Published online Aug 26, 2023. doi: 10.12998/wjcc.v11.i24.5797
Peer-review started: May 24, 2023
First decision: July 8, 2023
Revised: July 12, 2023
Accepted: August 3, 2023
Article in press: August 3, 2023
Published online: August 26, 2023
Processing time: 92 Days and 20 Hours
Papillary thyroid carcinoma (PTC) is regarded as a fairly common endocrine malignancy, which can be divided into different multiple variants due to wide morphologic differences. The majority of PTC variants have been reported, but PTC with nodular fasciitis-like stroma (NFS) is a rare pathological variant and has been infrequently reported in the relevant literature. This condition involves abundant reactive stromal components rich in spindle cells, which may account for 60%-80% of the tumor along with a typical papillary carcinoma.
A 44-year-old man presented with a 4-mo history of a palpable mass over the anterior aspect of the left neck, the tumor demonstrated gradual enlargement but was painless during the 4 mo prior to discovery. Thyroid function test results were normal. Physical examination showed an enormous and firm nodular mass in the left lobe of the thyroid gland extending to the level of the hyoid bone. Ultrasonography of the neck revealed a well-defined heterogeneous lesion measuring around 5.0 cm × 4.0 cm with a hypoechoic complex nodule, decreased vascularity and speckles of microcalcification. The patient underwent left thyroidectomy with central compartment lymph node dissection. Final histopathological examination confirmed the diagnosis of PTC with extensive fibromatosis-like stroma combined with typical PTC. The patient was asymptomatic at the 3-mo follow-up.
PTC-NFS is a rare pathological variant and its diagnosis and prognosis may be similar to typical papillary carcinoma.
Core Tip: Papillary thyroid carcinoma (PTC) with nodular fasciitis-like stroma (NFS) is a histological variant of PTC with a favorable prognosis. The diagnosis of PTC-NFS requires a combination of ultrasonography, fine-needle aspiration cytology, and histological examination. Total thyroidectomy combined with cervical lymph node dissection is the best treatment for PTC-NFS.
- Citation: Hu J, Wang F, Xue W, Jiang Y. Papillary thyroid carcinoma with nodular fasciitis-like stroma - an unusual variant with distinctive histopathology: A case report. World J Clin Cases 2023; 11(24): 5797-5803
- URL: https://www.wjgnet.com/2307-8960/full/v11/i24/5797.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i24.5797
Thyroid carcinoma is the most common malignancy of the endocrine system with an increasing incidence worldwide, and papillary thyroid carcinoma (PTC) is the most frequent histologic type[1,2]. PTC has many histological morphological variants; however, more attention has been paid to the typical type of PTC. According to World Health Organization (WHO) 2017 guidelines, PTC has 13 different subtypes in terms of histological morphology[3]. Some of these subtypes have special clinical biological behavior, while others have unique morphological characteristics. Understanding these variants is helpful for the doctor to decide on the correct diagnosis and clinical management. Among the numerous variants of PTC, PTC with nodular fasciitis-like stroma (PTC-NFS) is a relatively rare morphological unexplored variant of PTC, accounting for only 0.17%-0.5% of all cases[4]. PTC-NFS was first reported by Chan et al[5] in 1991. To date, no more than 30 cases have been published in the literature[6]. Most patients presented with a painless progressive enlargement of the mass with no thyroid function changes[7]. Histologically, PTC-NFS is characterized by extensive reactive stromal proliferation, which may occupy 60%-80% of the tumor[8]. Herein, we describe the case of a 44-year-old man who presented with a mixed thyroid carcinoma comprised of conventional PTC and co-existing PTC morphology with spindle cell hyperplasia. Our study illustrates the clinicopathological features, morphological identification, and differential diagnosis and prognosis of PTC-NFS to raise awareness of the disease.
A 44-year-old man presented with a 4-mo history of a gradually increasing swelling over the left anterior aspect of the neck, without any associated pain, hoarseness or dysphagia.
