Published online Aug 6, 2023. doi: 10.12998/wjcc.v11.i22.5322
Peer-review started: March 20, 2023
First decision: June 15, 2023
Revised: June 28, 2023
Accepted: July 7, 2023
Article in press: July 7, 2023
Published online: August 6, 2023
Processing time: 135 Days and 19.6 Hours
The development of anaplastic lymphoma kinase (ALK)-tyrosine kinase inhibitors (TKIs) has remarkably improved the prognosis of patients with ALK-positive advanced non-small cell lung cancer (NSCLC). Alectinib, the second-generation ALK-TKI, has been approved as first-line treatment for advanced or metastatic NSCLC patients with ALK rearrangement. Neoadjuvant therapy can achieve tumor downstaging and eradicate occult lesions in patients with potentially resectable disease. Whether neoadjuvant alectinib can be a conversion therapy in ALK-positive advanced NSCLC patients remains unclear.
A 41-year-old man was pathologically diagnosed with locally advanced ALK-positive stage IIIB NSCLC. Alectinib was prescribed to induce tumor down
This case sheds light on the feasibility and safety of alectinib as a neoadjuvant treatment for stage IIIB NSCLC patients with ALK rearrangement. Its efficacy needs to be validated in prospective clinical trials.
Core Tip: Whether neoadjuvant alectinib can serve as a conversion therapy in patients with anaplastic lymphoma kinase (ALK)-positive advanced non-small-cell lung cancer (NSCLC) remains unclear. We report the first case of ALK-positive stage IIIB NSCLC in which a pathological complete response was achieved after neoadjuvant alectinib therapy. Subsequently, left upper lobectomy with mediastinal lymphadenectomy was performed. Postoperatively, alectinib is being continued as adjuvant therapy with a recurrence-free survival of 29 mo as of date. This case report highlights the feasibility of alectinib as neoadjuvant therapy for unresectable ALK-positive locally advanced NSCLC.
- Citation: Wang LM, Zhao P, Sun XQ, Yan F, Guo Q. Pathological complete response to neoadjuvant alectinib in unresectable anaplastic lymphoma kinase positive non-small cell lung cancer: A case report. World J Clin Cases 2023; 11(22): 5322-5328
- URL: https://www.wjgnet.com/2307-8960/full/v11/i22/5322.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i22.5322
Lung cancer is the leading cause of cancer-related mortality worldwide, and non-small-cell lung cancer (NSCLC) accounts for approximately 85% cases of lung cancer[1]. Although curative resection is the optimal treatment for NSCLC, only 25% of these patients are eligible for surgical resection owing to delayed diagnosis. Compared with patients who are ineligible for surgery, NSCLC patients receiving surgical resection show significant survival benefit[2].
Neoadjuvant therapy refers to the administration of chemotherapy or radiotherapy before surgical resection of malignant tumors. With the advances in systemic and locoregional treatment modalities (such as targeted drugs and radiotherapy), neoadjuvant therapy has expanded the indications for surgical resection in cancer patients. Effective neoadjuvant therapy can reduce tumor size, achieve tumor downstaging, and eliminate occult micrometastases, thus improving surgical outcomes and prolonging the survival of patients with locally advanced NSCLC. Compared to clinical studies on neoadjuvant treatments for stage IIIA NSCLC, few studies have explored downstaging treatment regimens for patients with stage IIIB NSCLC[3]. Approximately 5%–6% of NSCLC patients have anaplastic lymphoma kinase (ALK) rearrangements, among which echinoderm microtubule-associated protein-like 4-ALK variant (EML4-ALK) is the most common fusion type[4]. EML4-ALK fusion protein is the therapeutic target for ALK-tyrosine kinase inhibitors (TKIs), and has shown promising results in NSCLC patients with ALK rearrangements[5]. In recent years, ALK inhibitors have shown better therapeutic efficacy compared to conventional chemotherapy in patients with ALK-positive NSCLC. Alectinib, a highly selective second-generation ALK inhibitor, is approved as a first-line therapy for ALK-positive advanced NSCLC. Here, we presented the application of neoadjuvant alectinib in a patient with ALK-positive locally advanced NSCLC (stage IIIB-N3), where the tumor was radically resected after downstaging and pathological complete response (pCR) was achieved.
