Published online Aug 6, 2023. doi: 10.12998/wjcc.v11.i22.5398
Peer-review started: May 27, 2023
First decision: June 13, 2023
Revised: June 24, 2023
Accepted: July 11, 2023
Article in press: July 11, 2023
Published online: August 6, 2023
Processing time: 67 Days and 21.7 Hours
Mitochondrial myopathy is a rare genetic disease with maternal inheritance that may involve multiple organ systems. Due to the lack of typical characteristics, its clinical diagnosis is difficult, and it is often misdiagnosed or even missed.
The patient was a young college student. When he presented at the hospital, he had severe lactic acidosis, respiratory failure, and shock with multiple organ dysfunction syndrome (MODS). He was treated by mechanical ventilation, veno-arterial extracorporeal membrane oxygenation, and other organ support. However, his condition continued to worsen. After a thorough and detailed medical and family history was taken, a mitochondrial crisis was suspected. A muscle biopsy was taken. Further genetic testing confirmed a mitochondrial gene mutation (TRNL1 3243A>G). The final diagnosis of mitochondrial myopathy was made. Although there is no known specific treatment, intravenous methylprednisone and intravenous immunoglobulin were started. The patient’s shock eventually improved. The further course was complicated by severe infection in multiple sites, severe muscle weakness, and recurrent MODS. After 2 mo of multidisciplinary management and intensive rehabilitation, the patient could walk with assistance 4 mo after admission and walk independently 6 mo after admission.
More attention should be paid to mitochondrial myopathy to avoid missed diagnosis and misdiagnosis.
Core Tip: Mitochondrial crisis is rare and difficult to diagnose and treat. This article reports a patient with mitochondrial crisis, hyperlactic acidemia, and respiratory failure at admission, complicated by multiple organ failure and limb muscle weakness, who survived the mitochondrial crisis after early high-dose hormone and high-dose intravenous human immunoglobulin therapy, and the patient finally recovered and was discharged after more than 2 mo of treatment.
- Citation: Chen L, Shuai TK, Gao YW, Li M, Fang PZ, Christian W, Liu LP. Treatment of a patient with severe lactic acidosis and multiple organ failure due to mitochondrial myopathy: A case report. World J Clin Cases 2023; 11(22): 5398-5406
- URL: https://www.wjgnet.com/2307-8960/full/v11/i22/5398.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i22.5398
Mitochondrial myopathy, accompanied by myasthenia as the first manifestation, is often associated with stroke and epilepsy-like symptoms. This patient was hospitalized with primary symptoms of hyperlactatemia, severe shock, and multiple visceral failures. In addition, it manifested as a mitochondrial crisis and poor microcirculation and organ support. However, only drugs to improve circulation and assist mitochondrial function could be given without specific treatment for the disease. The purpose of this article is to share the mechanisms behind the diagnosis and treatment of mitochondrial crisis in patients with mitochondrial myopathy to strengthen the understanding of mitochondrial myopathy. By reporting this case, more doctors can reduce the rates of missed diagnosis and misdiagnosis of mito
A 22-year-old man was hospitalized in June 2021 due to fatigue, chest tightness, and shortness of breath for half a month, which worsened for 4 d.
The patient caught a cold and developed fatigue, chest tightness, inability to walk, and gradual aggravation of shortness of breath.
A third-party testing institution and the family history led towards the diagnosis of the underlying disease. The patient had been hospitalized in 2016 and 2018 for muscle weakness, chest tightness, and shortness of breath after strenuous exercises such as running in junior high school. Electromyography indicated a decrease in the amplitude of the evoked potentials and myogenic changes in the right deltoid muscle, but no genetic testing was performed. The patient had responded favourably to the administration of methylprednisolone at that time.
The patient’s mother and uncle had undiagnosed muscle weakness and tachycardia after fatigue. A distant male relative in the father's family had been diagnosed with pseudohypertrophic muscular dystrophy. A male relative in the mother's family had hereditary muscular dystrophy (point mutation).
The patient reported progressive shortness of breath. The respiratory rate was 32/min with an SpO2 of 96% (with 5 L/min of oxygen per mask) and sinus tachycardia (pulse rate 115/min). The pCO2 was 65 mmHg. Blood pressure (16.7/9.04 Kpa) and body temperature (36.8 °C) were normal. Muscle strength in the extremities was symmetrically reduced with grade 4 of the medical research council scale (with grade 0 assigned to no muscle movement and grade 5 assigned to full strength). There was no aphasia. There were abnormal breath sounds, but the other physical examination was unremarkable.
