Published online Aug 16, 2023. doi: 10.12998/wjcc.v11.i23.5430
Peer-review started: May 24, 2023
First decision: June 19, 2023
Revised: July 4, 2023
Accepted: July 18, 2023
Article in press: July 18, 2023
Published online: August 16, 2023
Processing time: 77 Days and 23.8 Hours
Intracranial hemorrhage after spinal surgery is a rare and devastating compli
To investigate the economic burden, clinical characteristics, risk factors, and mechanisms of intracranial hemorrhage after spinal surgery.
A retrospective cohort study was conducted from January 1, 2015, to December 31, 2022. Patients aged ≥ 18 years, who had undergone spinal surgery were included. Intracranial hemorrhage patients were selected after spinal surgery during hospitalization. Based on the type of spinal surgery, patients with intra
A total of 24472 patients underwent spinal surgery. Six patients (3 males and 3 females, average age 71.3 years) developed intracranial hemorrhage after posterior spinal fusion procedures, with an incidence of 0.025% (6/24472). The prevailing type of intracranial hemorrhage was cerebellar hemorrhage. Two patients had a poor clinical outcome. Based on the type of surgery, 30 control patients were randomly matched in 1:5 ratio. The intracranial hemorrhage group showed significant differences compared with the control group with regard to age (71.33 ± 7.45 years vs 58.39 ± 8.07 years, P = 0.001), previous history of cerebrovascular disease (50% vs 6.7%, P = 0.024), spinal dura mater injury (50% vs 3.3%, P = 0.010), hospital expenses (RMB 242119.1 ± 87610.0 vs RMB 96290.7 ± 32029.9, P = 0.009), and discharge activity daily living score (40.00 ± 25.88 vs 75.40 ± 18.29, P = 0.019).
The incidence of intracranial hemorrhage after spinal surgery was extremely low, with poor clinical outcomes. Patient age, previous stroke history, and dura mater damage were possible risk factors. It is suggested that spinal dura mater injury should be avoided during surgery in high-risk patients.
Core Tip: The incidence of intracranial hemorrhage after spinal surgery was 0.025%. This resulted in high economic burden and poor clinical outcomes. Cerebellar hemorrhage was the most common imaging presentation. Age, previous stroke history, and dura mater damage were possible risk factors.
- Citation: Yan X, Yan LR, Ma ZG, Jiang M, Gao Y, Pang Y, Wang WW, Qin ZH, Han YT, You XF, Ruan W, Wang Q. Clinical characteristics and risk factors of intracranial hemorrhage after spinal surgery. World J Clin Cases 2023; 11(23): 5430-5439
- URL: https://www.wjgnet.com/2307-8960/full/v11/i23/5430.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i23.5430
Intracranial hemorrhage after spinal surgery is a rare but disastrous postoperative complication, with poor clinical prognosis[1]. It has a heavy economic and social burden but lacks clinical data in these areas. To date, there have been approximately 100 cases of intracranial hemorrhage after spinal surgery worldwide; however, most of these were case reports[2-6]. Due to the few number of cases, its risk factors and mechanisms are not apparent. Comorbidity, dura matter damage, intracranial pressure imbalance, patient’s surgery positioning, and intra-operative high blood pressure, etc. are possible mechanisms[2-6]. The purpose of this study was to investigate the incidence of intracranial hemorrhage after spinal surgery, the health economic burden of this complication, clinical and imaging manifestations, and the possible risk factors for intracranial hemorrhage. The findings of this study could be beneficial for surgical consultation and optimizing perioperative management of spinal surgery.
Patient data were retrospectively obtained from a spinal surgery database using the electronic health information system from January 1, 2015, to December 31, 2022. This database included both emergency and elective spinal surgery. Patients with acute spinal trauma accompanied by severe head trauma and intraspinal tumors were excluded from this analysis. All experimental protocols were approved by the Ethics Committee of Beijing Jishuitan Hospital (No. 202004-76).
Postoperative brain hemorrhage after spinal surgery was selected, including intracranial hemorrhage, subarachnoid hemorrhage, cerebellar hemorrhage, epidural hematoma, and subdural hematoma, which were confirmed by computed tomography (CT) and/or magnetic resonance imaging (MRI). Intracranial hemorrhage patients were identified from the database. Based on the same type of spinal surgery, we randomly matched control patients without intracranial hemorrhage in a 5:1 ratio with the intracranial hemorrhage patients, to identify the associated risk factors.
