Published online Aug 16, 2023. doi: 10.12998/wjcc.v11.i23.5494
Peer-review started: May 10, 2023
First decision: June 13, 2023
Revised: June 22, 2023
Accepted: July 17, 2023
Article in press: July 17, 2023
Published online: August 16, 2023
Processing time: 97 Days and 18.8 Hours
Isolated left ventricular apical hypoplasia (ILVAH), also known as truncated left ventricle (LV), is a very unusual cardiomyopathy. It is characterised by a trun
To analysing the so far 37 reported ILVAH cases worldwide.
The electronic databases PubMed and Scopus were investigated from their estab
The majority of cases reported occurred in males (52.7%). Mean age at diagnosis was 26.1 ± 19.6 years. More than a third of the patients were asymptomatic (35.1%). The most usual clinical presentation was breathlessness (40.5%). The most commonly detected electrocardiogram changes were T wave abnormalities (29.7%) and right axis deviation with poor R wave progression (24.3%). Atrial fibrillation/flutter was detected in 24.3%. Echocardiography was performed in 97.3% of cases and cardiac MRI in 91.9% of cases. Ejection fraction was reduced in more than a half of patients (56.7%). An associated congenital heart disease was found in 16.2%. Heart failure therapy was administered in 35.1% of patients. The outcome was favorable in the vast majority of patients, with just one death.
ILVAH is a multifaceted entity with a so far unpredictable course, ranging from benign until the elderly to sudden death during adolescence.
Core Tip: The manuscript is focused on an interesting topic, e.g. a rare form of cardiomyopathy which is called isolated left ventricular hypoplasia or truncated left ventricle. The so far 37 reported cases worldwide have been reviewed and analysed to help clinicians in the difficult management of this extremely rare congenital heart disease. Nice images from our personal archive have been added.
- Citation: Bassareo PP, Duignan S, James A, Dunne E, McMahon CJ, Walsh KP. Isolated left ventricular apical hypoplasia: Systematic review and analysis of the 37 cases reported so far. World J Clin Cases 2023; 11(23): 5494-5503
- URL: https://www.wjgnet.com/2307-8960/full/v11/i23/5494.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i23.5494
Isolated LV apical hypoplasia (ILVAH) is an extremely rare and unclassified cardiomyopathy that has been recognized as a distinct entity since 2004[1]. ILVAH is also known as truncated left ventricle (LV). It is a very unusual cardiomyopathy which can be easily detected on echocardiography. It is characterised by a truncated, spherical, and non-apex forming LV with some degree of systolic dysfunction[2]. The true apex is occupied by the right ventricle, which wraps around the distal LV and whose systolic function is usually normal[2]. ILVAH is usually not accompanied by other abnormalities[3]. Its diagnosis is often refined by means of computed tomography or cardiac magnetic resonance imaging (MRI)[4]. Clinical presentation varies a lot, ranging from the lack of symptoms to fatigue, breathlessness, palpitations, chest pain, syncope[5,6]. The death of an adolescent patient was reported in literature. He suffered from arrhythmia with fulminant cardiac insufficiency and non-responsive to therapy pulmonary hypertension[7].
Due to the rarity of the disease, just a few case reports and limited case series have been published in the field. The aim of this paper is making a literature review concerning to so far reported cases of ILVAH with related features and outcomes.
