Published online Aug 26, 2023. doi: 10.12998/wjcc.v11.i24.5830
Peer-review started: June 18, 2023
First decision: July 4, 2023
Revised: July 5, 2023
Accepted: July 25, 2023
Article in press: July 25, 2023
Published online: August 26, 2023
Processing time: 67 Days and 10.2 Hours
Immunotherapy has revolutionized the treatment of metastatic melanoma, but a significant proportion of patients still experience treatment resistance. Fecal microbiota transplantation (FMT) has emerged as a potential strategy to overcome immunotherapy resistance by modulating the gut microbiome.
We present a case report of a 57-year-old male with metastatic melanoma refractory to immunotherapy who received FMT in combination with anti-programmed death-ligand 1 (PD-L1) immunotherapy (pembrolizumab). After failing multiple lines of treatment, the patient underwent a single FMT procedure by colonoscopy using fecal material from a female metastatic melanoma donor who successfully responded to immunotherapy. Following FMT, the patient demonstrated a response with decreased subcutaneous disease and subsequently underwent surgery to remove the residual disease. Despite a subsequent recurrence in the small bowel that was resected, the patient remained on pembrolizumab without evidence of melanoma recurrence at the time of writing.
The favorable clinical and long-lasting effect we saw in our patient without significant toxicity suggests that this procedure should be considered in similar patients with immunotherapy refractory melanomas.
Core Tip: This case report highlights the use of fecal microbiota transplantation (FMT) as a potential strategy to overcome immunotherapy resistance in metastatic melanoma patients. The case involves a 57-year-old male who had failed multiple lines of treatment and received FMT alongside anti-programmed death-ligand 1 immunotherapy. Following FMT, the patient showed a response with a decrease in disease burden and remained on immunotherapy more than two years. This suggests that FMT may restore sensitivity to immunotherapy in refractory cases. Further research is needed to understand the underlying mechanisms and optimize treatment protocols for FMT in metastatic melanoma.
- Citation: del Giglio A, Atui FC. Fecal transplantation in patient with metastatic melanoma refractory to immunotherapy: A case report. World J Clin Cases 2023; 11(24): 5830-5834
- URL: https://www.wjgnet.com/2307-8960/full/v11/i24/5830.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i24.5830
Melanoma accounted for 325000 new cases worldwide, causing about 57000 deaths in 2020[1], affecting males more frequently than females. It is estimated that Melanoma cases should increase by 50% in 2040, reaching 510000 new cases and 96000 deaths[1]. Despite the recent remarkable advances in the treatment of metastatic melanoma with immunotherapy and anti-Braf targeted therapies, about 40% of the patients will still die of their disease[2,3].
One possibility treating immune-refractory patients with metastatic melanoma is the manipulation of the gut microbiome through Fecal Microbiota Transplantation (FMT)[4-6]. FMT can restore sensitivity to anti-programmed death-ligand 1 (anti-PD-L1) immunotherapy in about 40% of patients previously refractory to this medication[5,6]. Based on these encouraging preliminary data[5,6], we report here a case of a 57-year-old patient refractory to immunotherapy who benefited from FMT added to anti-PD-L1 immunotherapy with Pembrolizumab, which he was previously refractory to.
We report the case of a 57-year-old man with metastatic BRAF V-600E mutated melanoma diagnosed in October 2019 and started on Nivolumab with a partial response.
The patient presented with intense musculoskeletal pain at the site of the bony metastasis in his right hip.
The patient had a previous history of a desmoid tumor controlled by surgery and a bariatric surgery, without any other relevant past medical history.
No relevant past family history.
Right inguinal lymphadenopathy, right axillar lymphadenopathy and atrophy of the right shoulder muscles (due to previous desmoid surgery) with no other relevant physical findings.
Normal blood cell count and biochemical profile.
Computed tomography scan showed right axillary lymphadenopathy and right iliac bone metastasis.
Metastatic malignant melanoma. Tissue was obtained from a percutaneous biopsy of the right axillary lymphadenopathy.
