Published online Jul 16, 2023. doi: 10.12998/wjcc.v11.i20.4890
Peer-review started: November 7, 2022
First decision: January 12, 2023
Revised: January 21, 2023
Accepted: June 13, 2023
Article in press: June 13, 2023
Published online: July 16, 2023
Processing time: 246 Days and 16.5 Hours
Acute spinal subdural haematoma (ASSH) is a rare and potentially devastating condition with a variable prognosis. Previously described subdural haematomas were thought to have occurred spontaneously or be related to major or minor iatrogenic or traumatic injuries caused by surgery, spinal puncture or epidural anaesthesia. Other contributing pathologies have been described, such as intradural tumours or spinal arteriovenous malformations. ASSH has also been associated with anticoagulation therapy, haemostatic abnormalities and risk factors such as pregnancy. To the best of our knowledge, this case study described the first reported occurrence of an ASSH during spinal surgery in a paediatric patient. The patient was not known to have any coagulopathies, and no obvious vascular lesions were documented. The surgical procedure did not directly involve the dura mater, and no evident intraoperative dural tears were found.
We reported and discussed a case of ASSH complicating a posterior spinal instrumented fusion during surgery for paediatric congenital scoliosis. This condition has not been previously described. We made recommendations for facing such an occurrence, explored its aetiology in the context of malformation and discussed the benefits of neuromonitoring during scoliosis correction and the management protocol. We conducted a PubMed literature review for cases of paediatric ASSH and other closely related disorders. We reviewed recommendations regarding neuromonitoring and treatment management in such cases.
ASSH is a rare complication of posterior spinal instrumented fusion. Published cases are more often associated with anticoagulation therapy or coagulopathy. Neuromonitoring is strongly recommended to detect and assess neurological status, thus enabling rapid diagnosis and treatment and facilitating early spinal decompression and a return to a normal neurological status.
Core Tip: Acute spinal subdural haematoma is a rare complication of posterior spinal instrumented fusion. The context of the spine deformity and associated congenital abnormalities, the use of neuromonitoring in such surgery and the management of acute spinal subdural haematoma are discussed. Published cases are more often associated with anticoagulation therapy or coagulopathy. Neuromonitoring is strongly recommended to detect and assess neurological status, enabling rapid diagnosis and treatment, thus allowing early spinal decompression and a return to a normal neurological status.
- Citation: Michon du Marais G, Tabard-Fougère A, Dayer R. Acute spinal subdural haematoma complicating a posterior spinal instrumented fusion for congenital scoliosis: A case report. World J Clin Cases 2023; 11(20): 4890-4896
- URL: https://www.wjgnet.com/2307-8960/full/v11/i20/4890.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i20.4890
Acute spinal subdural haematoma (ASSH) is a rare and potentially devastating condition with a variable prognosis. Previously described subdural haematomas were thought to have occurred spontaneously or be related to major or minor iatrogenic or traumatic injuries caused by surgery, spinal puncture or epidural anaesthesia. Other contributing pathologies have been described, such as intradural tumours or spinal arteriovenous malformations. ASSH has also been associated with anticoagulation therapy, haemostatic abnormalities and risk factors such as pregnancy[1-11].
Spontaneous postoperative spinal subdural bleeding has been described previously[5]. As with the present subject, who was not undergoing anticoagulation therapy and had no haemorrhagic disorders, acute bleeding should be considered when the clinical signs of acute spinal cord or cauda equina compression are observed. We described the case of a 14-year-old girl with an intraoperative ASSH complicating a posterior spinal instrumented fusion. We discussed the context of her spine deformity, her associated congenital abnormalities, the use of neuromonitoring during such surgery and the treatment management of ASSH.
A 14-year-old female presented with an intraoperative ASSH complicating a posterior spinal instrumented fusion.
The patient was not known to have any coagulopathies, and no obvious vascular lesions were documented. The surgical procedure did not directly involve the dura mater, and no evident intraoperative dural tears were found.
Concerning the haemostatic agent administered (as described in the literature)[1-11], the patient was not known to have had any coagulopathies or anticoagulant treatments, and all preoperative blood testing was normal.