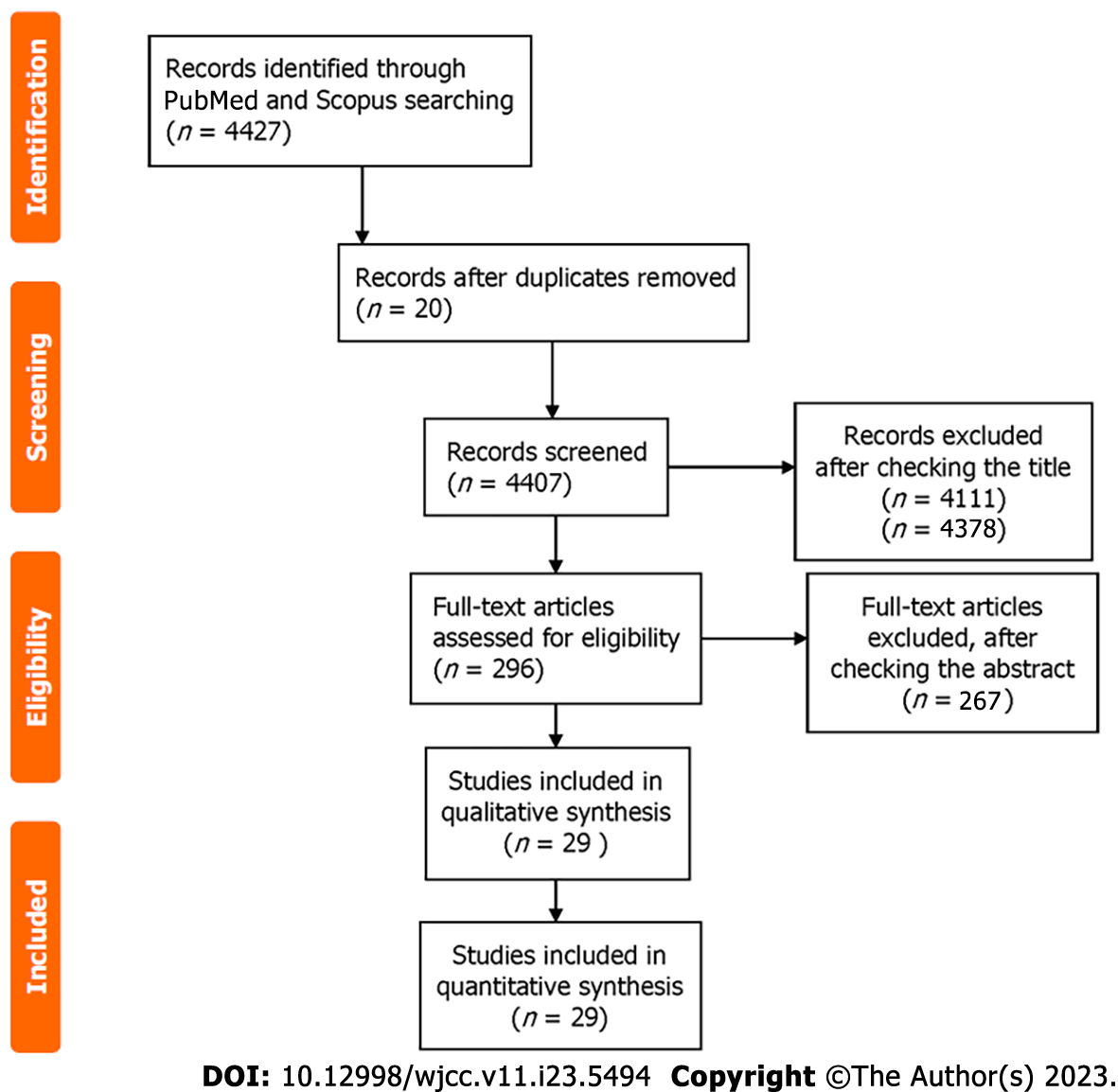
The electronic databases PubMed and Scopus were investigated from their establishment up to December 13, 2022. The MeSH (Medical Subject Headings) search terms were “case report” and/or “case series” and/or “isolated left ventricular hypoplasia” and/or “truncated left ventricle”. We excluded any animal studies, and papers with language other than English (Figure 1).
The authors separately looked into the selected abstracts and evaluated whether they were eligible. Full-texts were checked when all the reviewers of the abstracts felt that the latter might match the inclusion criteria.
Information from the selected single case reports and case series were taken. The reported points were: age at diagnosis, gender, clinical presentation, electrocardiographic features, imaging (ultrasounds and cardiac MRI), associated cardiac abnormalities, therapy, and outcome.
Data were presented in the form of mean ± SD. Chi square test and Mann-Whitney U test were used to check statistical significance when needed. Statistical significance was set to P < 0.05.
Overall, 4427 potential single case reports of ILVAH/truncated left ventricle were detected on the PubMed and Scopus databases. Twenty were duplicates. Other 4378 papers were excluded after checking the abstract. The remaining 29 manuscripts were used for analysis of the patients’ features and disease outcome (Table 1)[8-29].