Abnormal values in the laboratory tests were found for lactate (25 mmol/L), creatinine phosphokinase-MB (385 U/L), N-terminal pro-brain natriuretic peptide (1622 pg/mL), white blood cell count (WBC, 1.88 × 1010/L) with 84.4% of neutrophils, procalcitonin (5.6 ng/mL), and interleukin-6 (32 pg/mL).
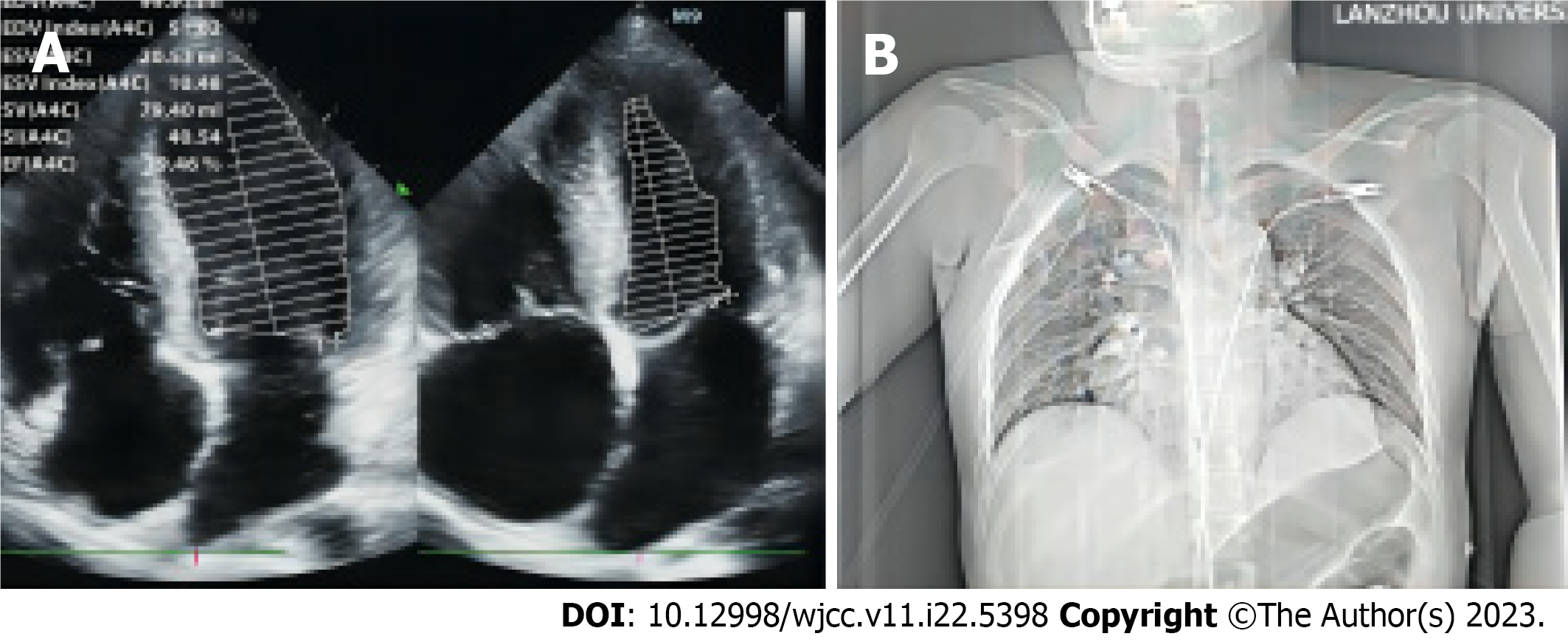
Cardiac ultrasound revealed global heart enlargement, mild mitral and tricuspid valve regurgitation, a pulmonary artery pressure of 3.86 Kpa, and small amounts of pericardial effusion. The left ventricular systolic function appeared normal (Figure 1A). The chest X-ray suggested enlargement of the right heart. No infiltrations could be seen (Figure 1B).