Four months before admission for spinal surgery, the patient successfully underwent the neurosurgical decompression of a Chiari malformation with associated syringomyelia; this consisted of a sub-occipital craniotomy, a C1 laminectomy and duraplasty. At the 3-mo follow-up, the neurosurgeon noted no contraindications to future correction surgery for scoliosis. The paediatric orthopaedic team planned a posterior spinal instrumented fusion from T3 to L4 associated with multilevel, Ponte-type (posterior elements) osteotomies around the apex of the kyphosis.
The patient’s known comorbidities secondary to multiple congenital malformations included ventricular (ORPHA-1480) and atrial (ostium secundum type, ORPHA-99103) septal defects, tricuspid atresia (ORPHA-1209), a congenital partial pulmonary venous return anomaly (ORPHA-99124), a persistent left superior vena cava connecting through the coronary sinus to the left-sided atrium (ORPHA 99-109), a right aortic arch (ORPHA-99081), major aortopulmonary collateral arteries (MAPCAs), moderate-to-severe dilatation of the ascending aorta, severe dilatation of the left ventricle and moderate mitral insufficiency.
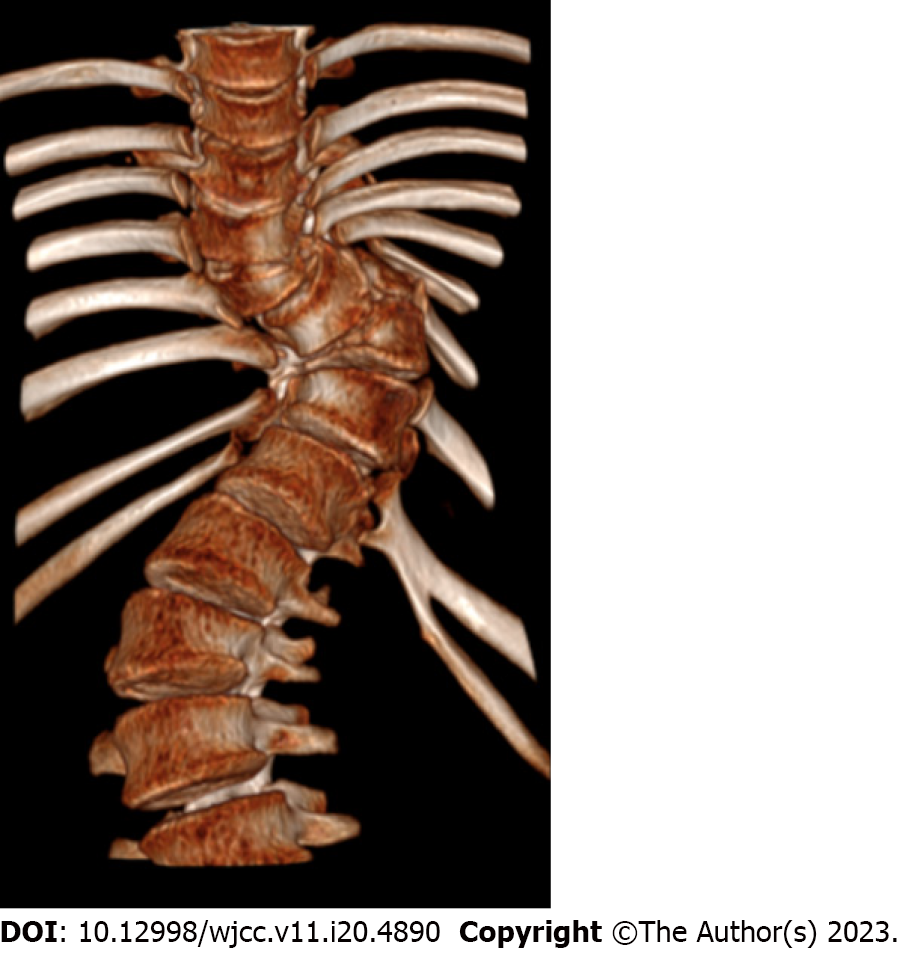
At the time of surgery, the triradiate cartilage was closed and the patient’s Risser sign was grade 1. The curves of the scoliosis consisted of a right proximal thoracic curve of 36°, a main left thoracic curve of 72° (the major curve) and a right thoracolumbar-lumbar curve of 58°. There was a T2-T12 hyperkyphosis of 68° in the sagittal plane (Figure 1).
A congenital Chiari malformation type I associated with a C5-D2 syringomyelia, a D6-D7 hemivertebra scoliosis and a D9 wedge-type vertebra.