| Ref. | Patient’s age (yr) | Patient’s gender | Clinical presentation | Electrocardiogram | Echocardiography | MRI | Associated cardiac abnormalities | Therapy | Outcome |
| Dattani et al[8] | 64 | Male | Shortness of breath (NYHA III) | Complete left bundle branch block | Severe LV systolic dysfunction with global hypokinesis | Yes | Hypertension, hypercholesterolaemia and asthma | Furosemide, perindopril, bisoprolol and anticoagulation for the suspected mural thrombus | CRT |
| Chaowu et al[9] | 22 | Female | Palpitations | Atrial fibrillation, right axis deviation and T-wave abnormality | EF not reported | Yes | Dextro-transposition of the great vessels, patency of ductus arteriosus | Not reported | Serial follow-up |
| Vanhecke et al[5] | 53 | Female | Palpitations | Normal sinus rhythm, inferolateral T wave abnormalities and poor R wave progression | Severe LV systolic dysfunction (EF 35%), mitral valve regurgitation | Yes | Hypertension | Ace inhibitor, beta-blocker, and diuretics | Serial follow-up |
| Meléndez et al[10] | 9 | Female | Heart murmur. No symptoms | Not done | EF not reported | Yes | None | None | Serial follow-up |
| Marin et al[4] | 3 months | Male | No symptoms | Not done | EF not reported | Yes | None | None | Serial follow-up |
| Patrianakos et al[11] | 11 | Female | Asymptomatic | Right axis deviation and decreasing R wave in precordial leads beyond V3 | Mild-to-moderate decreased contractility with a restrictive filling pattern and mild mitral regurgitation | Yes | None | None | Serial follow-up |
| 35 | Female | Previous peripartum pulmonary oedema | P mitrale, left axis deviation and decreasing R wave in precordial leads beyond V3 | Mild-to-moderate decreased contractility with a restrictive filling pattern and mild mitral regurgitation | Yes | Atrial fibrillation | ACE inhibitor, beta blocker, and low-dose furosemide | Serial follow-up | |
| Irving et al[7] | 19 | Male | Chest pain and palpitations | Atrial flutter and then ventricular fibrillation | LV systolic and diastolic function was severely impaired and RV function was also poor | Not done | Atrial and ventricular arrhythmias, refractory pulmonary hypertension | inotropic support (adrenaline, milrinone and vasopressin), inhaled nitric oxide and intravenous prostacyclin | Death |
| Braga et al[6] | 66 | Female | Atypical chest pain | Complete left bundle branch block | Mild LV systolic dysfunction (EF 48%), abnormal interventricular septal motion and elongated RV | Yes | Hypertension, dyslipidaemia and stable angina | None | Serial follow-up |
| Motwani et al[2] | 63 | Male | Exertional dyspnoea | Atrial fibrillation | Severely impaired LV systolic function | Yes | None | DC shock | Serial follow-up |
| Moon et al[3] | 33 | Male | Heart murmur. No symptoms | Right axis deviation, incomplete right bundle branch block, right atrial enlargement and RV hypertrophy | Good global LV systolic function | Yes | Mild infundibular pulmonary stenosis and moderate-to-severe aortic stenosis | None | Serial follow-up |
| Alizadeh Sani et al[12] | 13 | Male | Shortness of breath and chest discomfort | Right axis deviation and a low precordial voltage with poor R-wave progression | Severe LV systolic dysfunction, moderate mitral regurgitation, and enlarged left atrium | Yes | Developmental delay, family history of sudden cardiac death | Standard drugs for systolic heart failure | Serial follow-up |
| Zhao et al[13] | 19 | Male | Heart murmur. No symptoms | Right-axis deviation, poor R wave progression, and T wave abnormalities | Mild LV systolic dysfunction | Yes | RV outflow tract obstruction due to exaggerated rightward bulging of the basal-anterior septum during systole | ACE inhibitor and beta-blocker | Serial follow-up |
| Starmer et al[14] | 62 | Male | Shortness of breath | Atrial fibrillation and poor precordial R-wave progression | Severe LV systolic dyfunction | Yes | None | Standard heart failure therapy | Serial follow-up |
| Fernandez-Valls et al[1] | 22 | Female | Fatigue | Right axis deviation and low precordial voltages with poor R wave progression | Mild LV systolic dysfunction, moderate mitral regurgitation | Not done | None | Not reported | Serial follow-up |
| 46 | Female | Shortness of breath | Right axis deviation and low precordial voltages with poor R wave progression | Mild-to-moderate LV systolic dysfunction | Yes | None | Not reported | Serial follow-up | |
| 26 | Male | Chest discomfort | Right axis deviation and low precordial voltages with poor R wave progression | Moderate-to-severe LV systolic dysfunction, moderate mitral regurgitation | Yes | None | Not reported | Serial follow-up | |
| Hong et al[15] | 34 | Female | Chest discomfort | Q wave in leads V1-V4 | Mild systolic dysfunction (EF 44%) | Yes | None | ACE inhibitor | Serial follow-up |