A 41-year-old Chinese man presented to our hospital because of detection of pulmonary nodules in another hospital.
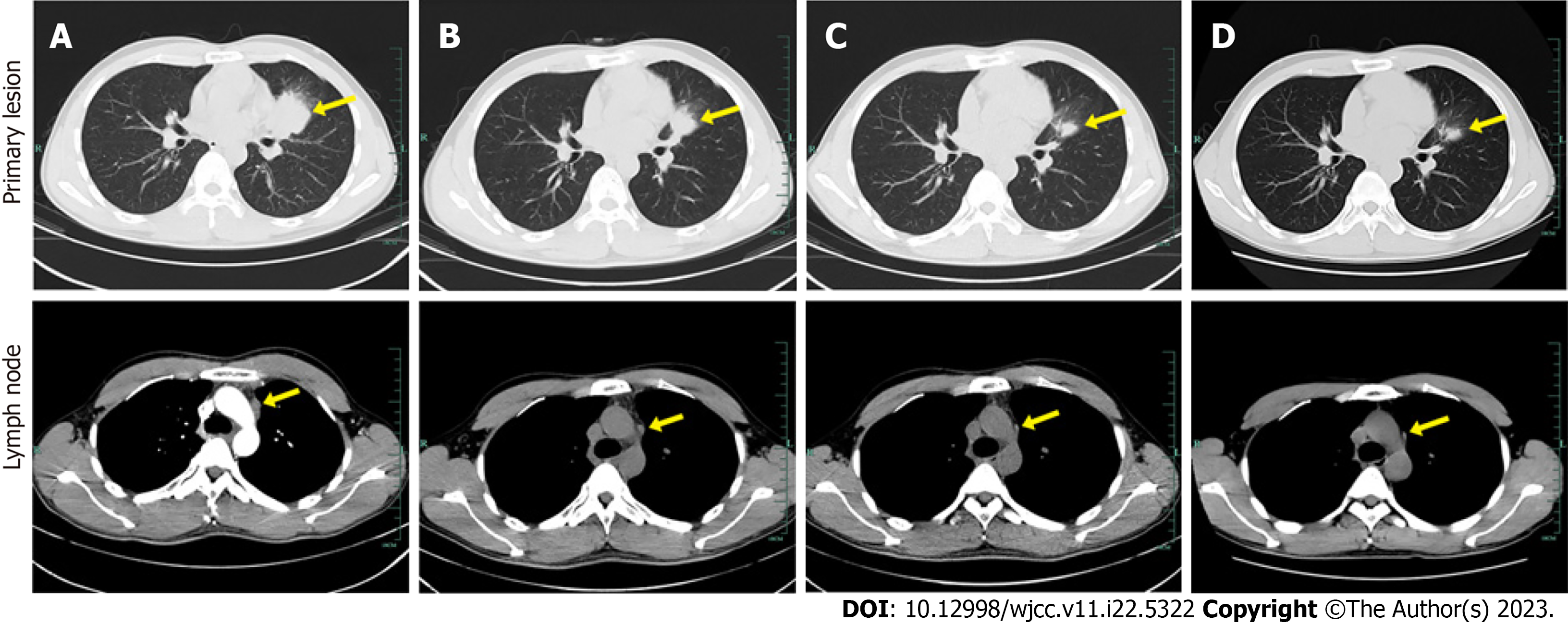
In August 2019, pulmonary nodules were detected on routine physical examination. He was preliminarily diagnosed with locally advanced NSCLC by bronchoalveolar lavage in a local hospital. Then the patient visited our hospital for further management. Chest computed tomography (CT) displayed a 39 mm × 34 mm lesion in the upper lobe of the left lung near the pulmonary hilum (Figure 1A). A CT-guided percutaneous biopsy was performed in the left lung neoplasm, and immunohistochemical examination showed the neoplasm was ALK positive.