Patients’ demographics, preoperative risk factors, and perioperative drug use (antiplatelet and anticoagulant medicine) were extracted from the database. We recorded health economic data, including length of hospital stay, hospital cost, and activity daily living (ADL) on admission and discharge. Patient’s operative and anesthetic data were collected, including type and duration of spinal surgery, surgical instrument, blood loss, and transfusion, maximum and fluctuation of systolic blood pressure, dura mater damage, subfascial drainage, and duration of intensive care unit (ICU) stay. Laboratory results were recorded. Finally, we recorded the time of intracranial hemorrhage onset, clinical manifestations, CT/MRI imaging, and prognosis, which was evaluated by the modified Rankin Scale (mRS).
Levin's variance equivalence test was conducted to determine whether the sample was homogeneous. Based on Levin's variance equivalence test, the T-test test was used to analyze continuous variables (mean ± standard deviation). The Chi-square test was used for categorical variables (%). All reported P values are two-sided. Statistical analyses were performed using SPSS 24.0. Statistical significance was set at P < 0.05.
In total, 24472 patients who underwent spinal surgery from January 1, 2015, to December 31, 2022, were recorded. Six patients (3 males and 3 females, average age 71.3 years) developed intracranial hemorrhage after spinal surgery, with an incidence of 0.025% (6/24472). All six patients underwent posterior spinal fusion procedures (one in the cervical, three in the thoracic, and two in the lumbar site) in the prone position. Anticoagulation or antiplatelet drugs were routinely discontinued preoperatively. Systolic blood pressure was less than 180 mmHg during the operation. Subfascial drainage was placed in all six patients.
The median interval time between spinal surgery and intracranial hemorrhage was 3 h (ranging from 0 to 24 h). The most common symptom of intracranial hemorrhage was consciousness disorder (n = 5), which varied from somnolence to coma. Other symptoms included dysphasia (n = 3), paralysis (n = 2), vomiting (n = 2), dizziness (n = 1), and headache (n = 1). Brain imaging demonstrated cerebellar hemorrhage in three patients, subarachnoid hemorrhage (SAH) in two patients, lobar hemorrhage in two patients, and intraventricular hemorrhage in one patient. One patient underwent cerebellar hematoma evacuation and decompressive craniectomy, one patient underwent digital subtraction angiography (DSA), and the other four patients were treated conservatively. On discharge, two patients were in a vegetative state (33.3%) with poor clinical outcomes.
Based on the same type of surgery, 30 control patients without intracranial hemorrhage were randomly matched in a 5:1 ratio with the six intracranial hemorrhage patients. Intracranial hemorrhage patients had a higher percentage of cerebrovascular disease history (50% vs 6.7%, P = 0.024) and significant dura matter damage during the operation (50% vs 3.3%, P = 0.010) than controls (Tables 1 and 2). There were significant differences in terms of age (71.33 ± 7.45 years vs 58.39 ± 8.07 years, P = 0.001), hospital cost (RMB 242119.1 ± 87610.0 vs 100, 192.4 ± 33, 556.3, P = 0.009), duration of ICU stay (310.18 ± 235.82 h vs 1.26 ± 4.85 h, P = 0. 024) and discharge ADL score (40.00 ± 25.88 vs 75.40 ± 18.29, P = 0.019) between the two groups (Tables 1 and 2). There were no differences in laboratory examinations between the two groups (Table 3).