| Ding et al[16] | 22 | Female | Lethargy and shortness of breath | Fragmented QRS and undetermined axis | Severe LV systolic dysfunction | Yes | Non sustained ventricular tachycardia | Not reported | Serial follow-up |
| Orsborne et al[17] | 17 | Female | Chest pain | Not done | Normal LV systolic function | Yes | None | Not reported | Serial follow-up |
| Tumabiene et al[18] | 21 | Female | Severe respiratory distress | Atrial flutter | Mild LV systolic dysfunction | Yes | None | ACE inhibitor, beta blocker, diuretics | Serial follow-up |
| Ong et al[19] | 11 | Female | Heart murmur. Asymptomatic | Normal ECG | Normal LV systolic function | Yes | None | None | Serial follow-up |
| Flett et al[20] | 37 | Female | Pulmonary oedema | Left bundle branch block | Not reported | Yes | Non sustained ventricular tachycardia | Ace inhibitor, beta blocker, diuretics, amiodarone, coumarin | Serial follow-up |
| Meng et al[21] | 24 | Female | Exercise intolerance | Atrial fibrillation, right axis deviation, and T wave abnormalities | Severe LV systolic dysfunction (EF 34%), bi-atrial enlargement, mild-to-moderate mitral valve regurgitation | Yes | PDA, severe pulmonary hypertension | Anti-pulmonary hypertension agents | Serial follow-up |
| 5 | Female | Exercise intolerance | Right axis deviation, and T wave abnormalities | Normal LV systolic function | Yes | None | None | Serial follow-up | |
| 3 | Male | Asymptomatic | T wave abnormalities | Normal LV systolic function | Yes | None | None | Serial follow-up | |
| 13 | Male | Asymptomatic | T wave abnormalities | Normal LV systolic function | Yes | None | None | Serial follow-up | |
| 15 | Male | Asymptomatic | T wave abnormalities | Normal LV systolic function, enlarged left atrium | Yes | PDA, severe pulmonary hypertension | Anti-pulmonary hypertension agents | Serial follow-up | |
| Haffajee et al[22] | 50 | Male | Asymptomatic | Non-specific intraventricular conduction delay with lateral T-wave abnormal | Severe LV systolic dysfunction | Yes | PDA, S/P ligation | ACE inhibitor, beta blocker | Serial follow-up |
| Liao et al[23] | 18 | Male | Shortness of breath | Atrial fibrillation and left ventricular hypertrophy | Severe LV systolic dysfunction (EF 27%), mild mitral regurgitation | Yes | None | ACE inhibitor, beta blocker, diuretics, trimetazidine, levocarnitine | Serial follow-up |
| 2 | Female | Asymptomatic | Normal ECG | Normal LV systolic function, mild mitral regurgitation | Yes | None | None | Serial follow-up | |
| Maidman et al[24] | 58 | Male | Bradycardia and lightheadedness | Sinus bradycardia, right axis deviation, low QRS voltages, mild intraventricular delay | Mildly reduced LV systolic function (EF 45%) | Yes | Not reported | Not reported | Not reported |
| Ramamurthy et al[25] | 16 months | Male | Asymptomatic | Raised ST segment, T wave inversion and q waves in lateral leads | Normal LV systolic function | Yes | None | None | Serial follow-up |
| Choh et al[26] | 2 | Male | Dyspnoea | Not reported | Normal LV systolic function | Yes | None | ACE inhibitor, beta blocker, diuretics | Serial follow-up |
| Skidan et al[27] | 32 | Male | Dyspnoea | Atrial fibrillation | Severe LV systolic dysfunction | Yes | LV non compaction | Ablation, ICD | Serial follow-up |
| Schapiro et al[28] | 17 | Male | Asymptomatic | Sinus bradycardia and nonspecific T wave changes | LV Systolic function not reported | Yes | None | Not reported | Serial follow-up |
| Mirdamadi et al[29] | 19 | Not reported | Mild dyspnoea | Normal ECG | Normal LV systolic function | Not done | None | None | Serial follow-up |
The majority of cases reported occurred in males (52.7%; male-to-female ratio 1.12/1). Mean age at diagnosis was 26.1 ± 19.6 years; range 3 months-66 years). For male patients the mean age at diagnosis was 26.8 ± 22.2 years, whereas female patients were slightly younger (25.7 ± 17.5 years) at diagnosis. This difference was not statistically significant. More than a third of the patients were asymptomatic (35.1%). The most usual clinical presentation was breathlessness (40.5%). There are no statistically significant differences between genders in terms of symptoms/absence of symptoms. The most commonly detected electrocardiogram (ECG) changes were T wave abnormalities (29.7%) and right axis deviation with poor R wave progression (24.3%). Atrial fibrillation/flutter was detected in 24.3%. Echocardiography was performed in 97.3% of cases and cardiac MRI in 91.9% of cases. Ejection fraction was reduced in more than a half of patients (56.7%). An associated congenital heart disease was found in 16.2%, most of all in the form of patency of ductus arteriosus with or without pulmonary hypertension. Heart failure therapy was administered in 35.1% of patients. The outcome was favorable in the vast majority of patients, with just one death. The results of the review are summarised in Table 2.