Neuromonitoring was used intraoperatively. Two electrodes were placed over the cortex and the C3 position. Peripherally, a track to the hand was used as a control (abductor pollicis brevis). Electrodes were also inserted into the quadriceps, tibialis anterior and abductor digiti minimi of both lower limbs. Both motor evoked potentials (MEPs) and somatosensory evoked potentials (SSEPs) were recorded during the scoliosis surgery, with baseline plots established beforehand.
During the surgical procedure after the insertion of the pedicle screws but before any attempts at kyphotic or scoliosis deformity correction, the neurophysiologist alerted the surgical team about a significant decrease in MEPs, starting in the left foot (the abductor digiti minimi muscle) and progressing to the left quadriceps. Over 15 min, the surgical team witnessed a total bilateral loss of MEPs, followed by a decrease in SSEPs. Fluoroscopy (anteroposterior and lateral views) was used to ensure that there was no pedicle screw misplacement at any of the instrumented levels.
The surgical procedure was stopped immediately after the loss of MEP, and all the pedicle screws were removed without any noticeable local bleeding or cerebrospinal fluid effusion. After the removal of the screws, however, there was no recovery of MEPs and SSEPs. The neurosurgical team thus decided to perform a wake-up test. This showed a partial improvement in SSEPs and secondarily in MEPs, which reached a maximum of 30% of their baseline level. Following this critical neuromonitoring alert, the surgery was aborted, and the operative site was closed.
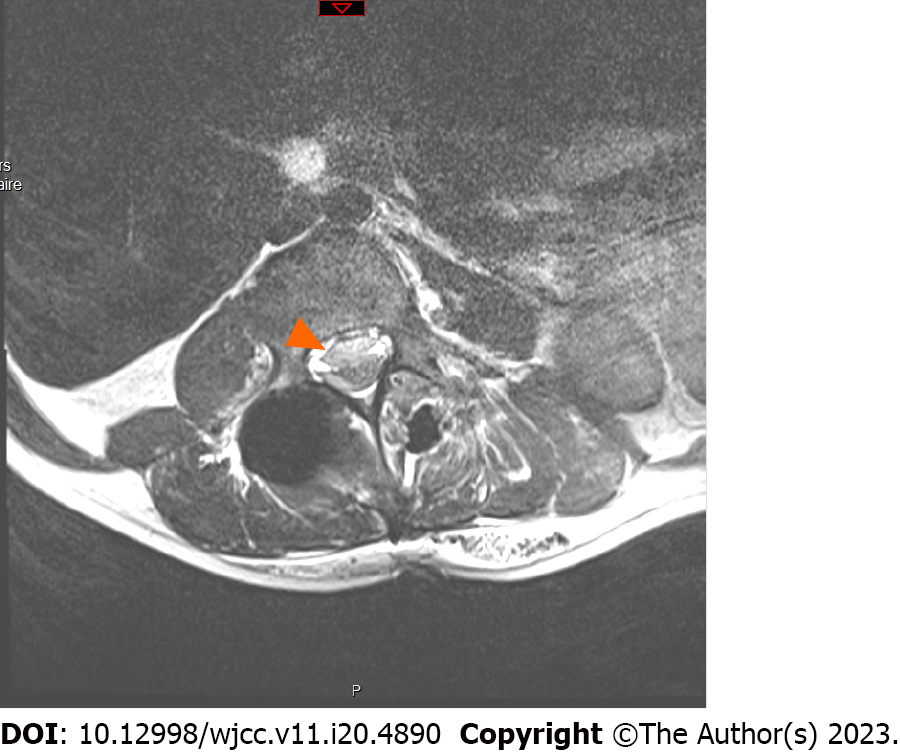
As recommended by the Scoliosis Research Society[12], an urgent magnetic resonance imaging scan of the complete neuraxis was performed while the patient was still under general anaesthesia. The images showed a compressive anterior subdural haematoma extending from D10 to L3 (Figure 2).
The neurosurgery team opted for a prompt open surgical drainage of the subdural haematoma, performing emergency bilateral L3 laminotomies, followed by a complete L3 laminectomy. An extended durotomy was carried out, allowing the haematoma to be drained and causing the immediate normalisation of the electrical MEPs. No active subdural bleeding was noted after the haematoma’s drainage. A Gore-Tex® duraplasty was performed.
