitalization and the necessity for prolonged mechanical ventilation. Importantly, these benefits are achieved without any attendant increase in the incidence of complications. This evidence provides a compelling case for the preferential use of HFOV+PS in the management of infant ARDS, to optimize patient outcomes and enhance the efficiency of care.
Our future research will be based on alleviating the economic pressure of patients and finding the cheapest treatment to treat Infant respiratory distress syndrome.
We would like to express our gratitude to all medical research institutes and patients who participated in this study, as well as to all reviewers for their review of this article.
| 1. | Froese AB, McCulloch PR, Sugiura M, Vaclavik S, Possmayer F, Moller F. Optimizing alveolar expansion prolongs the effectiveness of exogenous surfactant therapy in the adult rabbit. Am Rev Respir Dis. 1993;148:569-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 109] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 2. | Dreyfuss D, Saumon G. Ventilator-induced lung injury: lessons from experimental studies. Am J Respir Crit Care Med. 1998;157:294-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1587] [Cited by in RCA: 1443] [Article Influence: 51.5] [Reference Citation Analysis (0)] |
| 3. | Amatullah H, Maron-Gutierrez T, Shan Y, Gupta S, Tsoporis JN, Varkouhi AK, Teixeira Monteiro AP, He X, Yin J, Marshall JC, Rocco PRM, Zhang H, Kuebler WM, Dos Santos CC. Protective function of DJ-1/PARK7 in lipopolysaccharide and ventilator-induced acute lung injury. Redox Biol. 2021;38:101796. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 63] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 4. | Stephens RS, Shah AS, Whitman GJ. Lung injury and acute respiratory distress syndrome after cardiac surgery. Ann Thorac Surg. 2013;95:1122-1129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 126] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 5. | Rong LQ, Di Franco A, Gaudino M. Acute respiratory distress syndrome after cardiac surgery. J Thorac Dis. 2016;8:E1177-E1186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 59] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 6. | Fink MP. Role of reactive oxygen and nitrogen species in acute respiratory distress syndrome. Curr Opin Crit Care. 2002;8:6-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 85] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 7. | Acute Respiratory Distress Syndrome Network, Brower RG, Matthay MA, Morris A, Schoenfeld D, Thompson BT, Wheeler A. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342:1301-1308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8487] [Cited by in RCA: 8455] [Article Influence: 325.2] [Reference Citation Analysis (3)] |
| 8. | Yildizdas D, Yapicioglu H, Bayram I, Yilmaz L, Sertdemir Y. High-frequency oscillatory ventilation for acute respiratory distress syndrome. Indian J Pediatr. 2009;76:921-927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | Bojan M, Gioanni S, Mauriat P, Pouard P. High-frequency oscillatory ventilation and short-term outcome in neonates and infants undergoing cardiac surgery: a propensity score analysis. Crit Care. 2011;15:R259. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 10. | Rettwitz-Volk W, Veldman A, Roth B, Vierzig A, Kachel W, Varnholt V, Schlösser R, von Loewenich V. A prospective, randomized, multicenter trial of high-frequency oscillatory ventilation compared with conventional ventilation in preterm infants with respiratory distress syndrome receiving surfactant. J Pediatr. 1998;132:249-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 66] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 11. | Lin X-Z, Lai JD, Lv M, Zhu Y, Wang L, Chen C. [Clinical efficacy of high-frequency oscillatory ventilation combined with pulmonary surfactant in treatment of neonatal pulmonary hemorrhage]. Zhongguo Dang Dai Er Ke Za Zhi 2015; 17: 345–349. Available from: https://pubmed.ncbi.nlm.nih.gov/25919553/. |
| 12. | Khemani RG, Smith LS, Zimmerman JJ, Erickson S; Pediatric Acute Lung Injury Consensus Conference Group. Pediatric acute respiratory distress syndrome: definition, incidence, and epidemiology: proceedings from the Pediatric Acute Lung Injury Consensus Conference. Pediatr Crit Care Med. 2015;16:S23-S40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 293] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 13. | Willson DF, Thomas NJ, Markovitz BP, Bauman LA, DiCarlo JV, Pon S, Jacobs BR, Jefferson LS, Conaway MR, Egan EA; Pediatric Acute Lung Injury and Sepsis Investigators. Effect of exogenous surfactant (calfactant) in pediatric acute lung injury: a randomized controlled trial. JAMA. 2005;293:470-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 267] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 14. | Deterding RR. Infants and Young Children with Children's Interstitial Lung Disease. Pediatr Allergy Immunol Pulmonol. 2010;23:25-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 45] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 15. | Matthay MA, Zemans RL. The acute respiratory distress syndrome: pathogenesis and treatment. Annu Rev Pathol. 2011;6:147-163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 829] [Cited by in RCA: 820] [Article Influence: 54.7] [Reference Citation Analysis (0)] |
| 16. | Walsh BK, Daigle B, DiBlasi RM, Restrepo RD; American Association for Respiratory Care. AARC Clinical Practice Guideline. Surfactant replacement therapy: 2013. Respir Care. 2013;58:367-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 17. | De Luca D. Respiratory distress syndrome in preterm neonates in the era of precision medicine: A modern critical care-based approach. Pediatr Neonatol. 2021;62 Suppl 1:S3-S9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 18. | Amigoni A, Pettenazzo A, Stritoni V, Circelli M. Surfactants in Acute Respiratory Distress Syndrome in Infants and Children: Past, Present and Future. Clin Drug Investig. 2017;37:729-736. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 19. | Volsko TA, Parker SW, Deakins K, Walsh BK, Fedor KL, Valika T, Ginier E, Strickland SL. AARC Clinical Practice Guideline: Management of Pediatric Patients With Tracheostomy in the Acute Care Setting. Respir Care. 2021;66:144-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 20. | Möller JC, Schaible T, Roll C, Schiffmann JH, Bindl L, Schrod L, Reiss I, Kohl M, Demirakca S, Hentschel R, Paul T, Vierzig A, Groneck P, von Seefeld H, Schumacher H, Gortner L; Surfactant ARDS Study Group. Treatment with bovine surfactant in severe acute respiratory distress syndrome in children: a randomized multicenter study. Intensive Care Med. 2003;29:437-446. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 61] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 21. | Rodríguez-Moya VS, Gallo-Borrero CM, Santos-Áreas D, Prince-Martínez IA, Díaz-Casañas E, López-Herce Cid J. Exogenous surfactant and alveolar recruitment in the treatment of the acute respiratory distress syndrome. Clin Respir J. 2017;11:1032-1039. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 22. | Yapicioğlu H, Yildizdaş D, Bayram I, Sertdemir Y, Yilmaz HL. The use of surfactant in children with acute respiratory distress syndrome: efficacy in terms of oxygenation, ventilation and mortality. Pulm Pharmacol Ther. 2003;16:327-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 23. | Poddutoor PK, Chirla DK, Sachane K, Shaik FA, Venkatlakshmi A. Rescue high frequency oscillation in neonates with acute respiratory failure. Indian Pediatr. 2011;48:467-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 24. | Sun H, Cheng R, Kang W, Xiong H, Zhou C, Zhang Y, Wang X, Zhu C. High-frequency oscillatory ventilation versus synchronized intermittent mandatory ventilation plus pressure support in preterm infants with severe respiratory distress syndrome. Respir Care. 2014;59:159-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Pediatrics
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Lee S, Japan; Washio T, Japan S-Editor: Liu JH L-Editor: A P-Editor: Liu JH