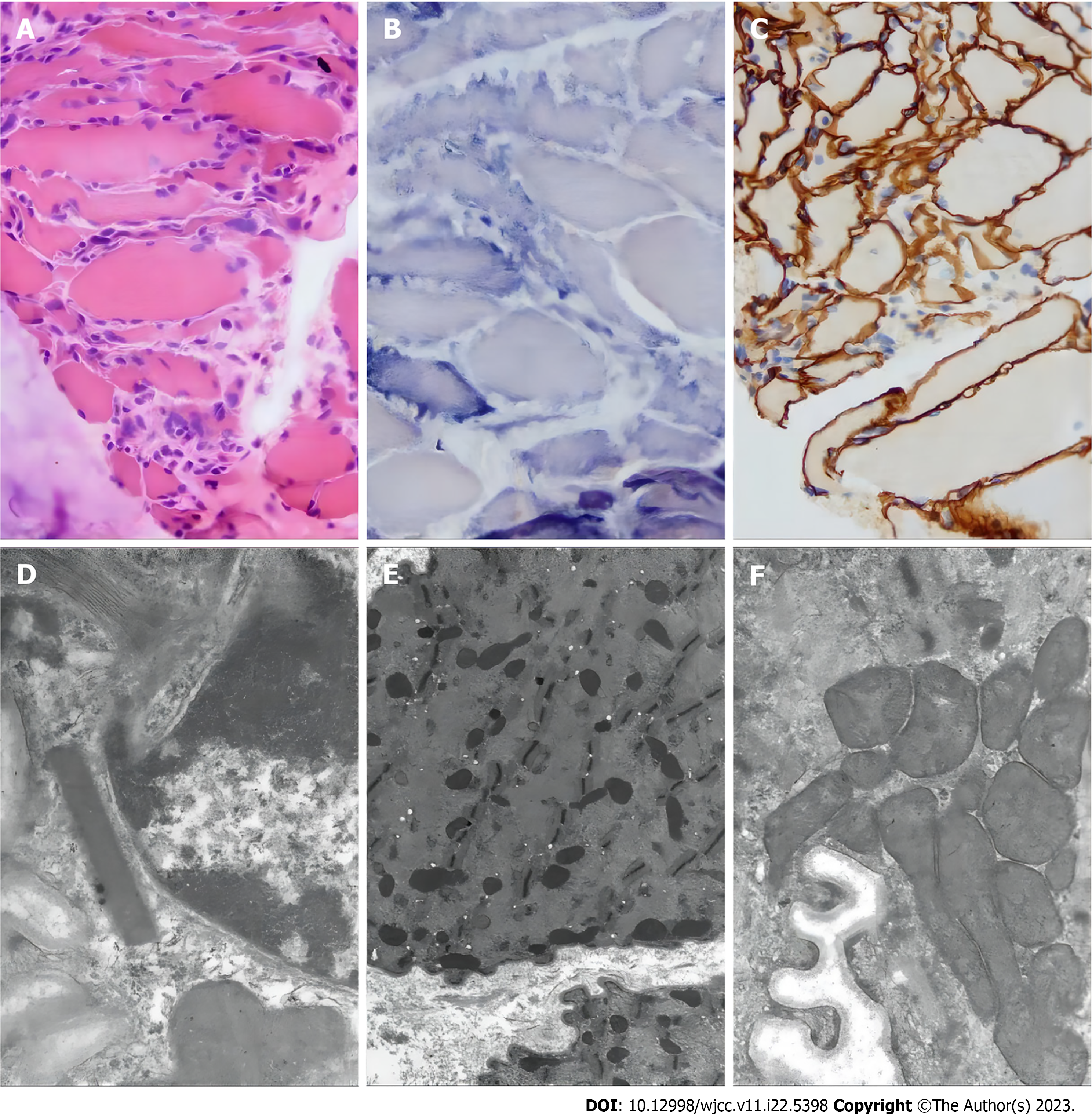
Based on the patient's symptoms, signs, pathology (Figure 2), and genetic testing, the patient received a diagnosis of mitochondrial myopathy and mitochondrial crisis.
In mitochondrial crisis, intravenous methylprednisone (IVMP) and intravenous immunoglobulin (IVIG) may be effective. In the maintenance period, the main treatment strategy is symptomatic supportive care and preventive care. There is no unified theory. It is mainly the application of high-dose B vitamins, coenzyme Q10, L-carnitine, etc. In addition, adenosine triphosphate, fat emulsion, amino acids, hormones, and other drugs can significantly improve the symptoms of muscle weakness and fatigue, and traditional Chinese medicine[1] (invigorating the spleen, supplementing qi, removing dampness, promoting blood circulation, and removing blood stasis) is also effective.
The patient was able to walk with assistance 4 mo and walk independently 6 mo after the initial admission. Now, the patient is completely normal.
Mitochondrial myopathy is a rare disease with mitochondrial dysfunction as the main feature of the genetic defect[2]. The global incidence rate is only 0.025%[3]. Mitochondrial damage mainly affects the energy supply. Therefore, the brain and muscles that are most sensitive to energy supply are particularly vulnerable in this disease. When both the brain and muscles are involved, the condition is called mitochondrial encephalomyopathy, while the simple invasion of the skeletal muscles is called mitochondrial myopathy[4]. Muscle weakness is a common symptom of several clinical syndromes, such as mitochondrial encephalomyopathy with lactic acidosis and stroke-like episodes (MELAS), chronic progressive external ophthalmoplegia, Kearns-Sayre syndrome, and myoclonus epilepsy ragged-red fibres[5]. Symptoms of exercise intolerance, paroxysmal muscle weakness, and muscle soreness could be corroborated with the diagnosis of mito-chondrial myopathy. The disease was predominantly chronic, and few patients developed severe mitochondrial myopathy. Severe mitochondrial myopathy presents as acute muscle pain, palpitation, dyspnoea, limb weakness, and lactic acidosis. In extreme cases, it can present as a life-threatening mitochondrial crisis[6].