The patient inadvertently discovered a neck mass 4 mo earlier, and the mass had gradually increased over the past 4 mo. The patient experienced neck tightness but no dysphagia or hoarseness.
The patient had no past medical history or family history of thyroid disease. He was a smoker and consumed alcohol without drug abuse. He had no exposure to radiation.
There was no significant family history and physical examination was normal.
Physical examination revealed that the left lobe of the thyroid gland was significantly enlarged, and an approximately 4.5 cm × 3.0 cm firm-to-hard nodule was palpable with poor activity. Another 5.0 cm × 4.0 cm mass was palpable extending from above the thyroid gland to the left carotid sheath space, which was tough and mobile, and had a certain boundary with surrounding tissues.
After admission, the patient underwent a series of laboratory examinations. The results of routine tests, including blood tests, liver and kidney function, coagulation function and cardiopulmonary function were normal. The representative diagnostic markers of thyroid function tests, including free triiodothyronine, free thyroxine and thyroid-stimulating hormone levels were within normal ranges. Anti-thyroglobulin antibodies were found to be negative, and Hashimoto's thyroiditis was excluded.
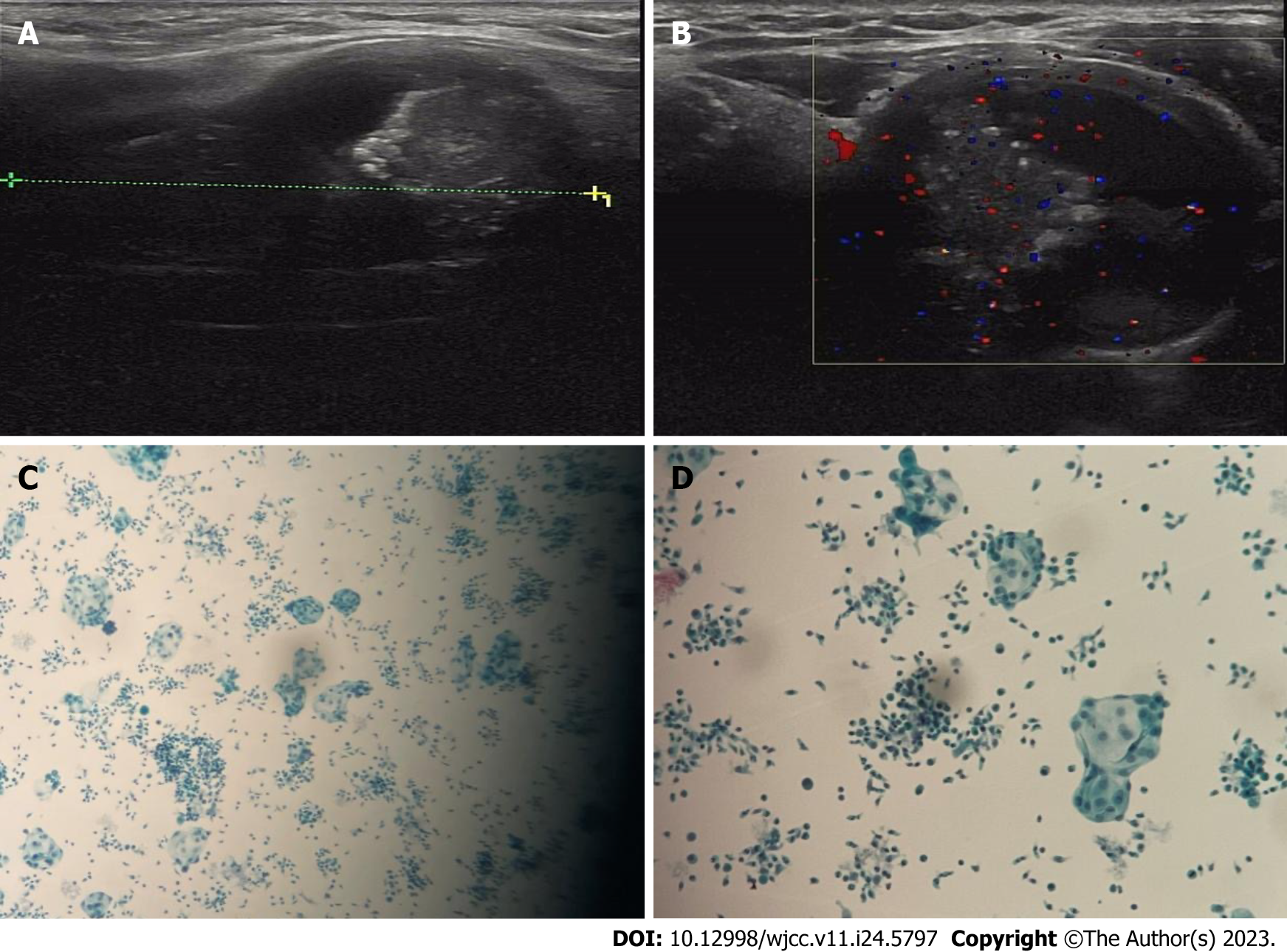