| Hemorrhage group, n = 6 | Control group, n = 30 | P value | |
| Age (yr) | 71.33 ± 7.45 | 58.39 ± 8.07 | 0.001a |
| Gender (Male) | 3 (50) | 17 (56.7) | 1.000 |
| BMI (kg/m2) | 27.16 ± 2.17 | 26.47 ± 3.72 | 0.667 |
| Hypertension | 5 (83.3) | 15 (60) | 0.169 |
| Diabetes mellitus | 1 (16.7) | 5 (16.7) | 1.000 |
| Hyperlipidemia | 1 (16.7) | 3 (10) | 0.535 |
| Coronary heart disease | 0 (0) | 2 (6.7) | 1.000 |
| Cerebral vascular disease | 3 (50) | 2 (6.7) | 0.024a |
| Smoking | 2 (33.3) | 3 (10) | 0.186 |
| Alcohol drinking | 1 (16.7) | 2 (6.7) | 0.431 |
| Hospital Cost (RMB) | 242119.1 ± 87610.0 | 96290.7 ± 32029.9 | 0.009a |
| Admission ADL | 78.33 ± 31.73 | 90.33 ± 14.50 | 0.403 |
| Discharge ADL | 40.00 ± 25.88 | 75.40 ± 18.29 | 0.019a |
| LOS (d) | 60.17 ± 74.71 | 13.27± 3.73 | 0.185 |
| ICU stay (h) | 310.18 ± 235.82 | 1.26 ± 4.85 | 0.024a |
| Hemorrhage group, n = 6 | Control group, n = 30 | P value | |
| Surgery time (min) | 177.50 ± 50.97 | 149.67 ± 69.06 | 0.357 |
| Blood loss during surgery (mL) | 433.33 ± 350.24 | 327.67 ± 301.94 | 0.451 |
| Max systolic BP (mmHg) | 150.00 ± 22.14 | 139.47 ± 27.34 | 0.381 |
| Fluctuating systolic BP (mmHg) | 47.50 ± 16.66 | 39.00 ± 17.49 | 0.282 |
| Dura damage | 3 (50.0) | 1 (3.3) | 0.010a |
| Fusion instrument | 4 (66.7) | 10 (33.3) | 0.181 |
| Pedicle screw fixation | 4 (66.7) | 23 (76.7) | 0.627 |
| Plasma transfusion (mL) | 633.33 ± 898.15 | 20.00 ± 109.55 | 0.155 |
| RBC transfusion (U) | 2.00 ± 2.53 | 0.20 ± 0.81 | 0.143 |
| Subfascial drainage (mL) | 1804.83 ± 1284.60 | 695.33 ± 507.85 | 0.088 |
| Hemorrhage group, n = 6 | Control group, n = 30 | P value | |