The personal and family history was unremarkable. There was no history of underlying diseases (such as hypertension) or smoking. He denied any family history of malignant tumors.
On physical examination, the vital signs were as follows: Body temperature, 36.6 °C; blood pressure, 130/80 mmHg; heart rate, 80 beats per min; respiratory rate, 20 breaths per min. Heart, lung, and abdominal examinations showed no remarkable changes.
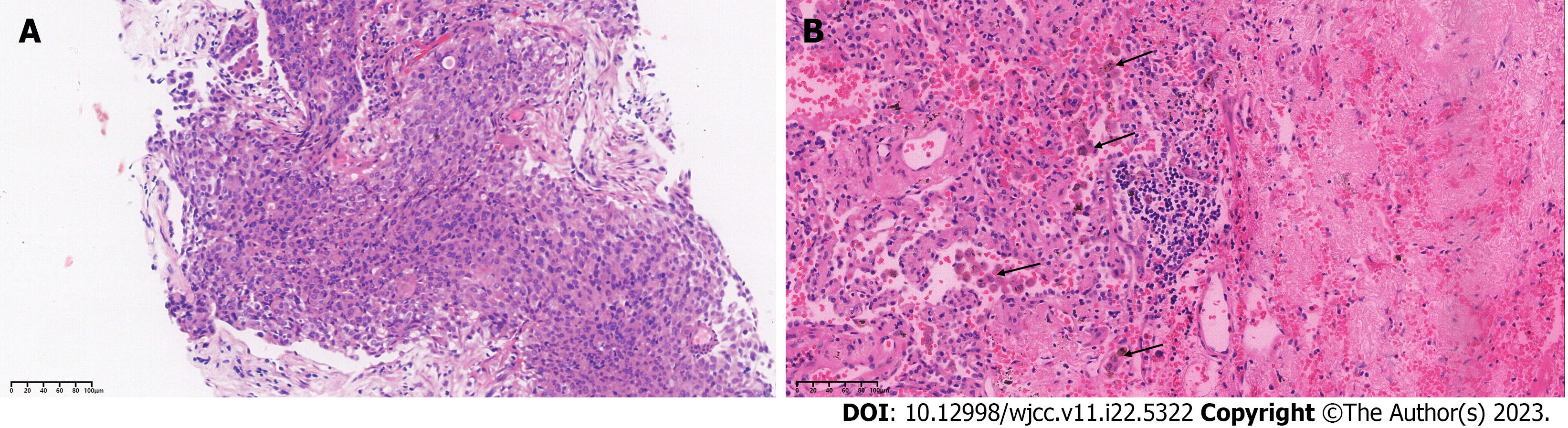
On immunohistochemical examination, the neoplasm was positive for CK7, TTF-1, and Napsina A, and negative for p40 and CK5/6. ALK rearrangement was detected by both immunohistochemistry and next-generation sequencing, which indicated EML4-ALK (E13:A20; abundance 17.1%) rearrangement (Figure 2A).