| Male-to-female ratio | 1.25/1 |
| Mean age at diagnosis | 26.1 ± 19.6 yr (range 3 mo-66 yr) |
| Asymptomatic (35.1%) | |
| Breathlessness (40.5%) | |
| ECG changes | T wave abnormalities (29.7%) |
| Right axis deviation with poor R wave progression (24.3%) | |
| Atrial fibrillation/flutter (24.3%) | |
| Non sustained ventricular tachycardia (8.1%) | |
| Diagnosis | By echocardiography (97.3%) |
| By cardiac magnetic resonance (91.9%) | |
| Reduced left ventricular ejection fraction | 56.7% |
| Associated congenital heart disease | 16.2% |
| Heart failure medical therapy | 35.1% |
| Implantable cardioverter device | 2.7% |
| Death | 2.7% |
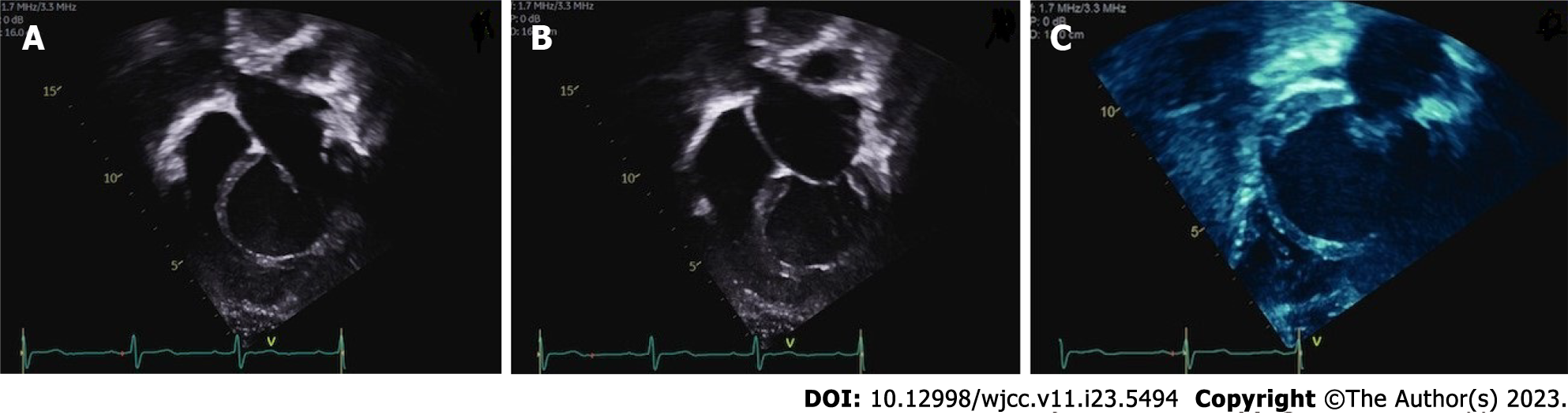
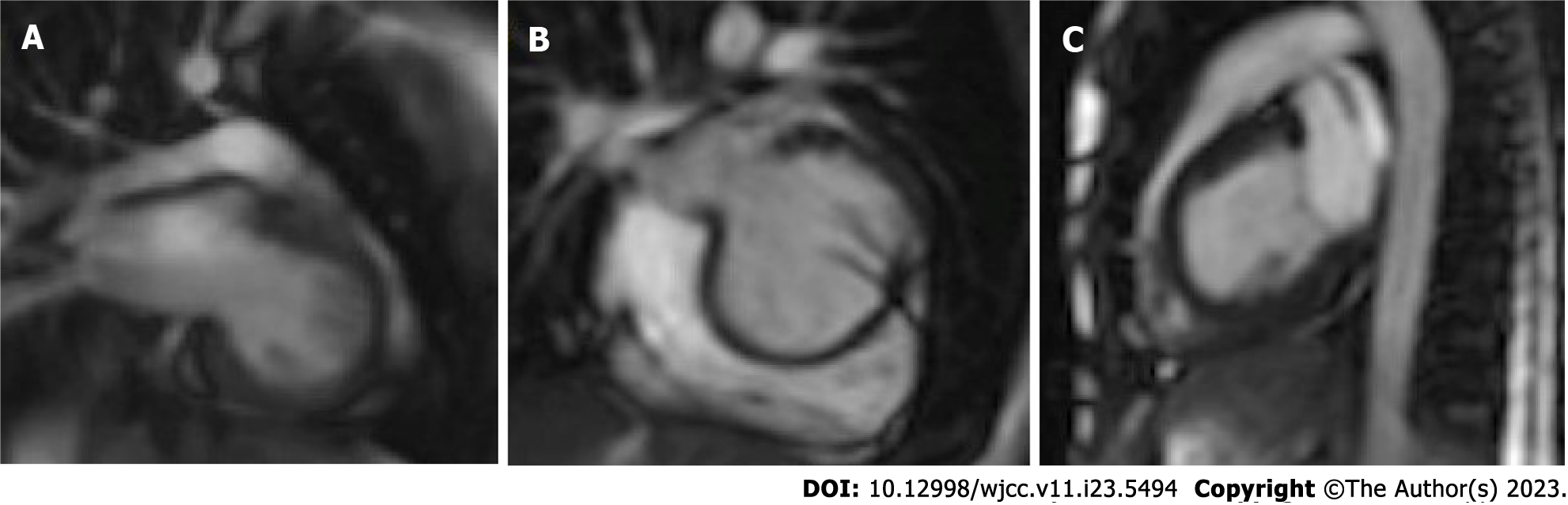
From the case series analysis, there is a slight prevalence of ILVAH in males, though the disease is quite equally distributed between genders. The age of diagnosis is extremely variable, ranging from infancy to elderly. ILVAH is easily detected by echocardiography and diagnosis is confirmed by cardiac MRI (Figures 2 and 3).