After surgery, the patient was transferred to the paediatric intensive care unit for continuous monitoring and maintenance of high mean arterial pressure. Over the following day, her neurological status improved favourably from paraparesis to completely normal. At this point, she had recovered a normal neurological status in her lower limbs, with no motor and sensitivity deficits. A magnetic resonance imaging scan was not repeated before the second deformity surgery because of the return and subsequent persistence of a normal neurological status after drainage of the subdural haematoma.
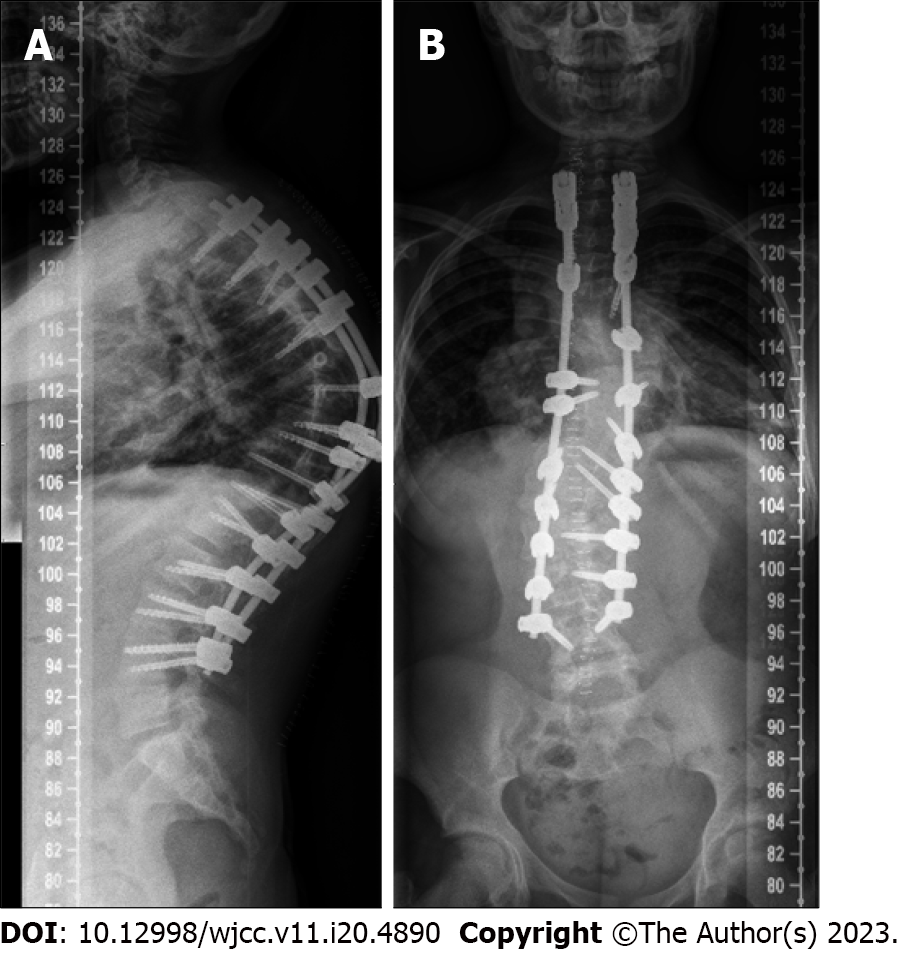
The posterior spinal instrumented fusion procedure was finally performed 7 d later. Pedicle screws were inserted on both sides, inferior facetectomies were performed from T3 to L3, and Ponte osteotomies were performed from T8 to T11.
Due to the transient loss of multiple MEPs on the right lower limb during our curve correction attempts, the correction of the kyphotic component was partially abandoned to focus on scoliotic correction in the coronal plane (Figure 3).
With no further signs of loss of MEPs or SSEPs, the procedure was completed, and the surgical site was closed. No intraoperative complications were noted apart from the transient loss of neuromonitoring; the postoperative neurological examination was normal.
No further complications were noted at the 3-mo and 2-year follow-ups. Although the initially planned correction of the kyphotic component had to be limited because urgent intraoperative neuromonitoring alerts triggered an additional surgical procedure, the end result was considered favourable by the patient and the surgical team.
To the best of our knowledge, this case study described the first reported occurrence of an ASSH during spinal surgery in a paediatric patient. The patient was not known to have any coagulopathies, and no obvious vascular lesions were documented. The surgical procedure did not directly involve the dura mater, and no evident intraoperative dural tears were found.