| WBC (109/L) | 6.05 ± 1.18 | 6.43 ± 1.60 | 0.584 |
| RBC (1012/L) | 4.72 ± 0.43 | 4.75 ± 0.39 | 0.852 |
| HBG (g/L) | 144.33 ± 10.73 | 146.23 ± 11.90 | 0.720 |
| PLT (109/L) | 189.83 ± 70.59 | 227.70 ± 48.17 | 0.113 |
| HCT (%) | 42.22 ± 3.60 | 44.24 ± 8.29 | 0.565 |
| ALT (IU/L) | 19.67 ± 9.73 | 21.20 ± 11.78 | 0.767 |
| AST (IU/L) | 19.33 ± 4.72 | 21.67 ± 6.40 | 0.404 |
| ALP (IU/L) | 69.83 ± 14.93 | 61.63 ± 15.88 | 0.252 |
| GLU (mmol/L) | 5.10 ± 0.49 | 5.69 ± 1.18 | 0.059 |
| UREA (mmol/L) | 5.45 ± 1.67 | 5.29 ± 1.29 | 0.798 |
| CREA (umol/L) | 67.00 ± 17.75 | 62.23 ± 15.00 | 0.495 |
| CHOL (mmol/L) | 4.61 ± 0.75 | 5.00 ± 1.01 | 0.375 |
| ESR (mm) | 9.67 ± 7.42 | 8.97 ± 8.22 | 0.848 |
| CRP (mg/dL) | 4.62 ± 5.36 | 2.58 ± 1.31 | 0.396 |
| PT (s) | 11.70 ± 0.42 | 11.35 ± 0.64 | 0.204 |
| PA (%) | 102.98 ± 7.89 | 109.86 ± 11.55 | 0.175 |
| INR | 0.98 ± 0.43 | 0.94 ± 0.50 | 0.092 |
| APTT (s) | 26.67 ± 3.12 | 25.17 ± 2.51 | 0.209 |
| FIB (mg/dL) | 306.95 ± 67.93 | 257.31 ± 68.43 | 0.114 |
| D-dimer | 0.36 ± 0.17 | 0.42 ± 0.51 | 0.786 |
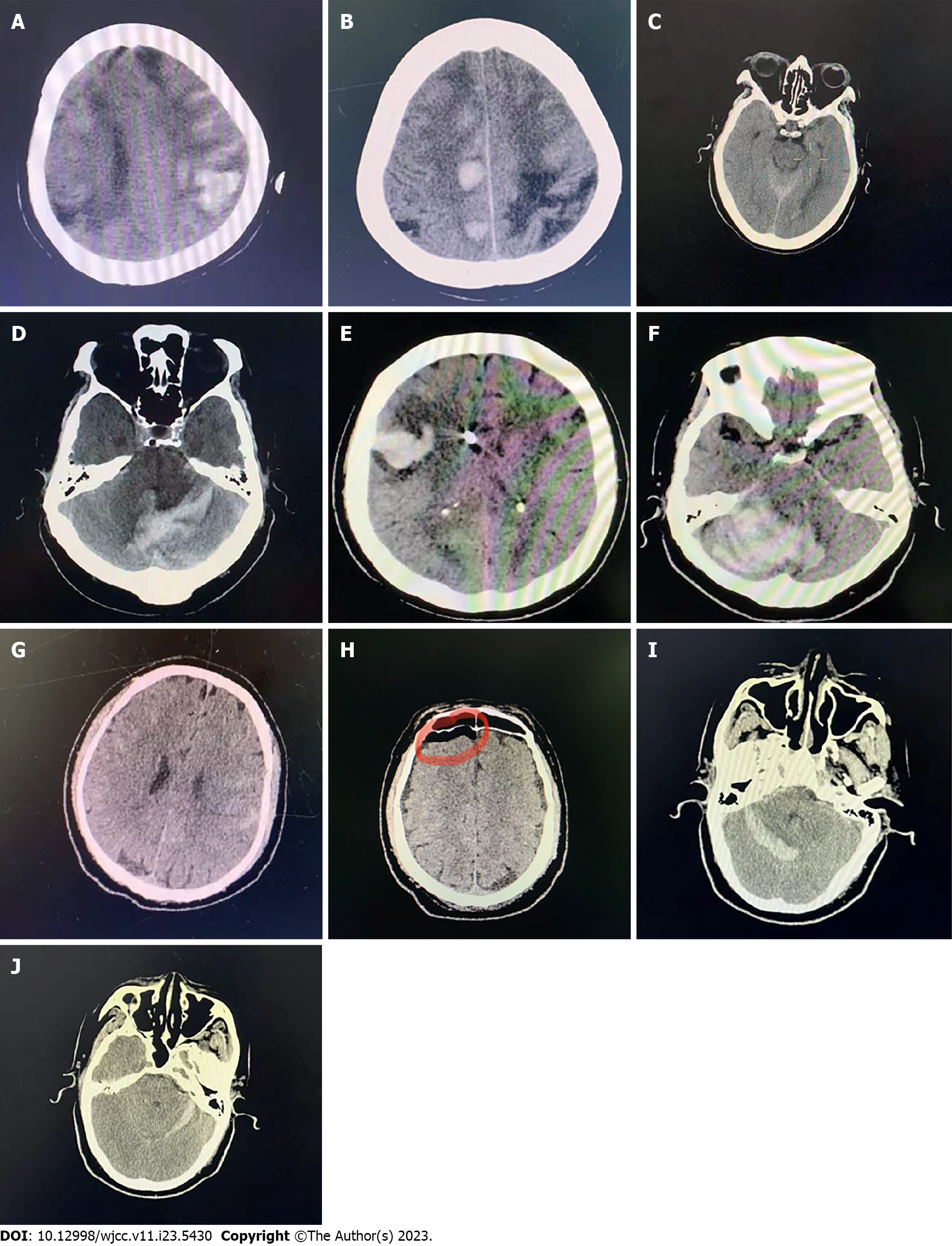
Case 1: A 78-year-old female patient with lumbar spinal stenosis (L4/5) underwent a posterior lumbar fusion procedure. Three hours after the operation, she developed apathy, disorientation, and limb paralysis (0/5) with a National Institute of Health stroke scale (NIHSS) score of 17. Brain CT showed bilateral parietal, frontal lobe hemorrhage, and SAH (Figure 1A). Cerebral amyloid angiopathy (CAA) was diagnosed. Lobar hemorrhage occurred another three times during hospitalization (Figure 1B). On discharge, the patient was transferred to a local hospital in a vegetative state.