Sonographic examination revealed a huge mass in the left thyroid gland representing a complex lesion including both a solid and a cystic component measuring approximately 5.1 cm × 3.1 cm × 2.9 cm. Moreover, ultrasonography of the left neck showed a well-defined heterogeneous lesion measuring approximately 5.0 cm × 4.0 cm with a hypoechoic complex nodule, decreased vascularity and speckles of microcalcification (Figure 1).
On the basis of biochemical parameters, combined with the clinical manifestations, the patient was diagnosed with PTC-NFS.
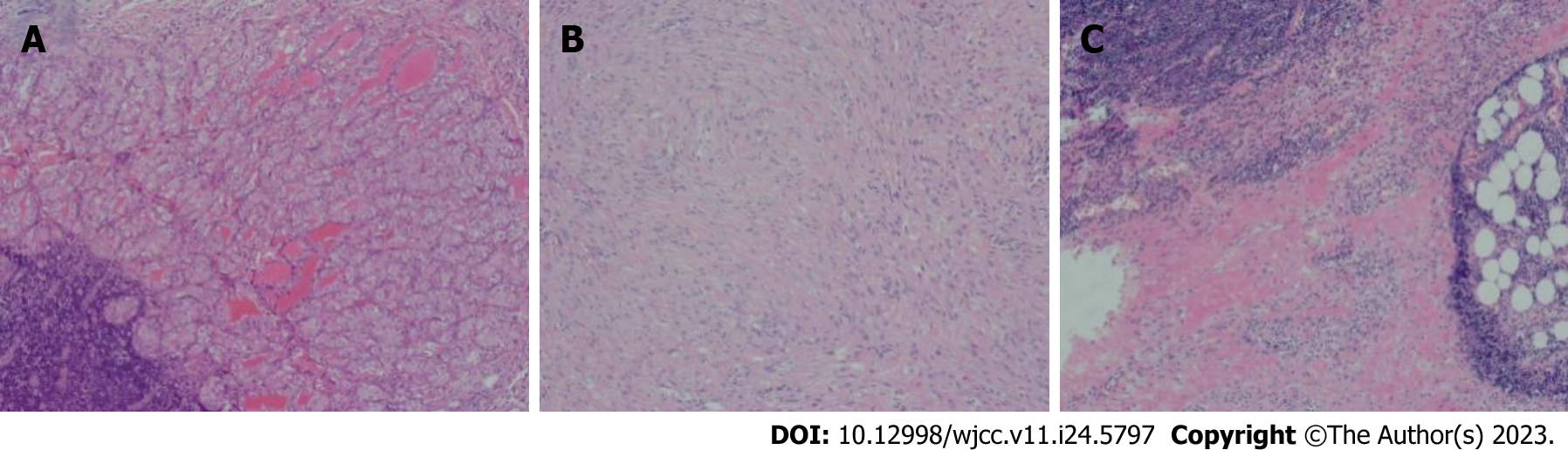
The patient underwent left thyroidectomy with central lymph node dissection and resection of the left neck mass after the completion of routine examination. On gross inspection, a poorly circumscribed tumor occupying almost the entire left lobe was observed measuring 4.5 cm at the greatest diameter. The cut surface of the tumor was firm and grayish white. At the side of the tumor, the left neck mass showed a well-circumscribed solid nodule with a predominantly firm white cut surface measuring 5.0 cm along its greatest axis. On microscopic examination, the thyroid carcinoma invaded the normal thyroid tissue and the boundary between them was indistinguishable. The left lobe thyroid tumor consisted of tumor follicular cells arranged in complex, dendritic and closely packed papillary structures. In the tumor follicular cells, enlarged overlapping nuclei, distinct chromatin dispersion, clear nuclear groove and cytoplasmic pseudoinclusions were observed. The left neck mass was mainly composed of two distinct components: epithelium and stroma. The epithelial component revealed glandular