Nivolumab with a partial response.
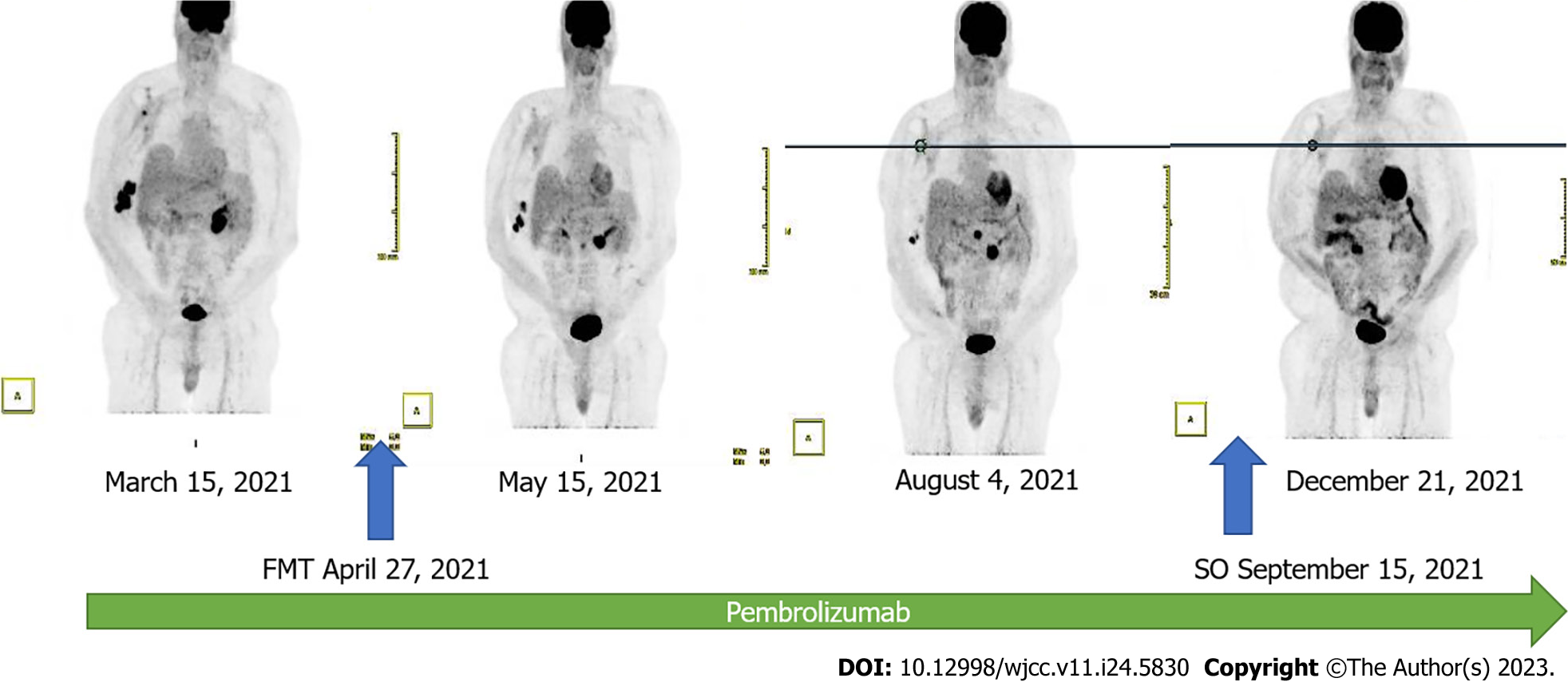
In October 2020, we noted disease progression of the melanoma in the subcutaneous tissue of the right thoracic region, and we started Cobimetinib and Vemurafenib without a response. In December 2020, we started Ipilimumab plus Pembrolizumab with a new progression. The Pembrolizumab was maintained, and in April 2021, he received only FMT by colonoscopy without prior antibiotic therapy. Fecal material was obtained from a female metastatic melanoma patient donor who achieved a longstanding complete response to Ipilimumab and Nivolumab and was off therapy in remission for more than 2 years. The donor had an entirely negative pre-donation screen for multiple infectious agents. The patient and donor formally consented to the FMT procedure.