Case 2: An 80-year-old female patient with thoracic ossification of the ligamentum flavum (T1-3) underwent a posterior thoracic fusion procedure. Immediately after the procedure, she developed lethargy, aphasia, gaze, quadriplegia (0/5), and neck rigidity with a NIHSS of 34. Brain CT scan demonstrated left intraventricular hemorrhage and SAH (Figure 1C). The patient recovered normally on discharge.
Case 3: A 68-year-old male patient with cervical spinal stenosis (C3-6) underwent a posterior cervical fusion procedure. Twenty-four hours after the operation, he developed drowsiness, vomiting, dysarthria, and left-side ataxia with a NIHSS of 5. Immediate brain CT demonstrated bilateral cerebellar hematoma and SAH (Figure 1D). This patient was discharged with slurred speech.
Case 4: A 74-year-old female patient with thoracic ossification of the ligamentum flavum (T10/11) underwent a posterior thoracic fusion procedure. The dura matter was damaged during the operation and was sutured immediately. Twenty-four hours later, she appeared drowsy, with vomiting, aphasia, and gaze with a NIHSS of 10. Brain CT scan showed right frontal lobe hemorrhage and right cerebellar hematoma (Figure 1E and F). The patient underwent hematoma evacuation and decompressive craniectomy. She was in coma with assisted ventilation. Her family chose to discharge against medical advice.
Case 5: A 68-year-old male was admitted for lumbar spondylolisthesis (L4) and underwent a posterior lumbar fusion procedure. Three hours after surgery, he developed coma, neck rigidity, and quadriplegia (0/5) with a NIHSS of 34. Brain CT showed SAH (Figure 1G). DSA was performed immediately with normal results. Intracranial pneumatosis was found on CT scan seven days later (Figure 1H). This patient was discharged with mild paralysis of the left limb.
Case 6: A 60-year-old male with thoracic spondylolisthesis (T12/L1) underwent a posterior lumbar fusion procedure. The dura matter was damaged and was sutured during the operation. Twenty-four hours after surgery, he developed headache, dysphasia, and numbness with a NIHSS of 2. Brain CT showed bilateral cerebellum hemorrhage (Figure 1I and J). This patient was discharged with mild dysphasia (Table 4).
| No | Gender | Age | Comorbidity | Diagnosis | Procedure | Surgery position | Dura damage | Pedicle screws | Fusion instrument | Clinical manifestations | Brain imaging | Treatment | Outcome (mRS) |
| 1 | F | 78 | CVD | Lumber spinal stenosis (L4/5) | Posterior lumbar spinal fusion, decompression and discectomy | Prone | No | Yes | Yes | Apathy, hemiparalysis 0/5. NIHSS 17 | Bilateral parietal and frontal lobe hemorrhage and SAH | Conservative treatment | 5 |
| 2 | M | 80 | HT | Ossification of thoracic ligamentum flavum (T1-3) | Posterior thoracic spinal fusion, resection of the ligamentum flavum ossification | Prone | No | No | No | Lethargy, aphasia, gaze, quadriplegia 0/5, neck rigidity. NIHSS 34 | Left intraventricular hemorrhage and SAH | Conservative treatment | 1 |
| 3 | F | 68 | HT | Cervical degenerative disc disease (C3-6) | Posterior cervical spinal fusion, laminoplasty procedure | Prone | No | Yes | No | Drowsiness, vomiting, dysarthria, ataxia. NIHSS 5 | Bilateral cerebellar hematoma and SAH | Conservative treatment | 3 |
| 4 | F | 74 | CVD, HT, HLP | Ossification of thoracic ligamentum flavum (T10/11) | Posterior thoracic spinal fusion, laminectomy and resection of ligamentum flavum ossification | Prone | Yes | Yes | Yes | Drowsy, vomiting, aphasia, gaze. NIHSS 10 | Right frontal lobe hemorrhage and right cerebellar hematoma | Hematoma evacuation, decompressive craniectomy | 5 |
| 5 | M | 68 | CVD, HT, DM | Lumber spondylolisthesis L4; degenerative disc disease (L5/S1) | Posterior cervical spinal fusion, decompression and discectomy | Prone | Yes | Yes | Yes | Coma, neck rigidity, quadriplegia 0/5. NIHSS 34. | SAH and intracranial pneumatosis | DSA and conservative treatment | 3 |
| 6 | M | 60 | HT | Thoracic spondylolisthesis (T12/L1) | Posterior lumbar fusion procedure, resection of ligamentum flavum ossification | Prone | Yes | Yes | Yes | Headache, dysphasia, numbness. NIHSS 2 | Bilateral cerebellum hemorrhage | Conservative treatment | 2 |
Intracranial hemorrhage after spinal surgery is a rare complication and its etiology and pathogenesis are unclear. The first reported case was in 1981 by Chadduck[7]. To date, only 100 cases have been published worldwide. Our study comprised a case series of intracranial hemorrhage after spinal surgery in a large Chinese database, with the aim of determining the health economic burden, clinical and imaging manifestations, investigate the associated risk factors, and provide useful information in order to prevent this complication.