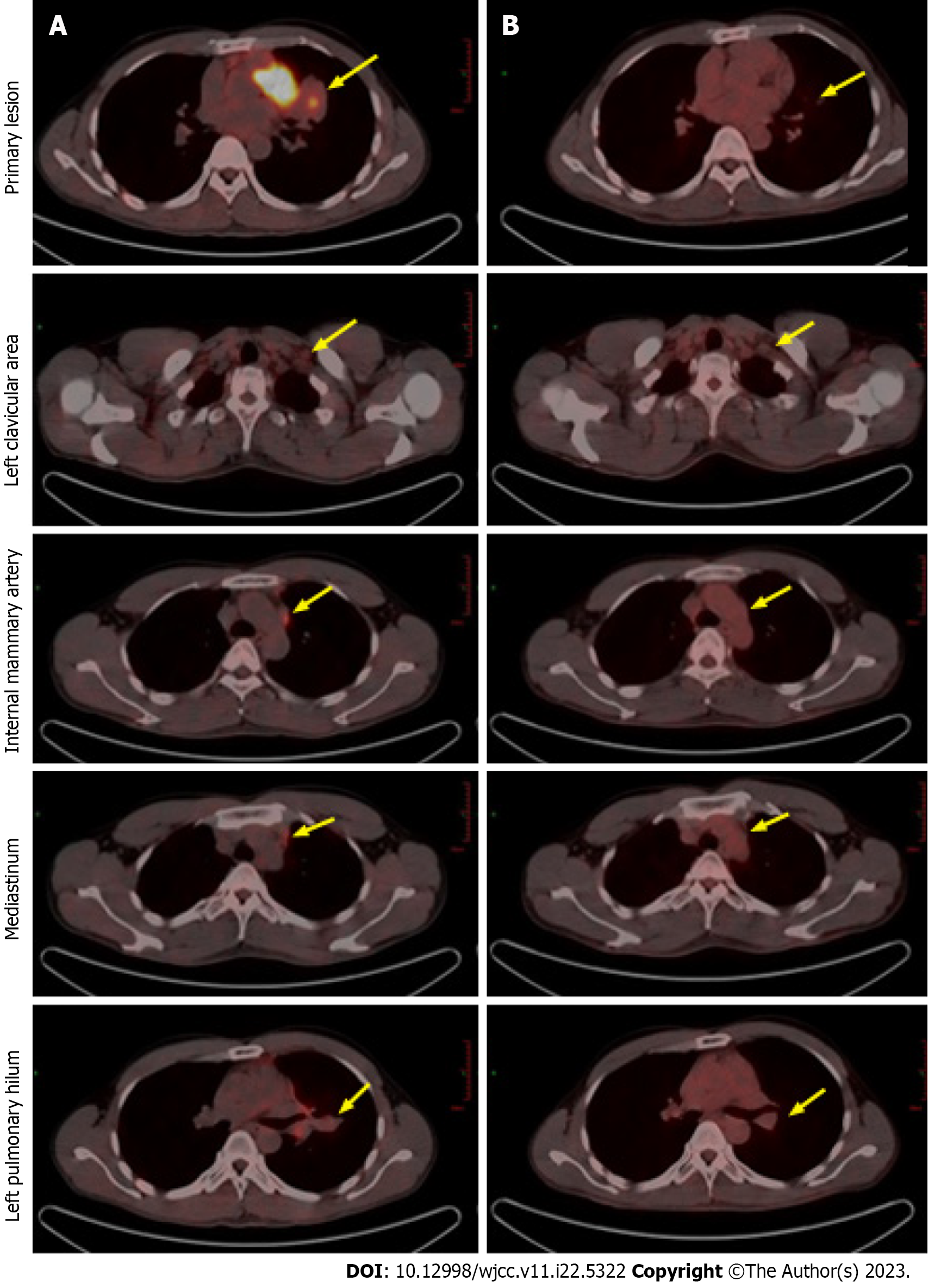
Positron emission tomography (PET) revealed multiple metastatic lymph nodes located in left clavicular, mediastinal, and left hilar region, with no distant metastasis (Figure 3A).
Based on the recommendations of multidisciplinary consultation and willingness of the patient, neoadjuvant alectinib was prescribed to induce tumor downstaging and facilitate the subsequent surgical resection.
Locally advanced ALK-positive stage IIIB-N3 NSCLC.