There was a complete return of MEPs during the neurosurgical procedure to relieve the spinal cord of the tension caused by the ASSH. An incidence of ASSH in a patient with no obvious pre-existing vascular lesions or coagulopathies can be considered rare[13].
Previous reports have suggested venous congestion followed by the rupture of radiculomedullary veins[14], in addition to various iatrogenic injuries[9,15,16], as possible causes of this type of bleeding. In this case, the aetiology was most likely an iatrogenic injury due to instrumentation or the pedicle screws, although this remains uncertain because no active bleeding or dural tears were visualized intraoperatively.
The Scoliosis Research Society[12] describes four causes of iatrogenic postoperative neurological deficit: (1) Mechanical compression of the spinal cord due to instrumentation or an epidural haematoma; (2) Compression by an infolding of a ligamentum flavum, a posterior longitudinal ligament or post-correction disc material; (3) Spinal cord distraction following instrumentation; and (4) Vascular insufficiency, without a mechanical component, resulting in a reduction in the blood supply to the cord.
In cases of paediatric spinal surgery, the spinal biomechanics differ from those of healthy adults, with a relative increase in ligament laxity. This laxity can affect the stability or competency of the facet joints. In the present case, the patient’s congenital spine malformations may have increased the risk of iatrogenic neurological injuries. These congenital malformations can also affect the patient’s biomechanics, increasing the risks of segmental instability or dynamic encroachment on the space available for the cord[12].
Hence, the patient’s paediatric status added with her congenital malformations complicated the analysis of the origins of the ASSH. Even if the precise cause of her ASSH is uncertain, the presence of congenital scoliosis itself could be considered a risk factor[12]. Congenital cardiac malformations also increase the risk of neurological damage by increasing the risk of blood clot formation and possible transient intraoperative hypotension.
Neurophysiological monitoring has been demonstrated to effectively assist spinal surgery through the early detection and possible prevention of postoperative complications. A retrospective analysis of 60366 surgical procedures, conducted by the Scoliosis Research Society and the European Spinal Deformity Society[17], found 364 cases of postoperative neurological deficit, 263 of which were revealed by SSEPs and 101 which were not identified using SSEP monitoring (i.e. false-negatives). The combined use of SSEP and MEP monitoring was proven effective and facilitated early response to spinal cord deficits[17,18]. A significant decrease in false-negative rates of cord deficits was described using bimodal neuromonitoring instead of unimodal neuromonitoring[19].
We chose not to continue the deformity correction surgery after draining the patient’s haematoma. We concluded that this was the safest option, considering her increased blood loss (with the potential risk of adding a secondary ischaemic spinal cord lesion) and surgeon fatigue (by the end of the haematoma drainage, it was 23:00), and the fact that a complete clinical recovery of her lower limbs, confirmed by a neurological examination, would be required before returning to the operation room. The downsides to this two-stage surgical strategy were a potential increase in the anaesthesia risk and the financial cost.
As reported in previously published studies, impaired neurological functions secondary to an ASSH can recover spontaneously[4,13]. A rapid redistribution of the haematoma, including its craniocaudal expansion within the subdural space, may occur without compromising the spinal cord, and this could explain such spontaneous recoveries. A relative dilution of the haematoma within the cerebrospinal fluid could also contribute to relieving spinal cord compression. Only a few patients, those with minimal neurological deficits, could be treated using a conservative approach[1]. We think it is reasonable to believe that a spontaneous recovery could only occur in a minority of patients with ASSH.
In cases of surgical decompression, prompt relief of the pressure in the subdural space using laminectomy and the evacuation of the haematoma is key to a fast and lasting neurological recovery. Moreover, in cases involving residual postoperative haemorrhages, the redistribution and dilution of blood could also contribute to a patient’s total neurological recovery.
In the present case, three key features were observed: (1) The rapid evolution of neuromonitoring data; (2) The short delays between the interruption of surgery, magnetic resonance imaging and neurosurgical decompression; and (3) The rapid, full neurological recovery.
Our management of the intraoperative neuromonitoring alert secondary to the ASSH was consistent with the Scoliosis Research Society’s recommendations[12]. Using bimodal SSEP and MEP monitoring was key to managing the ASSH promptly, shortening the time required for diagnosis and treatment and thus enhancing the chances of a full recovery.