Increased reactive oxygen species generation, hormonal changes, and altered mitochondrial gene transcription are associated with mitochondrial dysfunction in patients with severe sepsis[7]. Therefore, the patient presented with mitochondrial crisis, respiratory insufficiency, muscle weakness, and severe lactic acidosis.
Lactic acidosis can be the result of a large variety of conditions and diseases. Lactic acidosis from tissue hypoxia and hypoperfusion is very common in clinical practice in the intensive care setting. Septic shock could not be completely ruled out in our patient (elevated WBC and procalcitonin). However, there was no hypoxemia, and blood pressure was normal. Furthermore, the initial diagnostic workup did not show an obvious infectious focus. Some cardiac compromise was identified on echocardiography, but this was not consistent with cardiogenic shock. No seizures were reported or observed. Considering type B lactic acidosis, most conditions could be ruled out, including intoxications, poisoning, and adverse effects from drugs. In a quiet state, the lactic acid content of the body mainly arises from the skin (25%), red blood cells (20%), the central nervous system (20%), muscles (25%), and the gastrointestinal tract (10%). Under strain, the elevated lactic acid mainly arises from the skeletal muscles. Studies have confirmed that mitochondrial diseases are metabolic disorders caused by pathogenic genetic variants affecting mitochondrial function[8]. These pathogenic variants alter mitochondrial structure and function as well as various mitochondrial processes, such as oxidative phosphorylation, mitochondrial transmembrane ion transport, and mitochondrial division and fusion[9]. Therefore, the cellular energy supply is affected, and lactic acid may accumulate. The change in plasma lactate level over time is a reliable marker of severity and mortality of the disease[10,11]. Recent studies have shown that lactate is a potential biomarker for predicting patients with poor prognoses from septic shock[12]. Relative hyperlactatemia (blood lactate concentration > 0.75 mmol/L) has been shown to be associated with increased in-hospital mortality[13,14].
Rare causes of lactic acidosis, such as mitochondrial myopathy, were considered as the cause of muscle weakness and respiratory distress with hypercapnia.
The third-party testing institution and the family history with recurrent fatigue and weakness after exercise in the patient and established or suspected (mitochondrial) myopathy in other family members pointed us to the clinical suspicion. Autoantibodies, muscle biopsy, and genetic testing were performed.
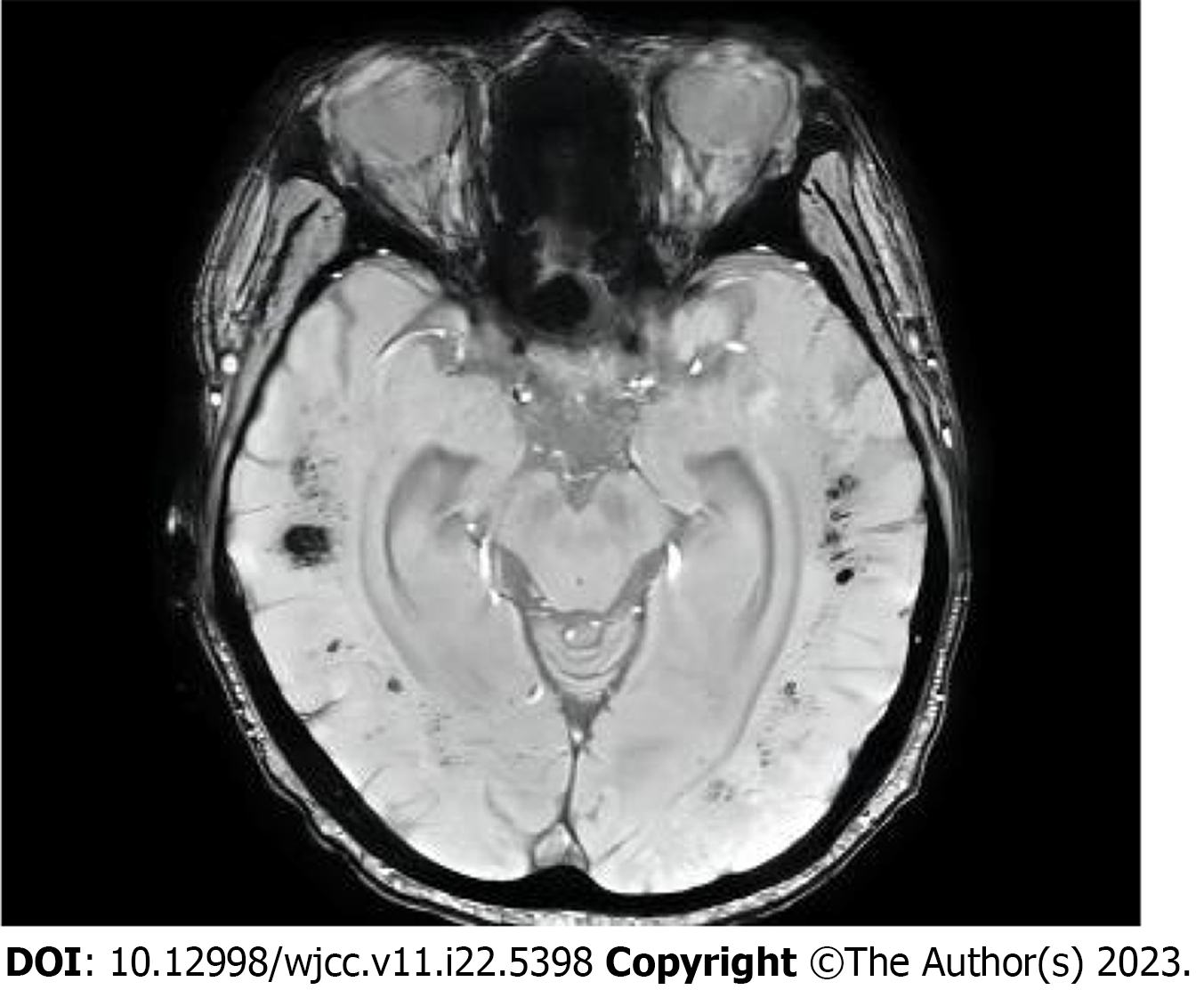