Based on the recommendations of multidisciplinary consultation and willingness of the patient, neoadjuvant alectinib was prescribed to induce tumor downstaging and facilitate the subsequent surgical resection. From September 17, 2019, the patient received 600 mg alectinib twice per day. After 10 wk, the patient showed a partial response (PR) at the first radiologic evaluation with the size of target lesion shrinking to 27 mm × 24 mm (Figure 1B). Routine biochemical screening detected asymptomatic elevation of liver enzymes (serum alanine aminotransferase: 118 U/L; serum aspartate aminotransferase: 71 U/L). Therefore, the patient received hepatoprotective therapy with diammonium glycyrrhizinate at a dose of 150 mg tid for two weeks, then serum liver enzymes became normal. Repeat radiological assessments conducted regularly during treatment showed constant PR. In June 2020, the size of the primary lesion had decreased to 15 mm × 10 mm (Figure 1C). A second multidisciplinary discussion yielded the following two recommendations: (1) Continuation of alectinib; or (2) suspending the usage of alectinib and receiving surgical resection. After through consideration, the patient opted for continuation of alectinib with close radiological surveillance. On follow-up chest CT examination in November 2020, only a slight change was found in the tumor size (12 mm × 11 mm) (Figure 1D). Compared with baseline PET-CT, the PET-CT performed at 14 mo after initiation of alectinib showed significant reduction in tumor size and uptake of 18F-fluorodeoxyglucose as well as disappearance of metastatic hypermetabolic lymph nodes (Figure 3). Owing to concerns about drug resistance, video-assisted thoracoscopic surgery was performed for left upper lobectomy with mediastinal lymph node dissection at two weeks after discontinuation of alectinib. After an uneventful postoperative course, the patient was discharged on the fifth day after operation. Histopathological examination of the surgical specimen showed no viable tumor cell, indicating pCR. Interstitial vascular and fibrous tissue hyperplasia, eosinophil infiltration, and chronic inflammation were detected in the lung tissue (Figure 2B).
Postoperatively, the patient has continued to receive adjuvant alectinib till date. The recurrence-free survival as of writing this report is more than 29 mo.
Lung cancer is one of the most common malignancies worldwide in terms of morbidity and mortality. Stage IIIB NSCLC, also known as inoperable locally advanced NSCLC, is a highly heterogenous disease with a poor prognosis. Approximately 20% of lung cancer patients are initially diagnosed at this stage[6]. For years, concurrent chemoradiotherapy has been the standard treatment for inoperable locally advanced NSCLC. However, the prognosis remains dismal with the median progression-free survival (PFS) of 8–12 mo and the 5-year overall survival rate of 15%–25%[6]. Since ALK-TKI treatment was proven superior to chemotherapy for advanced ALK-positive NSCLC, the indications for ALK-TKI have tentatively been extended to the neoadjuvant settings[7]. This case report describes the application of neoadjuvant alectinib in a patient with ALK-positive locally advanced NSCLC (stage IIIB-N3), where the tumor was radically resected after downstaging and pCR was achieved.
ALK inhibitors have become the first-line treatment for advanced or metastatic ALK-positive NSCLC. Alectinib, a novel ALK TKI, has shown good efficacy and safety as first-line treatment in patients with advanced ALK-positive NSCLC[7-9]. The majority of available data regarding targeted drugs as neoadjuvant therapy are limited to patients with epidermal growth factor receptor (EGFR)-mutated NSCLC[10,11]. There is a paucity of evidence on the feasibility and safety of ALK inhibitors as neoadjuvant treatments in ALK-positive NSCLC patients (Table 1). Zhang et al[12] first reported 11 cases of pathologically confirmed N3 ALK-positive NSCLC treated with neoadjuvant crizotinib followed by surgical resection, among whom 10 patients achieved R0 resection and 2 patients achieved pCR. Zhang et al[13] reported a patient with ALK-positive stage IIIB NSCLC in whom tumor downstaging (to stage Ib) was achieved after two cycles of neoadjuvant alectinib; the patient subsequently underwent radical surgical resection and a partial response (PR) was achieved with a tumor shrinkage of 47%. Yue et al[14] reported a patient with stage IIIA ALK-positive NSCLC in whom tumor shrinkage of 42.2% was achieved after a single cycle of neoadjuvant alectinib therapy. PR was achieved without any adverse events, although the tumor stage did not downgrade and major pathologic response (MPR) was not achieved. Leonetti et al[15] performed a phase II multicenter study to evaluate the efficacy and safety of neoadjuvant alectinib in resectable ALK-positive NSCLC. They reported a patient with stage IIIA ALK-positive NSCLC who received two cycles of neoadjuvant alectinib followed by surgery and achieved a MPR. Recently, Hu et al[16] reported a case of stage IIIA resectable ALK-positive NSCLC in which pCR was achieved with neoadjuvant aletinib. In the present case, neoadjuvant alectinib was prescribed to a patient with unresectable ALK-positive stage IIIB-N3 NSCLC, and the tumor was radically resected after successful downstaging. To the best of our knowledge, this is the first reported case of unresectable ALK-positive NSCLC in which pCR was achieved after receiving alectinib as neoadjuvant therapy.