After FMT, as seen in Figure 1, the right lateral thoracic subcutaneous disease decreased in size while we maintained Pembrolizumab. In September 2021, the patient underwent surgery to remove the residual disease. In July 2022, he had a PET scan that showed a small intestinal loop with increased FDG uptake, and we also noted a decrease in the ferritin level. A small intestinal survey with an endoscopic capsule revealed an abnormality in the small intestine judged as a melanoma recurrence which was resected and confirmed by the pathological report. As there were no other foci of disease, we maintained Pembrolizumab until this writing (June 23, 2023) and the patient is presently in remission without evidence of melanoma recurrence.
The gut microbiome represents a highly dynamic environment where diet, medication intake, or emotional stress can induce significant changes[7]. There are strong correlations between the gut microbiome and the nervous and immune systems[7]. Intestinal dysbiosis occurs when there is a disturbance in the complex commensal microbial communities in the digestive tract, including the overgrowth of certain microorganisms (e.g., bacteria or fungi). Existing evidence has shown that gut dysbiosis may contribute to the etiology of numerous human diseases[7,8], including diabetes, atherosclerosis, inflammatory bowel disease, atopic dermatitis, autism, or even cancer development. Furthermore, mice with tumors but no gut microbiome showed different responses when treated with cancer immunotherapy[7,8].
Several authors showed that FMT could circumvent immunotherapy resistance in metastatic melanoma patients rendering about about 30% of them susceptible again to anti-PD1 agents to which they were resistant[4,5]. The mechanism whereby FMT can restore sensitivity to anti-PD1 monoclonal antibodies is not completely understood. Davar et al[5] demonstrated that FMT induced rapid and durable changes in the gut microbiota. The responders showed increased abundance of certain taxa associated with response to anti-PD-1, increased activation of CD8+- T cells with higher cytolytic functions, and decreased frequency of interleukin-8-expressing myeloid cells which may have immunossupressive activity. Proteomic and metabolomic analyses revealed distinct signatures in the responders, and network analyses confirmed that the gut microbiome regulated these changes[5]. In addition, Baruch et al[4] reported that gut sample analysis demonstrated post-treatment up-regulation of gene sets related to adenomatous polyposis colis via major histocompatibility complex (MHC) class 1 and IL-1 mediated signaling. Furthermore, tumor sample analysis showed post-treatment up-regulation of multiple immune-related gene sets (Interferon gamma-, T cell activation, MHC class II protein complex, dendritic cell differentiation, and T helper 1 type immune response)[4].
Our patient had disease resistant to Nivolumab, Pembrolizumab (anti-PD1 monoclonal antibodies), and Ipilimumab (an anti-CTL4 monoclonal antibody). After FMT, Pembrolizumab, to which its disease was resistant, regained its effectivity, as seen by the decrease of his right lateral thoracic subcutaneous disease. We did only one FMT by colonoscopy without prior antibiotic treatment, as did Davar et al[5]. Baruch et al[4], however, did serial FMTs in the patients they treated by capsules or colonoscopy and used antibiotics pre-FMT. If more than one procedure is needed, whether colonoscopy or capsules may be the best way of doing the FMT, and if antibiotic pretreatment is needed, all require further research. In addition, both Davar et al[5] and Baruch et al[4] reported changes in the microbiome of patients who underwent FMT and did not show any significant toxicity due to this procedure.
This report has limitations. Besides the fact that we reported here one only case of restoring sensitivity to anti-PD1 treatment through FMT, we also did not pursue immunological studies or fecal microbiome analysis.