and papillary configuration and comprised approximately 30% of the tumor mass. The epithelial component displayed representative cytological features of classical PTC. The nuclei of epithelial cells showed ground-glass changes with nuclear grooves and occasional intranuclear inclusions. Cellular crowding and nuclear elongation were also found. The stromal component, which comprised the great majority of the mass, consisted of bland spindle cells. In the central region of the tumor, the epithelial component was almost absent and replaced by long spindle cells arranged in braided, fascicles and nodules. Spindle cells had abundant cytoplasm, oval or long rod-shaped nuclei, delicate chromatin and clear small nucleoli. In some areas, spindle cells showed mild atypia and occasional mitosis. Small thick-walled blood vessels and occasional erythrocyte extravasation were also found in the spindle cells (Figure 2).
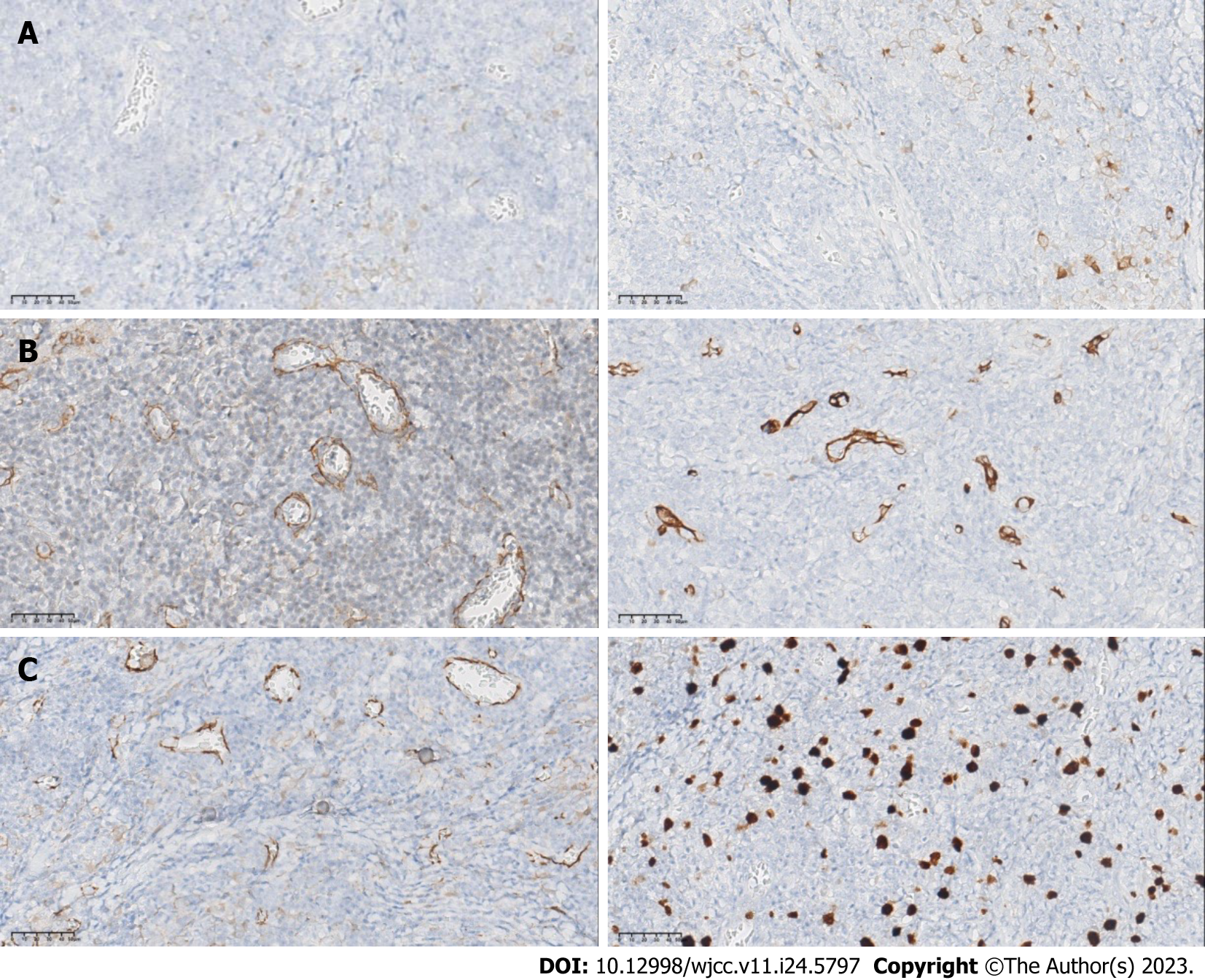
On immunohistochemistry (IHC) assessment, the epithelial component showed significant positive expression of thyroid transcription factor-1 (TTF-1), thyroglobulin, Galectin-3 and CK19. The stromal spindle cells of the tumor demonstrated prominent cytoplasmic staining with smooth muscle actin (SMA), Vimentin, CD34 and Calponin. Significant β-catenin staining was also found in nuclear and cytoplasmic regions. The Ki-67 proliferation index was < 3% both in the epithelial and stromal components (Figure 3). Moreover, BRAF V600E mutation was found by molecular analysis of the tumor. Based on these pathologic features, the diagnosis of PTC-NFS was confirmed.
During the postoperative period, the patient was asymptomatic and he gradually recovered over the following week. Histopathological examination confirmed the diagnosis of PTC-NFS. He was discharged with regular follow-up for 9 mo.
PTC-NFS has been confirmed as a rare histological subtype of PTC, and was also known as PTC with fibromatosis-like stroma or PTC forming a myofibroblastic tumor[9,10]. There is a paucity of related reports regarding this entity. It was first reported in 1991 by Chan et al[5], and officially defined in the 2017 edition of WHO Classification of Tumors of Endocrine Organs[11]. To the best of our knowledge, only around 30 cases have been reported in the form of case reports and case series in the national and international literature[12-16]. The tumor mainly occurred in adults aged 20 to 82 years, with an average age of 44.3 years (our patient was 44 years old); Women have a greater preponderance than men, with a female-to-male ratio of 3:1[17]. No racial predilection has been observed. The main clinical manifestations are a neck mass, neck discomfort, dyspnea, dysphagia, hoarseness and so on. Most tumors are located in the neck, and mediastinal tumors have also been reported. The lesions vary in size and the maximum diameter ranged from 2 to 10 cm. Lymph node metastases at presentation were described in a few patients. However, there is no relevant literature on distant metastases.