| Zhang et al[13] | Yue et al[14] | Leonetti et al[15] | Hu et al[16] | Present case | |
| Age/gender | 46/male | 51/male | 62/male | 58/female | 41/male |
| Symptoms | Cough and hemoptysis | None | NA | Hemoptysis | None |
| Smoking status | Nonsmoker | Nonsmoker | Former smoker | Nonsmoker | Nonsmoker |
| Location | Left lower lobe | Right upper lobe | Left upper lobe | Right lower lobe | Left upper lobe |
| Tumor size (cm) | 6.6 | 3.1 | NA | 4.2 | 3.9 |
| Baseline cTNM | cIIIB (cT3N2M0) | cIIIA (cT2N2M0) | cIIIA (cT2aN2M0) | cIIIA (cT2bN2M0) | cIIIB (cT2aN3M0) |
| Operable/inoperable | Inoperable | Operable | Operable | Operable | Inoperable |
| Cycles | 2 | 1 | 2 | 2 | 14 |
| Radiologic response | PR | PR | PR | PR | PR |
| Pathologic response | Non-MPR | Non-MPR | MPR | pCR | pCR |
| Downstaging after alectinib therapy | Yes | No | Yes | Yes | Yes |
| Adverse effects | Grade 1 constipation | None | None | Grade 1 constipation; Grade 1 erythema | Grade 1 abnormal liver function |
| Follow-up | NA | Free of disease for 6 mo | NA | Free of disease for 8 mo | Free of disease for 26 mo |
Although the incidence of pCR is very low (approximately 5%) after neoadjuvant chemotherapy in resectable NSCLC, successful downstaging and pCR were achieved in this ALK-positive stage IIIB-N3 NSCLC patient after neoadjuvant alectinib[17]. The patient continued to receive 600 mg alectinib twice daily with good medication compliance. The PFS in this case (up to 29 mo) is longer than that in previously reported cases. Some plausible explanations for this good prognosis are as follows: (1) Sufficient preoperative dosing schedule: the patient received neoadjuvant alectinib for 14 mo which was significantly longer than that in previously reported cases; (2) achievement of pCR, which is known to confer a longer survival time in NSCLC patients after neoadjuvant treatments[18]; (3) Infiltration of activated eosinophils detected in pathological specimens (Figure 2B). Studies have shown that chemokines secreted by eosinophils can induce recruitment of CD8+T cells into tumor tissues. In addition, activated eosinophils can remodel the tumor microenvironment by inducing macrophage polarization and normalizing the tumor vasculature, which ultimately promotes elimination of tumor cells[19]. Based on our experience, we speculate that alectinib targeted therapy may have induced eosinophilia and thus promoted CD8+T recruitment, which may be one of the potential mechanisms of pCR in this patient.
This is the first case report describing the achievement of pCR in a patient with ALK-positive stage IIIB-N3 NSCLC after neoadjuvant alectinib. This case highlights the feasibility of alectinib as neoadjuvant therapy for unresectable ALK-positive locally advanced NSCLC. Further clinical trials are warranted to confirm these findings.