Myasthenia gravis was suspected, but the immune-related antibody was negative. Strenuous exercise, fatigue, drinking, and sedative drugs may induce respiratory failure in patients with mitochondrial myopathy. This patient had a cold and fatigue 3 d before admission, which could be the primary inducement of the disease. MELAS is a multisystem disease with various neurological, muscular, liver, gastrointestinal, and other symptoms. It leads to exercise intolerance, cramps, and fatigue. Proximal myasthenia is the primary manifestation of myopathy, and progressive exacerbation involves the respiratory muscle and the diaphragm. The main diagnoses of the patient were as follows: (1) Clinical manifestations in the nervous system, eye symptoms, and metabolic disorders; (2) Lactic acidosis; (3) Abnormal mitochondrial morphology in muscle biopsy and ultrastructural examination; and (4) Mutated nuclear or mitochondrial DNA. However, there was no history of seizures or stroke-like symptoms. Cranial magnetic resonance imaging did not show characteristic manifestations of MELAS-like lesions (Figure 3). Therefore, MELAS syndrome was considered unlikely.
Typical morphological findings are broken red muscle fibres that can be visualized using Gomori triple staining, especially when ragged red fibers > 4%-10%, with great diagnostic significance[15]. The findings in the patient’s muscle biopsy were suggestive of mitochondrial myopathy but not pathognomonic for MELAS. However, it has been reported that muscle biopsy results can be normal in patients with mitochondrial myopathy[16].
Genetic testing indicated a mitochondrial TRNL1 3243A>G mutation. Mitochondrial disease caused by the m.3243A>G mutation is associated with complex and highly variable types of clinical phenotypes. It can involve various tissues and organs, including the nervous system, skeletal muscle and myocardium, ear, eye, kidney, liver, and endocrine system. However, in most cases, it presents with only a single symptom. On the other hand, it may be associated with other diseases or even present as sudden death of unknown cause[17].
On admission, the patient was in critical condition due to sepsis, with hyperlactatemia, respiratory insufficiency, and circulatory failure (table) within a few hours, mainly due to mitochondrial disease with sepsis, excessive mitochondrial oxidative stress, and mitochondrial crisis[18]. Zhou et al[6] reported three cases of severe mitochondrial myopathy. One patient died of cardiac arrest at the first onset, and the other two patients were treated for a long time after surviving the metabolic crisis. Pan et al[19] summarized the clinical characteristics of five patients with respiratory failure due to the m.3243A>G mutation. They found that acute respiratory failure was the first manifestation of type II respiratory failure and restrictive ventilation dysfunction.