Despite these limitations, the favorable clinical and long-lasting effect we saw in our patient without significant toxicity suggests that this procedure should be considered in similar patients with immunotherapy refractory melanomas.
| 1. | Arnold M, Singh D, Laversanne M, Vignat J, Vaccarella S, Meheus F, Cust AE, de Vries E, Whiteman DC, Bray F. Global Burden of Cutaneous Melanoma in 2020 and Projections to 2040. JAMA Dermatol. 2022;158:495-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 405] [Cited by in RCA: 796] [Article Influence: 199.0] [Reference Citation Analysis (0)] |
| 2. | Switzer B, Puzanov I, Skitzki JJ, Hamad L, Ernstoff MS. Managing Metastatic Melanoma in 2022: A Clinical Review. JCO Oncol Pract. 2022;18:335-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 138] [Article Influence: 34.5] [Reference Citation Analysis (0)] |
| 3. | Wolchok JD, Chiarion-Sileni V, Gonzalez R, Grob JJ, Rutkowski P, Lao CD, Cowey CL, Schadendorf D, Wagstaff J, Dummer R, Ferrucci PF, Smylie M, Butler MO, Hill A, Márquez-Rodas I, Haanen JBAG, Guidoboni M, Maio M, Schöffski P, Carlino MS, Lebbé C, McArthur G, Ascierto PA, Daniels GA, Long GV, Bas T, Ritchings C, Larkin J, Hodi FS. Long-Term Outcomes With Nivolumab Plus Ipilimumab or Nivolumab Alone Versus Ipilimumab in Patients With Advanced Melanoma. J Clin Oncol. 2022;40:127-137. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 119] [Cited by in RCA: 860] [Article Influence: 215.0] [Reference Citation Analysis (0)] |
| 4. | Baruch EN, Youngster I, Ben-Betzalel G, Ortenberg R, Lahat A, Katz L, Adler K, Dick-Necula D, Raskin S, Bloch N, Rotin D, Anafi L, Avivi C, Melnichenko J, Steinberg-Silman Y, Mamtani R, Harati H, Asher N, Shapira-Frommer R, Brosh-Nissimov T, Eshet Y, Ben-Simon S, Ziv O, Khan MAW, Amit M, Ajami NJ, Barshack I, Schachter J, Wargo JA, Koren O, Markel G, Boursi B. Fecal microbiota transplant promotes response in immunotherapy-refractory melanoma patients. Science. 2021;371:602-609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 357] [Cited by in RCA: 1153] [Article Influence: 192.2] [Reference Citation Analysis (0)] |
| 5. | Davar D, Dzutsev AK, McCulloch JA, Rodrigues RR, Chauvin JM, Morrison RM, Deblasio RN, Menna C, Ding Q, Pagliano O, Zidi B, Zhang S, Badger JH, Vetizou M, Cole AM, Fernandes MR, Prescott S, Costa RGF, Balaji AK, Morgun A, Vujkovic-Cvijin I, Wang H, Borhani AA, Schwartz MB, Dubner HM, Ernst SJ, Rose A, Najjar YG, Belkaid Y, Kirkwood JM, Trinchieri G, Zarour HM. Fecal microbiota transplant overcomes resistance to anti-PD-1 therapy in melanoma patients. Science. 2021;371:595-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 843] [Cited by in RCA: 1204] [Article Influence: 240.8] [Reference Citation Analysis (0)] |
| 6. | Ferreira A, Neves MT, Baleiras A, Malheiro M, Martins A. Fecal Microbiota Transplant in Immunotherapy-Resistant Melanoma: What Can We Expect in the Near Future? Cureus. 2022;14:e32586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 7. | de Vos WM, Tilg H, Van Hul M, Cani PD. Gut microbiome and health: mechanistic insights. Gut. 2022;71:1020-1032. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 168] [Cited by in RCA: 1538] [Article Influence: 384.5] [Reference Citation Analysis (0)] |
| 8. | Durack J, Lynch SV. The gut microbiome: Relationships with disease and opportunities for therapy. J Exp Med. 2019;216:20-40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 614] [Cited by in RCA: 649] [Article Influence: 92.7] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: Brazil
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Qin Y, China; Safarzadeh Kozani P, Iran S-Editor: Liu JH L-Editor: A P-Editor: Yu HG