The incidence of intracranial hemorrhage after spinal surgery in our study was 0.025% (6/24472), which was extremely low. An Egyptian study demonstrated that the incidence of intracranial hemorrhage was 0.066% (8/12185)[1]. A six-year retrospective study in Japan showed that 53 of 167106 (0.03%) patients developed hemorrhagic stroke after spinal surgery[8]. Zhao et al reported that the incidence of subarachnoid hemorrhage after spinal surgery was 0.16% (23/14526) in a single-center study in China[9]. Depending on the patients selected and their medical conditions, the incidence varied from 0.025% to 0.16% in different studies, which further confirmed the rarity of this disease.
In our study, the hospitalization cost of intracranial hemorrhage patients was twice that of the controls. Patients with intracranial hemorrhage after spinal surgery had significantly decreased ADL on discharge, and most patients were transferred to rehabilitation hospitals for further treatment. One-third of patients had poor clinical outcomes. Previous studies also found that nearly 30%-50% of patients with intracranial hemorrhage after spinal surgery had a severe prognosis, which was similar to our findings, and the mortality was 8%-15%[10,11]. Thus, this rare complication results in a heavy economic and caregiver burden to the patients and their families.
Intracranial hemorrhage after spinal surgery had an acute onset and rapid progression. The median interval time between spinal surgery and intracranial hemorrhage was 3 h, which was shorter than for postoperative ischemia (1-3 d after the operation) in our database. Brockmann et al[11] found that intracranial hemorrhage occurred within 30 h after the operation in 80% of patients. The most frequent clinical manifestations of intracranial hemorrhage were disturbance of consciousness, slurred speech, limb weakness, and vomiting, which were non-specific and easily confused with anesthetics and postoperative pain. It is necessary to pay close attention to the patient's symptoms within 24 h of surgery and perform an imaging examination immediately.
In our study, cerebellar hemorrhage was prevalent, and most cases were bilateral. The typical manifestation of cerebellar hemorrhage was the “zebra sign” strip, which referred to the bleeding zone on the cerebellum sulcus[12]. This is possibly the venous origin of intracranial bleeding[2,9,10], due to stretching the bridge or supracerebellar veins.
In Case 5, brain CT scan demonstrated SAH and brain pneumatosis, which was related to occult dura damage, although the patient had no dura impairment in his medical record. The possible underlying mechanisms include: First, the inverted bottle theory, cerebrospinal fluid leakage formed negative pressure in the subarachnoid space, thus leading air into the brain; second, the ball valve theory, the dura damage performs as a one-way valve, inducing air stuck in the intracranial cavity[13-15].
We found that dura matter damage was a risk factor for intracranial hemorrhage after spinal surgery. Half of the patients had definite or occult dura deficit, which was significantly higher than the controls. Dura damage can induce cerebrospinal fluid (CSF) fistula, which leads to the excessive loss of cerebrospinal fluid, and/or an imbalance of intracranial pressure[16]. It can lead to compensatory dilatation of the venous system and downward brain shift, thus inducing intracranial hemorrhage[1]. Previous studies demonstrated that 60%-93% of intracranial hemorrhage patients had dura matter damage[1,2,17]. Therefore, surgeons must pay close attention to avoid dura tears, and if a tear occurs, they should repair the dura immediately with a waterproof closure. For high-risk dura deficit patients (revision surgery/severe adhesion), it was necessary to monitor the amount and speed of CSF drainage, to avoid a permanent deficit. Some studies showed that fusion instruments, screw pedicle fixation, and intraoperative positioning could also be risk factors for intracranial hemorrhage after spinal surgery; however, this was not found in our study.