At present, there is no effective or gene-modified treatment for most patients with mitochondrial myopathy. Therefore, the existing regimens focus on symptomatic support. Several clinical trials have studied the therapeutic effects of various vitamins, adjuvant factors, and small molecules. However, they have not provided clear results[20-23]. Recent research shows that new gene and molecular therapy strategies, especially mitochondrial zinc finger nucleases[24] and mitochondrially targeted transcription activator-like effector nuclease[16], create cell lines carrying different pathogenic mito-chondrial DNA mutations that produce beneficial heterogeneous metastasis, providing hope for the future. In addition, recent advances in reproductive selection in patients with mitochondrial myopathy facilitate some families to prevent the transmission of mutation to the next generation.
Methylprednisolone was given because the patient had responded favourably to it in the past. We can only speculate why it was effective or whether adrenal insufficiency was present. For patients with specific mitochondrial crisis, IVMP and IVIG may be effective. They both ameliorate oxidative stress, are both therapeutic agents for idiopathic myopathy, and can also regulate immunity[25-27]. Subsequent treatment of infection and organ support with mechanical ventilation, veno-arterial extracorporeal membrane oxygenation, continuous renal replacement therapy (CRRT), antibiotics, nutrition, and other supportive measures stabilized the patient’s condition in the acute phase, and he entered a more stable stage.
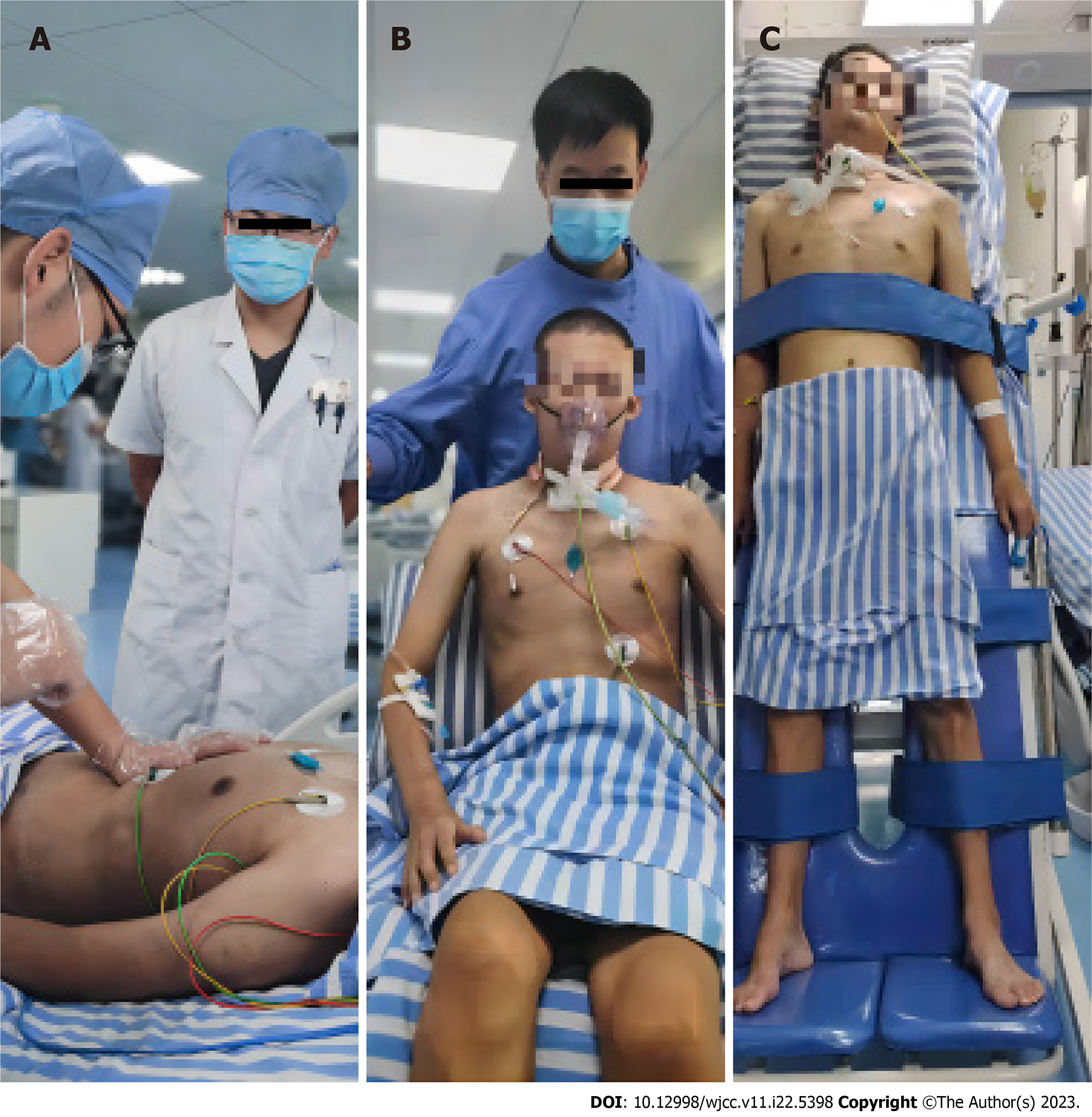
Over the next couple of days, the patient continuously stabilized (Table 1). He regained consciousness but had severe respiratory and limb muscle weakness. After more than 2 mo of intensive rehabilitation with functional active exercises (Figure 4), including diaphragmatic pacing, rehabilitation training, and muscle strength training, the patient could be disengaged from the ventilator and was eventually able to stand and walk again (Table 2).