| 1. | Wen M, Wang X, Sun Y, Xia J, Fan L, Xing H, Zhang Z, Li X. Detection of EML4-ALK fusion gene and features associated with EGFR mutations in Chinese patients with non-small-cell lung cancer. Onco Targets Ther. 2016;9:1989-1995. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 2. | David EA, Canter RJ, Chen Y, Cooke DT, Cress RD. Surgical Management of Advanced Non-Small Cell Lung Cancer Is Decreasing But Is Associated With Improved Survival. Ann Thorac Surg. 2016;102:1101-1109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 3. | Yeh J, Marrone KA, Forde PM. Neoadjuvant and consolidation immuno-oncology therapy in stage III non-small cell lung cancer. J Thorac Dis. 2018;10:S451-S459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 4. | Kwak EL, Bang YJ, Camidge DR, Shaw AT, Solomon B, Maki RG, Ou SH, Dezube BJ, Jänne PA, Costa DB, Varella-Garcia M, Kim WH, Lynch TJ, Fidias P, Stubbs H, Engelman JA, Sequist LV, Tan W, Gandhi L, Mino-Kenudson M, Wei GC, Shreeve SM, Ratain MJ, Settleman J, Christensen JG, Haber DA, Wilner K, Salgia R, Shapiro GI, Clark JW, Iafrate AJ. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med. 2010;363:1693-1703. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3613] [Cited by in RCA: 3574] [Article Influence: 223.4] [Reference Citation Analysis (0)] |
| 5. | Shaw AT, Yeap BY, Solomon BJ, Riely GJ, Gainor J, Engelman JA, Shapiro GI, Costa DB, Ou SH, Butaney M, Salgia R, Maki RG, Varella-Garcia M, Doebele RC, Bang YJ, Kulig K, Selaru P, Tang Y, Wilner KD, Kwak EL, Clark JW, Iafrate AJ, Camidge DR. Effect of crizotinib on overall survival in patients with advanced non-small-cell lung cancer harbouring ALK gene rearrangement: a retrospective analysis. Lancet Oncol. 2011;12:1004-1012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 709] [Cited by in RCA: 721] [Article Influence: 48.1] [Reference Citation Analysis (0)] |
| 6. | Cheema PK, Rothenstein J, Melosky B, Brade A, Hirsh V. Perspectives on treatment advances for stage III locally advanced unresectable non-small-cell lung cancer. Curr Oncol. 2019;26:37-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 53] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 7. | Hida T, Nokihara H, Kondo M, Kim YH, Azuma K, Seto T, Takiguchi Y, Nishio M, Yoshioka H, Imamura F, Hotta K, Watanabe S, Goto K, Satouchi M, Kozuki T, Shukuya T, Nakagawa K, Mitsudomi T, Yamamoto N, Asakawa T, Asabe R, Tanaka T, Tamura T. Alectinib vs crizotinib in patients with ALK-positive non-small-cell lung cancer (J-ALEX): an open-label, randomised phase 3 trial. Lancet. 2017;390:29-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 555] [Cited by in RCA: 701] [Article Influence: 77.9] [Reference Citation Analysis (0)] |
| 8. | Shaw AT, Kim DW, Nakagawa K, Seto T, Crinó L, Ahn MJ, De Pas T, Besse B, Solomon BJ, Blackhall F, Wu YL, Thomas M, O'Byrne KJ, Moro-Sibilot D, Camidge DR, Mok T, Hirsh V, Riely GJ, Iyer S, Tassell V, Polli A, Wilner KD, Jänne PA. Crizotinib vs chemotherapy in advanced ALK-positive lung cancer. N Engl J Med. 2013;368:2385-2394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2534] [Cited by in RCA: 2734] [Article Influence: 210.3] [Reference Citation Analysis (0)] |
| 9. | Zhou C, Kim SW, Reungwetwattana T, Zhou J, Zhang Y, He J, Yang JJ, Cheng Y, Lee SH, Bu L, Xu T, Yang L, Wang C, Liu T, Morcos PN, Lu Y, Zhang L. Alectinib vs crizotinib in untreated Asian patients with anaplastic lymphoma kinase-positive non-small-cell lung cancer (ALESIA): a randomised phase 3 study. Lancet Respir Med. 2019;7:437-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 203] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 10. | Rizvi NA, Rusch V, Pao W, Chaft JE, Ladanyi M, Miller VA, Krug LM, Azzoli CG, Bains M, Downey R, Flores R, Park B, Singh B, Zakowski M, Heelan RT, Shen R, Kris MG. Molecular characteristics predict clinical outcomes: prospective trial correlating response to the EGFR tyrosine kinase inhibitor gefitinib with the presence of sensitizing mutations in the tyrosine binding domain of the EGFR gene. Clin Cancer Res. 2011;17:3500-3506. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 60] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 11. | Zhong WZ, Chen KN, Chen C, Gu CD, Wang J, Yang XN, Mao WM, Wang Q, Qiao GB, Cheng Y, Xu L, Wang CL, Chen MW, Kang X, Yan W, Yan HH, Liao RQ, Yang JJ, Zhang XC, Zhou Q, Wu YL. Erlotinib Versus Gemcitabine Plus Cisplatin as Neoadjuvant Treatment of Stage IIIA-N2 EGFR-Mutant Non-Small-Cell Lung Cancer (EMERGING-CTONG 1103): A Randomized Phase II Study. J Clin Oncol. 2019;37:2235-2245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 212] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 12. | Zhang C, Li SL, Nie Q, Dong S, Shao Y, Yang XN, Wu YL, Yang Y, Zhong WZ. Neoadjuvant Crizotinib in Resectable Locally Advanced Non-Small Cell Lung Cancer with ALK Rearrangement. J Thorac Oncol. 2019;14:726-731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 74] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 13. | Zhang C, Yan LX, Jiang BY, Wu YL, Zhong WZ. Feasibility and Safety of Neoadjuvant Alectinib in a Patient With ALK-Positive Locally Advanced NSCLC. J Thorac Oncol. 2020;15:e95-e99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 14. | Yue P, Zhang S, Zhou L, Xiang J, Zhao S, Chen X, Dong L, Yang W, Xiang Y. Perioperative alectinib in a patient with locally advanced anaplastic lymphoma kinase positive non-small cell lung cancer (NSCLC): a case report. Transl Cancer Res. 2021;10:3856-3863. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 15. | Leonetti A, Minari R, Boni L, Gnetti L, Verzè M, Ventura L, Musini L, Tognetto M, Tiseo M. Phase II, Open-label, Single-arm, Multicenter Study to Assess the Activity and Safety of Alectinib as Neoadjuvant Treatment in Surgically Resectable Stage III ALK-positive NSCLC: ALNEO Trial. Clin Lung Cancer. 2021;22:473-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 16. | Hu Y, Ren S, Wang R, Han W, Xiao P, Wang L, Yu F, Liu W. Case Report: Pathological Complete Response to Neoadjuvant Alectinib in a Patient With Resectable ALK-Positive Non-Small Cell Lung Cancer. Front Pharmacol. 2022;13:816683. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 17. | Hellmann MD, Chaft JE, William WN Jr, Rusch V, Pisters KM, Kalhor N, Pataer A, Travis WD, Swisher SG, Kris MG; University of Texas MD Anderson Lung Cancer Collaborative Group. Pathological response after neoadjuvant chemotherapy in resectable non-small-cell lung cancers: proposal for the use of major pathological response as a surrogate endpoint. Lancet Oncol. 2014;15:e42-e50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 520] [Article Influence: 43.3] [Reference Citation Analysis (0)] |
| 18. | Betticher DC, Hsu Schmitz SF, Tötsch M, Hansen E, Joss C, von Briel C, Schmid RA, Pless M, Habicht J, Roth AD, Spiliopoulos A, Stahel R, Weder W, Stupp R, Egli F, Furrer M, Honegger H, Wernli M, Cerny T, Ris HB; Swiss Group for Clinical Cancer Research (SAKK). Prognostic factors affecting long-term outcomes in patients with resected stage IIIA pN2 non-small-cell lung cancer: 5-year follow-up of a phase II study. Br J Cancer. 2006;94:1099-1106. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 147] [Cited by in RCA: 168] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 19. | Carretero R, Sektioglu IM, Garbi N, Salgado OC, Beckhove P, Hämmerling GJ. Eosinophils orchestrate cancer rejection by normalizing tumor vessels and enhancing infiltration of CD8(+) T cells. Nat Immunol. 2015;16:609-617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 411] [Article Influence: 37.4] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bustamante-Lopez LA, Brazil; Chiu ML, United States; Rajer M, Slovenia S-Editor: Liu JH L-Editor: A P-Editor: Zhao S