In contrast, our case fulfills the pathological criteria for PTC-NFS. Histologically, the tumor is composed of mesenchymal and follicular epithelium of classical PCT. The abundant mesenchyme constitutes the main body of the tumor. The spindle mesenchymal nuclei have sparse or vacuolated chromatin, and small nucleoli are visible. In some areas, the interstitium is loose and mucous degeneration occurs. Spindle cells are arranged in parallel, wavy or bundles, and interwoven with collagen fibers. In addition, a small number of lymphocytes infiltrate thyroid tissue around the lesion. The stroma accounts for 60% to 80% of the total tumor volume, and in most cases is greater than 80%. Immunohistochemistry and electron microscopy have confirmed that spindle stromal cells were fibroblasts and myofibroblasts, which could produce collagen. Despite the obvious predominance of spindle cells in the tumor, lymph node metastases have been reported in cases with only epithelial and no spindle cell component metastases. However, the mechanisms of this phenomenon have not been fully elucidated. The epithelial cells mainly show the morphology of typical papillary carcinoma, with elongated and crowded nuclei or ground-glass nuclei. Nuclear grooves have also been seen in the epithelium. Squamous differentiation has been found in a few cases. On IHC, papillary carcinoma showed the immunophenotypic characteristics of typical papillary carcinoma, including CKl9, TTF-1, HBME-1, and Galectin-3 positive expression in tumor cells, while Vimentin, SMA and β-catenin expression in the nucleus or cytoplasm is also positive in spindle cells.
Different from the classical PCT, PTC-NFS has its own unique histopathological characteristics. However, the mechanisms of these features have not been fully elucidated. In order to speculate on the underlying mechanism, extensive research has been conducted. Some researchers hold the view that the formation of mesenchymal components is an excessive allergic reaction to tumor invasion in the mesenchyme resulting in damage. It has also been suggested that the mesenchymal component is essentially hormone-dependent tumorigenesis. Researchers found that the high expression of receptors of transforming growth factor (TGF)-β types 1 and 2 and platelet-derived growth factor receptor α- and β-subunits have been found in early stromal cells adjacent to malignant thyroid tumor cells, which were significantly reduced or disappeared in scar-like, dense collagenous stroma, suggesting that stromal cells lack the ability to mature into scar-like changes in early interstitial response[18]. In addition, previous reports have found that TGF-β is immunoreactive in stromal tumor cells, and this fundamental cytokine mediates scarring and activation of myofibroblasts[19]. The loss of E-cadherin and CK19 immunoreactivity in spindle cells may imply that it could be a mesenchymal-like metaplasia in follicular cell elements[20]. Furthermore, some researchers have suggested that the mutations in exon 3 of CTNNB1, which are the main cause of aberrant β-catenin nuclear staining, are very common in desmoid-type fibromatosis and desmoid tumors, and are closely associated with recurrence[21]. There are also studies indicating different levels of expression of programmed death-ligand 1 (PD-L1) in various PTC subtypes. And it is positively correlated with the reduction of disease-free survival, which can be used as a potential biomarker for the management of patients with PTC[22].
As PTC-NFS displays some pathophysiological similarities to other thyroid diseases, it is necessary to distinguish PTC-NFS from these other thyroid diseases, such as classical thyroid carcinoma, the fibrous variant of Hashimoto thyroiditis, Riedel thyroiditis, de Quervain thyroiditis, undifferentiated carcinoma and carcinosarcoma, and soft tissue tumor of the thyroid gland. Typical PTC is often accompanied by stromal fibrosis or cicatricial reactions, which never form carcinomatous nodules. Extensive fibrosis has also been found in various types of thyroiditis, but the composition of PTC has not appeared in this situation. Spindle cells in anaplastic carcinoma or carcinosarcoma have shown marked atypia and mitosis, and coagulation necrosis is common, which is significantly different to the mildly proliferative spindle cells in PTC-NFS. In addition, thyroid soft tissue tumors do not have PTC components. In recent years, with the continuous progress and development of technology, fine-needle aspiration (FNA) cytology has been widely used in the diagnosis of thyroid diseases. The requirements for FNA diagnosis of PTC-NFS are also becoming higher and higher. In addition to the diagnostic cytological features of classical PTC, it may also contain spindle cells with mild monomorphism in FNA specimens. However, in fine needle or coarse needle puncture specimens, the morphology of the lesion is closely related to the type of tumor. When only spindle fibromatosis is seen in the puncture tissue, it can be misdiagnosed as fibromatosis, solitary fibrous tumor, spindle cell carcinoma, etc. When the puncture tissue shows papillary carcinoma, the specific subtype may not be diagnosed. If the puncture tissue contains papillary carcinoma and hyperplastic spindle cells, the possible diagnosis of PTC-NFS should be considered. Furthermore, some neck diseases, such as thyroglossal duct cysts, exhibit similar symptoms and signs that need to be distinguished from PTC-NFS. Due to the non-specific imaging findings of cystic neck nodules, close attention needs to be paid to the patient's age, lesion location, and association with surrounding structures to exclude the possibility of other diseases. If necessary, more advanced radiologic procedures such as CT and MR imaging can be used for differential diagnosis[23].