| Days | Norepinephrine (µg/kg/min) | Epinephrine (µg/kg/min) | Lactic acid (mmol/L) | Blood pressure (mmHg) | ECMO |
| 1 | 18.2 | 1 | 31 | 70/30 | + |
| 2 | 10.8 | 0.5 | 27 | 110/70 | + |
| 3 | 3.2 | 0.1 | 15 | 120/65 | + |
| 4 | 1.8 | 0.05 | 11.5 | 125/73 | + |
| 5 | 0.16 | - | 9.2 | 130/75 | + |
| 6 | 0.04 | - | 6.8 | 140/75 | + |
| 7 | - | - | 3.4 | 120/73 | - |
| Days | Cervical muscle paralysis | Respiratory muscle paralysis | Proximal muscle strength | Distal muscle strength | Myalgia | Amyotrophy |
| On admission | - | - | 4 | 4 | - | - |
| 7 | + | + | 0 | 0 | + | - |
| 10 | + | + | 0 | 1 | + | - |
| 20 | - | + | 0 | 1-2 | + | + |
| 30 | - | + | 0 | 2 | + | + |
| 40 | - | + | 1-2 | 3 | + | + |
| 60 | - | - | 2-3 | 3-4 | - | + |
| 90 | - | - | 4 | 5 | - | + |
| 120 | Walk with assistance | |||||
| 180 | Walk independently | |||||
Although very rare, mitochondrial myopathy can present as a life-threatening disease. The diagnosis can be made by ruling out other causes of lactic acidosis and muscle weakness by taking a thorough family history and performing diagnostic tests such as muscle biopsy and genetic testing. Although there is currently no specific therapy available, supportive intensive care comprising steroid and vitamin therapy and extensive organ support (including extracorporeal membrane oxygenation, long-term mechanical ventilation, CRRT, and others) in combination with a dedicated rehabilitation team and program might result in favourable outcomes.
| 1. | She M, Huang M, Zhang J, Yan Y, Zhou L, Zhang M, Yang Y, Wang D. Astragulus embranaceus (Fisch.) Bge-Dioscorea opposita Thunb herb pair ameliorates sarcopenia in senile type 2 diabetes mellitus through Rab5a/mTOR-mediated mitochondrial dysfunction. J Ethnopharmacol. 2023;317:116737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 2. | Tinker RJ, Lim AZ, Stefanetti RJ, McFarland R. Current and Emerging Clinical Treatment in Mitochondrial Disease. Mol Diagn Ther. 2021;25:181-206. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 61] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 3. | Pitceathly RD, McFarland R. Mitochondrial myopathies in adults and children: management and therapy development. Curr Opin Neurol. 2014;27:576-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 4. | Attardi G, Yoneda M, Chomyn A. Complementation and segregation behavior of disease-causing mitochondrial DNA mutations in cellular model systems. Biochim Biophys Acta. 1995;1271:241-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 85] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 5. | Montano V, Gruosso F, Carelli V, Comi GP, Filosto M, Lamperti C, Mongini T, Musumeci O, Servidei S, Tonin P, Toscano A, Modenese A, Primiano G, Valentino ML, Bortolani S, Marchet S, Meneri M, Tavilla G, Siciliano G, Mancuso M. Primary mitochondrial myopathy: Clinical features and outcome measures in 118 cases from Italy. Neurol Genet. 2020;6:e519. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 6. | Zhou Y, Yi J, Liu L, Wang X, Dong L, Du A. Acute mitochondrial myopathy with respiratory insufficiency and motor axonal polyneuropathy. Int J Neurosci. 2018;128:231-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 7. | Singer M. The role of mitochondrial dysfunction in sepsis-induced multi-organ failure. Virulence. 2014;5:66-72. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 319] [Cited by in RCA: 428] [Article Influence: 32.9] [Reference Citation Analysis (0)] |
| 8. | Russell OM, Gorman GS, Lightowlers RN, Turnbull DM. Mitochondrial Diseases: Hope for the Future. Cell. 2020;181:168-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 312] [Article Influence: 52.0] [Reference Citation Analysis (0)] |
| 9. | Wallace DC, Fan W. The pathophysiology of mitochondrial disease as modeled in the mouse. Genes Dev. 2009;23:1714-1736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 158] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 10. | Gaieski DF, Goyal M. Serum lactate as a predictor of mortality in emergency department patients with infection: does the lactate level tell the whole story? Ann Emerg Med. 2005;46:561-2; author reply 562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 11. | Nichol A, Bailey M, Egi M, Pettila V, French C, Stachowski E, Reade MC, Cooper DJ, Bellomo R. Dynamic lactate indices as predictors of outcome in critically ill patients. Crit Care. 2011;15:R242. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 144] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 12. | Wong HR, Lindsell CJ, Pettilä V, Meyer NJ, Thair SA, Karlsson S, Russell JA, Fjell CD, Boyd JH, Ruokonen E, Shashaty MG, Christie JD, Hart KW, Lahni P, Walley KR. A multibiomarker-based outcome risk stratification model for adult septic shock*. Crit Care Med. 2014;42:781-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 99] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 13. | Nichol AD, Egi M, Pettila V, Bellomo R, French C, Hart G, Davies A, Stachowski E, Reade MC, Bailey M, Cooper DJ. Relative hyperlactatemia and hospital mortality in critically ill patients: a retrospective multi-centre study. Crit Care. 2010;14:R25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 208] [Cited by in RCA: 261] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 14. | Wacharasint P, Nakada TA, Boyd JH, Russell JA, Walley KR. Normal-range blood lactate concentration in septic shock is prognostic and predictive. Shock. 2012;38:4-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 126] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 15. | Mito T, Vincent AE, Faitg J, Taylor RW, Khan NA, McWilliams TG, Suomalainen A. Mosaic dysfunction of mitophagy in mitochondrial muscle disease. Cell Metab. 2022;34:197-208.e5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 67] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 16. | Ahmed ST, Craven L, Russell OM, Turnbull DM, Vincent AE. Diagnosis and Treatment of Mitochondrial Myopathies. Neurotherapeutics. 2018;15:943-953. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 105] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 17. | Li D, Liang C, Zhang T, Marley JL, Zou W, Lian M, Ji D. Pathogenic mitochondrial DNA 3243A>G mutation: From genetics to phenotype. Front Genet. 2022;13:951185. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 33] [Reference Citation Analysis (0)] |
| 18. | San-Millán I. The Key Role of Mitochondrial Function in Health and Disease. Antioxidants (Basel). 2023;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 131] [Reference Citation Analysis (0)] |
| 19. | Pan X, Wang L, Fei G, Dong J, Zhong C, Lu J, Jin L. Acute Respiratory Failure Is the Initial Manifestation in the Adult-Onset A3243G tRNALeu mtDNA Mutation: A Case Report and the Literature Review. Front Neurol. 2019;10:780. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 20. | de Barcelos IP, Emmanuele V, Hirano M. Advances in primary mitochondrial myopathies. Curr Opin Neurol. 2019;32:715-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 21. | Rodriguez MC, MacDonald JR, Mahoney DJ, Parise G, Beal MF, Tarnopolsky MA. Beneficial effects of creatine, CoQ10, and lipoic acid in mitochondrial disorders. Muscle Nerve. 2007;35:235-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 197] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 22. | Parikh S, Saneto R, Falk MJ, Anselm I, Cohen BH, Haas R, Medicine Society TM. A modern approach to the treatment of mitochondrial disease. Curr Treat Options Neurol. 2009;11:414-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 253] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 23. | DiMauro S, Mancuso M. Mitochondrial diseases: therapeutic approaches. Biosci Rep. 2007;27:125-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 83] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 24. | Gammage PA, Gaude E, Van Haute L, Rebelo-Guiomar P, Jackson CB, Rorbach J, Pekalski ML, Robinson AJ, Charpentier M, Concordet JP, Frezza C, Minczuk M. Near-complete elimination of mutant mtDNA by iterative or dynamic dose-controlled treatment with mtZFNs. Nucleic Acids Res. 2016;44:7804-7816. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 100] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 25. | MICKS RH, MULLANEY J. Dermatomyositis successfully treated by prednisone. Ir J Med Sci. 1958;333-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 26. | Ernste FC, Reed AM. Idiopathic inflammatory myopathies: current trends in pathogenesis, clinical features, and up-to-date treatment recommendations. Mayo Clin Proc. 2013;88:83-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 121] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 27. | Ben Mkaddem S, Aloulou M, Benhamou M, Monteiro RC. Role of FcγRIIIA (CD16) in IVIg-mediated anti-inflammatory function. J Clin Immunol. 2014;34 Suppl 1:S46-S50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bernardes A, Portugal; Juneja D, India S-Editor: Lin C L-Editor: Wang TQ P-Editor: Ji MX