Stroke history was another risk factor in our study, which has been proven in several studies[8,18,19]. Patients with previous stroke had poor collateral circulation, vascular stenosis, and/or malformation. It was necessary to obtain detailed information on the previous stroke (etiology and location) and perform a comprehensive cerebrovascular examination preoperatively, including routine blood tests, coagulation tests, brain CT/MR scan, etc. to identify potential vascular stenosis, malformation and microbleeds (T2* sequence)[10,11]. Patients with suspected CAA should be informed about the high risk of intracranial hemorrhage before the operation, and if unnecessary, surgery should be avoided[20].
Advancing age is also a well-known risk factor. We speculated that elderly patients may have severe ossification and adhesion of the spine, which increased the difficulty of surgery, and was prone to intra-operative dura mater damage[10]. Numerous studies have demonstrated that advancing age (≥ 65 years) was a key risk factor for postoperative stroke[18,21].
There are several limitations in our study. First, the study was retrospective based on medical records with incomplete and omission errors in a single hospital, which could have led to selection bias. Second, brain CT/MRI was not a routine examination after spinal surgery; thus, asymptomatic intracranial hemorrhage could have been missed, and the incidence of intracranial hemorrhage underestimated. Third, intracranial hemorrhage after spinal surgery was an extremely rare complication and the case number was relatively small. However, the entire cohort of spinal surgery patients was large, which could make up for this limitation to some extent. A multicentric and prospective study should be conducted in the future.
The incidence of intracranial hemorrhage after spinal surgery was 0.025%. It is a heavy economic and social burden for the patients and their families. Patient age, history of cerebrovascular disease, and dura damage may be risk factors. It might be helpful to perform a detailed cerebral vascular examination preoperatively and avoid dura damage during surgery in high-risk patients to prevent intracranial hemorrhage.
Intracranial hemorrhage after spinal surgery is a rare but disastrous postoperative complication.
To reduce the complication of intracranial hemorrhage after spinal surgery.
This study aimed to investigate the incidence and the health economic burden of intracranial hemorrhage after spinal surgery, clarify clinical and imaging manifestations and possible risk factors.
Intracranial hemorrhage patients were selected in this retrospective cohort study. Based on the type of spinal surgery, hemorrhage patients were randomly matched in a 1:5 ratio with control patients. The pre-, intra-, and post-operative associated risk factors were analyzed.
The incidence of intracranial hemorrhage after spinal surgery was 0.025% (6/24472). Cerebellar hemorrhage was the prevailing type of intracranial hemorrhage. Patient age, previous stroke history, and dura mater damage were possible risk factors.
Intracranial hemorrhage after spinal surgery was extremely rare, with poor clinical outcomes. It is suggested that spinal dura mater injury should be avoided during surgery in high-risk patients.
A multicentric and prospective study should be conducted in the future.
| 1. | Al-Saadi T, Al-Kindi Y, Allawati M, Al-Saadi H. Intracranial Hemorrhage following Spinal Surgery: A Systematic Review of a Rare Complication. Surg J (N Y). 2022;8:e98-e107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 2. | Allouch H, Abu Nahleh K, Mursch K, Shousha M, Alhashash M, Boehm H. Symptomatic Intracranial Hemorrhage after Dural Tear in Spinal Surgery-A Series of 10 Cases and Review of the Literature. World Neurosurg. 2021;150:e52-e65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 3. | Pham MH, Tuchman A, Platt A, Hsieh PC. Intracranial complications associated with spinal surgery. Eur Spine J. 2016;25:888-894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 4. | Kaloostian PE, Kim JE, Bydon A, Sciubba DM, Wolinsky JP, Gokaslan ZL, Witham TF. Intracranial hemorrhage after spine surgery. J Neurosurg Spine. 2013;19:370-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 5. | Yang F, Zhao J, Xu H. Characteristics of Hemorrhagic Stroke following Spine and Joint Surgeries. Biomed Res Int. 2017;2017:5390839. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 6. | Sánchez Zamora P, Gómez Del Pulgar Vázquez B, Gholamian Ovejero S. Intraventricular hemorrhage as complication after spinal surgery. Case report. Rev Esp Anestesiol Reanim (Engl Ed). 2022;69:183-186. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 7. | Chadduck WM. Cerebellar hemorrhage complicating cervical laminectomy. Neurosurgery. 9(2):185-9. [PubMed] [DOI] [Full Text] |