At present, total thyroidectomy combined with cervical lymph node dissection is the best treatment for PTC-NFS. Iodine131 therapy should be considered for the presence of local or distant spread, advanced age, BRAF gene mutation and other poor prognostic factors to consolidate the efficacy. Surgical treatment should ensure a negative margin to effectively reduce tumor recurrence. Further studies are required to delineate tumor prognosis due to the lack of available data in recent literature. According to the existing data, the follow-up time in the majority of cases is approximately 1 year, and the prognosis of the patients is satisfactory. These results indicate that PTC-NFS prognosis may be similar to typical papillary carcinoma. However, additional future studies are needed to help recognize this unusual entity.
| 1. | Paulson VA, Rudzinski ER, Hawkins DS. Thyroid Cancer in the Pediatric Population. Genes (Basel). 2019;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 165] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 2. | Higashi T. Cancer epidemiology and treatment patterns for older persons in Japan: a review of nationwide data and statistics. Jpn J Clin Oncol. 2022;52:303-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 3. | Elder JB, Sherman JH, Prevedello DM, Szerlip NJ, Spratt DE, Shaikhouni A, Mohyeldin A, Perez-Roman RJ, Buttrick SS, Ali SC, Komotar RJ, Todeschini A, Shahein M, Revuelta JM, Hardesty D, Carrau RL, Zada G, Giannotta S, Dornbos D, Lonser RR. Tumor. Oper Neurosurg (Hagerstown). 2019;17:S119-S152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (1)] |
| 4. | Ginter PS, Scognamiglio T. Papillary thyroid carcinoma with nodular fasciitis-like stroma: a usual entity with distinctive morphology. Int J Surg Pathol. 2015;23:305-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 5. | Chan JK, Carcangiu ML, Rosai J. Papillary carcinoma of thyroid with exuberant nodular fasciitis-like stroma. Report of three cases. Am J Clin Pathol. 1991;95:309-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 60] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 6. | Ma X, Xia C, Liu H, Zhu W. Primary thyroid spindle cell tumors: spindle cell variant of papillary thyroid carcinoma? Int J Clin Exp Pathol. 2015;8:13528-13531. [PubMed] |
| 7. | Zand V, Moghimi M, Sadeghi E, Kamal P, Vaziribozorg S. Papillary Thyroid Carcinoma with Nodular Fasciitis-Like Stroma in a 28-Year-Old Patient. Iran J Pathol. 2022;17:225-228. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 8. | Us-Krasovec M, Golouh R. Papillary thyroid carcinoma with exuberant nodular fasciitis-like stroma in a fine needle aspirate. A case report. Acta Cytol. 1999;43:1101-1104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 9. | Mizukami Y, Nonomura A, Matsubara F, Michigishi T, Ohmura K, Hashimoto T. Papillary carcinoma of the thyroid gland with fibromatosis-like stroma. Histopathology. 1992;20:355-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 10. | Mizukami Y, Kurumaya H, Kitagawa T, Minato H, Nonomura A, Michigishi T, Noguchi M. Papillary carcinoma of the thyroid gland with fibromatosis-like stroma: a case report and review of the literature. Mod Pathol. 1995;8:366-370. [PubMed] |
| 11. | Lam AK. Papillary Thyroid Carcinoma: Current Position in Epidemiology, Genomics, and Classification. Methods Mol Biol. 2022;2534:1-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 44] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 12. | Wong SBJ, Nga ME, Michal M, Vanecek T, Seet JE, Petersson F. SOX11 expression in a case of papillary thyroid carcinoma with fibromatosis/fasciitis-like stroma containing BRAF c.1799_1801delTGA and CTNNB1 c.133T>C mutations. Virchows Arch. 2019;475:519-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 13. | Zhou L, Shi L, Jiang Z, Xie L. Papillary thyroid carcinoma with nodular fasciitis-like stroma and β-catenin gene mutations: report of a recurrent case. Int J Clin Exp Pathol. 2018;11:2879-2883. [PubMed] |