On cardiac MRI the established features of ILVAH are four, namely: (1) A truncated and spherical LV shape with systolic and/or diastolic functions which are often impaired and rightward bulging of the interventricular septum in diastole; (2) Defective LV apex with adipose tissue infiltrating it; (3) Anomalous origin of the papillary muscles in the flattened LV apex; and (4) Elongation of the right ventricle wrapping around the deficient LV apex. In this report, we demonstrate these characteristic features with cardiac magnetic resonance imaging and summarize the existing information on isolated LV apical hypoplasia[5].
(1) Hypoplastic left heart syndrome, which is characterised by underdevelopment of the aortic valve and artery, and the whole LV[30]. Mitral valve is stenotic or atretic in most of the cases[31]; (2) LV non-compaction, resulting from interrupted endomyocardial morphogenesis leading to LV dysfunction. It is characterised by a diffusely enlarged LV with a markedly trabeculated endocardium (“spongy” appearance)[32]. Interestingly, in Table 1, a patient with a unique combination on ILVAH and LV non-compaction is reported; (3) Congenital LV aneurysm, which is an idiopathic anomaly of the endocardial and myocardial layers, and LV diverticulum, belonging to a syndrome with multiple defects. However, LV is elongated rather than truncated with involvement of the papillary muscles and surrounding myocardium (as opposed to isolated apical involvement). These conditions are usually deadly early in life owing to associated intracardiac and extracardiac abnormalities[33,34]; and (4) Congenital LV dysplasia with or without right ventricular dysplasia. On cardiac MRI, at tissue characterisation, diffuse transmural fibrofatty replacement is noted, whereas in ILVAH it is predominantly apical[35,36].
Of note, isolated hypoplasia can also involve just the right ventricle, with lack of trabeculated apex[37].
The clinical course of ILVAH is variable. It can be benign, but complications, such as heart failure, supraventricular and ventricular arrhythmias, and pulmonary hypertension, have been described even in young patients[2,7,9]. Only one death has been reported so far[7].
A possible association with patency of ductus arteriosus is suggested by the analysis of the cases reported in this review.
ILVAH embryonic aetiology is purely speculative. However, in an article written not in English, and as such not included in the present analysis, a mutation of the lamin A/C gene (p.Arg644Cys) responsible for dilated cardiomyopathy was found associated to the disease[38].
On balance, the Authors hope that more cases may be published in the field to increase scientific knowledge on ILVAH. The latter is a multifaceted entity with a so far unpredictable course, ranging from benign until the elderly to sudden death during adolescence.
Isolated left ventricular hypoplasia is an extremely rare form of cardiomyopathy.
We aimed at shedding light on the disease outcome.
The aim of this paper is making a literature review about to so far reported cases of isolated left ventricular apical hypoplasia with related features and outcomes.
A literature review was carried out. The electronic databases PubMed and Scopus were investigated from their establishment up to December 13, 2022.
From the initial 4427 papers, 29 manuscripts were selected.
The most usual clinical presentation was breathlessness (40.5%). There are no statistically significant differences between genders in terms of symptoms/absence of symptoms. The most commonly detected ECG changes were T wave abnormalities (29.7%) and right axis deviation with poor R wave progression (24.3%). Atrial fibrillation/flutter was detected in 24.3%. Echocardiography was performed in 97.3% of cases and cardiac MRI in 91.9% of cases. Ejection fraction was reduced in more than a half of patients (56.7%). An associated congenital heart disease was found in 16.2%, most of all in the form of patency of ductus arteriosus with or without pulmonary hypertension. Heart failure therapy was administered in 35.1% of patients. The outcome was favorable in the vast majority of patients, with just one death.