| 8. | Ohya J, Chikuda H, Oichi T, Horiguchi H, Takeshita K, Tanaka S, Yasunaga H. Perioperative stroke in patients undergoing elective spinal surgery: a retrospective analysis using the Japanese diagnosis procedure combination database. BMC Musculoskelet Disord. 2015;16:276. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 9. | Zhao J, Jiang H, Meng Y, Gao R, Ma J, Wang C, Zhou X. Analysis of Risk Factors Related to Acute Subarachnoid Hemorrhage After Spinal Surgery. World Neurosurg. 2022;160:e111-e117. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 10. | Sturiale CL, Rossetto M, Ermani M, Baro V, Volpin F, Milanese L, Denaro L, d'Avella D. Remote cerebellar hemorrhage after spinal procedures (part 2): a systematic review. Neurosurg Rev. 2016;39:369-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 11. | Brockmann MA, Groden C. Remote cerebellar hemorrhage: a review. Cerebellum. 2006;5:64-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 75] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 12. | Mallio CA, Sarà M, Pistoia ML, Occhicone F, Errante Y, Giona A, Zobel BB, Quattrocchi CC. Bilateral remote cerebellar haemorrhage after spinal surgery: a case study and review of the literature. Brain Inj. 2014;28:1216-1222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 13. | Akyüz O, Gökpınar D, Aydın E, Aydın S, Duymuş M, Çığşar G, Özdemir M. Pneumocephalus and Pneumorrhachis After Spinal Surgery. Pol J Radiol. 2016;81:34-35. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Karavelioglu E, Eser O, Haktanir A. Pneumocephalus and pneumorrhachis after spinal surgery: case report and review of the literature. Neurol Med Chir (Tokyo). 2014;54:405-407. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 15. | Abu-Hamdiyah OJ, Al Sharie S, Awadi S, Khamees A, Athamneh MJ. Pneumocephalus secondary to a spinal surgery: A literature review and a case report. Int J Surg Case Rep. 2021;86:106342. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 16. | Worm PV, Dalla-Corte A, Brasil AVB, Perondi G, Sfreddo E, Vial ADM, Gago G, da Costa PRF. Cerebellar hemorrhage as a complication of spine surgery. Surg Neurol Int. 2019;10:85. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Numaguchi D, Wada K, Yui M, Tamaki R, Okazaki K. Incidence of Remote Cerebellar Hemorrhage in Patients with a Dural Tear during Spinal Surgery: A Retrospective Observational Analysis. Spine Surg Relat Res. 2019;3:141-145. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 18. | Benesch C, Glance LG, Derdeyn CP, Fleisher LA, Holloway RG, Messé SR, Mijalski C, Nelson MT, Power M, Welch BG; American Heart Association Stroke Council; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology; and Council on Epidemiology and Prevention. Perioperative Neurological Evaluation and Management to Lower the Risk of Acute Stroke in Patients Undergoing Noncardiac, Nonneurological Surgery: A Scientific Statement From the American Heart Association/American Stroke Association. Circulation. 2021;143:e923-e946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 89] [Article Influence: 17.8] [Reference Citation Analysis (1)] |
| 19. | Vlisides PE, Moore LE. Stroke in Surgical Patients. Anesthesiology. 2021;134:480-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 20. | Charidimou A, Boulouis G, Gurol ME, Ayata C, Bacskai BJ, Frosch MP, Viswanathan A, Greenberg SM. Emerging concepts in sporadic cerebral amyloid angiopathy. Brain. 2017;140:1829-1850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 380] [Article Influence: 42.2] [Reference Citation Analysis (0)] |
| 21. | Petersen PB, Kehlet H, Jørgensen CC; Lundbeck Foundation Center for Fast-Track Hip and Knee Replacement Collaborative Group. Incidence and Risk Factors for Stroke in Fast-Track Hip and Knee Arthroplasty-A Clinical Registry Study of 24,862 Procedures. J Arthroplasty. 2019;34:743-749.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Neurosciences
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chrastina J, Czech Republic; Viswanathan VK, United States S-Editor: Liu JH L-Editor: Webster JR P-Editor: Yu HG