| 14. | Suster D, Michal M, Nishino M, Piana S, Bongiovanni M, Blatnik O, Hájková V, Ptáková N, Suster S. Papillary thyroid carcinoma with prominent myofibroblastic stromal component: clinicopathologic, immunohistochemical and next-generation sequencing study of seven cases. Mod Pathol. 2020;33:1702-1711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 15. | Roth EM, Barrows CE, Nishino M, Sacks B, Hasselgren PO, James BC. Papillary thyroid cancer with extrathyroidal extension of desmoid-type fibromatosis. A case report of an aggressive presentation of an uncommon pathologic entity. Int J Surg Case Rep. 2019;63:5-9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 16. | Takada N, Hirokawa M, Ito M, Ito A, Suzuki A, Higuchi M, Kuma S, Hayashi T, Kishikawa M, Horikawa S, Miyauchi A. Papillary thyroid carcinoma with desmoid-type fibromatosis: A clinical, pathological, and immunohistochemical study of 14 cases. Endocr J. 2017;64:1017-1023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 17. | Basu S, Nair N, Shet T, Borges AM. Papillary thyroid carcinoma with exuberant nodular fasciitis-like stroma: treatment outcome and prognosis. J Laryngol Otol. 2006;120:338-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 18. | Naganuma H, Iwama N, Nakamura Y, Ohtani N, Ohtani H, Takaya K, Sakai N. Papillary carcinoma of the thyroid gland forming a myofibroblastic nodular tumor: report of two cases and review of the literature. Pathol Int. 2002;52:54-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 19. | Toti P, Tanganelli P, Schürfeld K, Stumpo M, Barbagli L, Vatti R, Luzi P. Scarring in papillary carcinoma of the thyroid: report of two new cases with exuberant nodular fasciitis-like stroma. Histopathology. 1999;35:418-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 20. | Woenckhaus C, Cameselle-Teijeiro J, Ruiz-Ponte C, Abdulkader I, Reyes-Santías R, Sobrinho-Simões M. Spindle cell variant of papillary thyroid carcinoma. Histopathology. 2004;45:424-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 21. | Lazar AJ, Tuvin D, Hajibashi S, Habeeb S, Bolshakov S, Mayordomo-Aranda E, Warneke CL, Lopez-Terrada D, Pollock RE, Lev D. Specific mutations in the beta-catenin gene (CTNNB1) correlate with local recurrence in sporadic desmoid tumors. Am J Pathol. 2008;173:1518-1527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 337] [Cited by in RCA: 342] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 22. | Girolami I, Pantanowitz L, Mete O, Brunelli M, Marletta S, Colato C, Trimboli P, Crescenzi A, Bongiovanni M, Barbareschi M, Eccher A. Programmed Death-Ligand 1 (PD-L1) Is a Potential Biomarker of Disease-Free Survival in Papillary Thyroid Carcinoma: a Systematic Review and Meta-Analysis of PD-L1 Immunoexpression in Follicular Epithelial Derived Thyroid Carcinoma. Endocr Pathol. 2020;31:291-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 23. | Corvino A, Pignata S, Campanino MR, Corvino F, Giurazza F, Tafuri D, Pinto F, Catalano O. Thyroglossal duct cysts and site-specific differential diagnoses: imaging findings with emphasis on ultrasound assessment. J Ultrasound. 2020;23:139-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Surgery
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Al-Ani RM, Iraq; Mokni M, Tunisia S-Editor: Yan JP L-Editor: A P-Editor: Yan JP