The search strategy will be repeated after 10 years.
| 1. | Fernandez-Valls M, Srichai MB, Stillman AE, White RD. Isolated left ventricular apical hypoplasia: a new congenital anomaly described with cardiac tomography. Heart. 2004;90:552-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 39] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 2. | Motwani M, Witte KK, Plein S, Greenwood JP. Isolated left ventricular apical hypoplasia evaluated by cardiovascular magnetic resonance and gadolinium enhancement techniques. J Am Coll Cardiol. 2011;58:2355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 3. | Moon JI, Jeong YJ, Lee G, Choi JH, Lee JW. Isolated left ventricular apical hypoplasia with infundibular pulmonary and aortic stenosis: a rare combination. Korean J Radiol. 2013;14:874-877. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 4. | Marin C, Sanchez ML, Maroto E, Ossaba S, Ruiz Y, Zabala JI. MR imaging of isolated left ventricular apical hypoplasia. Pediatr Radiol. 2007;37:703-705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 5. | Vanhecke TE, Decker J, Leonowicz N, Chinnaiyan KM. Isolated left ventricular apical hypoplasia. Congenit Heart Dis. 2011;6:646-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 6. | Braga CG, Silva P, Magalhães S, Bettencourt N, Themudo R. Isolated left ventricular apical hypoplasia. Eur Heart J Cardiovasc Imaging. 2014;15:1399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 7. | Irving CA, Chaudhari MP. Fatal presentation of congenital isolated left ventricular apical hypoplasia. Eur J Cardiothorac Surg. 2009;35:368-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Dattani A, Prasad R. Isolated Left Ventricular Apical Hypoplasia. Card Fail Rev. 2021;7:e21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 9. | Chaowu Y, Xin S, Shihua Z, Jianrong L, Hao W. Complete transposition of the atrioventricular valves associated with left ventricular apical hypoplasia. Circulation. 2011;124:538-539. [PubMed] [DOI] [Full Text] |
| 10. | Meléndez G, Muñoz L, Meave A. Isolated left ventricular apical hypoplasia. Rev Esp Cardiol. 2010;63:984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 11. | Patrianakos AP, Protonotarios N, Zacharaki A, Tsatsopoulou A, Parthenakis FI, Vardas PE. Isolated left ventricular apical hypoplasia: a newly recognized unclassified cardiomyopathy. J Am Soc Echocardiogr. 2010;23:1336.e1-1336.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 12. | Alizadeh Sani Z, Vojdanparast M, Rezaeian N, Seifi A, Omidvar Tehrani S, Nezafati P. Left ventricular apical hypoplasia: Case report on cardiomyopathy and a history of sudden cardiac death. ARYA Atheroscler. 2016;12:50-54. [PubMed] |
| 13. | Zhao Y, Zhang J. Isolated Left Ventricular Apical Hypoplasia with Right Ventricular Outflow Tract Obstruction: A Rare Combination. Ann Noninvasive Electrocardiol. 2015;20:502-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 14. | Starmer G, Younger JF, Stewart P. Multimodality imaging of isolated left ventricular apical hypoplasia. Eur Heart J. 2012;33:675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 15. | Hong SH, Kim YM, Lee HJ. Three-Dimensional Endo-Cardiovascular Volume-Rendered Cine Computed Tomography of Isolated Left Ventricular Apical Hypoplasia: A Case Report and Literature Review. Korean J Radiol. 2016;17:79-82. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 16. | Ding WY, Meah M, Rao A, Fairbairn T, Hasleton J. Isolated Left Ventricular Hypoplasia in a Postpartum Patient. Can J Cardiol. 2016;32:829.e15-829.e17. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 17. | Orsborne C, Schmitt M. Isolated left ventricular apical hypoplasia, characterized by cardiac magnetic resonance imaging. Eur Heart J. 2014;35:3303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 18. | Tumabiene KD, Magpali AE Jr, Chiong L, Jara RD, Abarquez RF Jr, Abelardo NS. A plump and fatty heart: isolated left ventricular apical hypoplasia. Echocardiography. 2012;29:E193-E196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 19. | Ong CC, Hia CP, Lim TC, Teo LL. Isolated left-ventricular apical hypoplasia presenting as a left-ventricular mass on echocardiography. Pediatr Cardiol. 2012;33:1456-1457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 20. | Flett AS, Elliott PM, Moon JC. Images in cardiovascular medicine. Cardiovascular magnetic resonance of isolated left ventricular apical hypoplasia. Circulation. 2008;117:e504-e505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 21. | Meng H, Li JR, Sun X. Left ventricular apical hypoplasia: a case series and review of the literature. Acta Cardiol. 2013;68:339-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 22. | Haffajee JA, Finley JJ, Brooks EL, Kuvin JT, Patel AR. Echocardiographic characterization of left ventricular apical hypoplasia accompanied by a patent ductus arteriosus. Eur J Echocardiogr. 2011;12:E17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 23. | Liao HQ, Peng ZF, Zhang M, Tan Y, Ouyang MZ, Zhou D, Tang K, Tang SX, Shang QL. Isolated ventricular apical hypoplasia: A report of four cases and literature review. J Clin Ultrasound. 2021;49:78-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 24. | Maidman SD, Salerno WD, Halpern DG, Donnino R, Saric M. Isolated Left Ventricular Apical Hypoplasia: A Very Rare Congenital Anomaly Characterized by Multimodality Imaging and Invasive Testing. Circ Cardiovasc Imaging. 2023;16:e014789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 25. | Ramamurthy HR, Auti O, Raj V, Viralam K. Isolated left ventricular apical hypoplasia in a young child. BMJ Case Rep. 2021;14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 26. | Choh NA, Amreen S, Mir AB, Malik AH, Hameed M, Shaheen F, Gojwari TA. Isolated left ventricular hypoplasia - A singularity. Ann Pediatr Cardiol. 2020;13:337-339. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 27. | Skidan VI, Kuznetsova T, Pavlyukova EN, Nartsissova GP. Isolated left ventricular apical hypoplasia with myocardial non-compaction: a case report. Eur Heart J Case Rep. 2020;4:1-6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 28. | Schapiro AH, Rattan MS, Moore RA, Dillman JR. Case 262: Isolated Left Ventricular Apical Hypoplasia. Radiology. 2019;290:569-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 29. | Mirdamadi A, Ashrafi S. Isolated Left Ventricular Apical Hypoplasia: Reporting a Case With Mild Manifestations and Different Echocardiography Features. Iran Red Crescent Med J. 2016;18:e26065. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 30. | Tchervenkov CI, Jacobs JP, Weinberg PM, Aiello VD, Béland MJ, Colan SD, Elliott MJ, Franklin RC, Gaynor JW, Krogmann ON, Kurosawa H, Maruszewski B, Stellin G. The nomenclature, definition and classification of hypoplastic left heart syndrome. Cardiol Young. 2006;16:339-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 127] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 31. | Freedom RM, Black MD, Benson LN. Hypoplastic left heart syndrome. In: Allen HD, Gutgesell HP, Clark EB, et al, eds. Moss and Adams’ heart disease in infants, children, and adolescents, including the fetus and young adult. Philadelphia: Lippincott Williams & Wilkins, 2001: 1011–1126. |
| 32. | Agmon Y, Connolly HM, Olson LJ, Khandheria BK, Seward JB. Noncompaction of the ventricular myocardium. J Am Soc Echocardiogr. 1999;12:859-863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 122] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 33. | Papagiannis J, Van Praagh R, Schwint O, D'Orsogna L, Qureshi F, Reynolds J, Kallfelz C, Nozar J. Congenital left ventricular aneurysm: clinical, imaging, pathologic, and surgical findings in seven new cases. Am Heart J. 2001;141:491-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 66] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 34. | Edgett JW Jr, Nelson WP, Hall RJ, Fishback ME, Jahnke EJ. Diverticulum of the heart. Part of the syndrome of congenital cardiac and midline thoracic and abdominal defects. Am J Cardiol. 1969;24:580-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 35. | De Pasquale CG, Heddle WF. Left sided arrhythmogenic ventricular dysplasia in siblings. Heart. 2001;86:128-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 43] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 36. | Zhang X, Ye RY, Chen XP. Dilated left ventricle with multiple outpouchings - a severe congenital ventricular diverticulum or left-dominant arrhythmogenic cardiomyopathy: A case report. World J Clin Cases. 2022;10:6289-6297. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (1)] |
| 37. | Van der Hauwaert LG, Michaelsson M. Isolated right ventricular hypoplasia. Circulation. 1971;44:466-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 38. | Pica S, Ghio S, Raineri C, Scelsi L, Turco A, Visconti LO. [Mutation of the lamin A/C gene associated with left ventricular apical hypoplasia: a new phenotype for laminopathies?]. G Ital Cardiol (Rome). 2014;15:717-719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
Funding: nothing to declare.
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cardiac and cardiovascular systems
Country/Territory of origin: Ireland
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Barison A, Italy; Ueda H, Japan S-Editor: Liu JH L-Editor: A P-Editor: Wu RR