According to one well-documented case report[4], it may be difficult to differentiate between purely subdural haemorrhages, where blood vessels are much more sporadic, and subdural-subarachnoid mixed haemorrhages. As another study suggested[13], the proportion of spinal subdural haematomas associated with subarachnoid haemorrhages might be underestimated. The proportion of spinal subdural haematomas associated with subarachnoid haemorrhages may even be as high as 25% of all spinal subdural haematomas[13,20].
In this case, the origin of the haemorrhage was likely to have been purely subdural because the clot was clearly visible in the subdural compartment and in the chronology of the evolving neurological deficit.
For cases involving specific mild neurological deficits, some authors[4,13,20-22] have suggested that a conservative approach is both appropriate and effective; however, there is no unanimous consensus on the optimal management of patients with an incomplete neurological deficit. However, patients who show signs of deterioration or a severe neurological deficit are more likely to benefit from percutaneous drainage or surgery[5,16,21].
Due to the present case’s special circumstances, severity and rapid progression, a conservative option was not considered. Future research, including comparable cases, will hopefully help to build more conclusive recommendations for the treatment of intraoperative ASSH.
ASSH is a rare complication of posterior spinal instrumented fusion. Published case studies are more often associated with anticoagulation therapy or coagulopathies. Neuromonitoring is strongly recommended to detect and assess neurological status, thus enabling rapid diagnosis and treatment, early spinal decompression and a return to a normal neurological status.
We would like to thank Mr. Darren Hart for the English language editing.
| 1. | Domenicucci M, Ramieri A, Ciappetta P, Delfini R. Nontraumatic acute spinal subdural haematoma: report of five cases and review of the literature. J Neurosurg. 1999;91:65-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 57] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 2. | Kim YH, Cho KT, Chung CK, Kim HJ. Idiopathic spontaneous spinal subarachnoid hemorrhage. Spinal Cord. 2004;42:545-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 3. | de Beer MH, Eysink Smeets MM, Koppen H. Spontaneous Spinal Subdural Hematoma. Neurologist. 2017;22:34-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 4. | Yang NR, Kim SJ, Cho YJ, Cho DS. Spontaneous resolution of nontraumatic acute spinal subdural hematoma. J Korean Neurosurg Soc. 2011;50:268-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 5. | Chang KC, Samartzis D, Luk KD, Cheung KM, Wong YW. Acute spinal subdural hematoma complicating lumbar decompressive surgery. Evid Based Spine Care J. 2012;3:57-62. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 6. | Tropeano MP, La Pira B, Pescatori L, Piccirilli M. Vertebroplasty and delayed subdural cauda equina hematoma: Review of literature and case report. World J Clin Cases. 2017;5:333-339. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 7] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 7. | Cha YH, Chi JH, Barbaro NM. Spontaneous spinal subdural hematoma associated with low-molecular-weight heparin. Case report. J Neurosurg Spine. 2005;2:612-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 8. | Nakamura T, Watanabe G, Harada R, Kawasaki E, Tsukita K, Suzuki Y. Acute Intracranial and Spinal Subdural Hematoma Associated with Vardenafil. J Stroke Cerebrovasc Dis. 2018;27:e201-e202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | Benyaich Z, Laghmari M, Lmejjati M, Aniba K, Ghannane H, Ait Benali S. Acute Lumbar Spinal Subdural Hematoma Inducing Paraplegia After Lumbar Spinal Manipulation: Case Report and Literature Review. World Neurosurg. 2019;128:182-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 10. | Boe CC, Freedman BA, Kumar R, Lee K, McDonald R, Port J. Spinal subdural hematoma: a rare case of spinal subdural hematoma complicating routine, minimally invasive lumbar discectomy and decompression and relevant literature review. J Spine Surg. 2017;3:112-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 11. | Yamada K, Nakahara T, Yamamato K, Muranaka T, Ushio Y. Nontraumatic spinal subdural haematoma occurring in a postpartum period. Acta Neurochir (Wien). 2003;145:151-5; discussion 155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 12. | Mummaneni PV; SRS Committee E. The Scoliosis Research Society (SRS) E-Text (Online). Milwaukee WI : Scoliosis Research Society - SRS. |
| 13. | Oh YM, Eun JP. Idiopathic spontaneous spinal subdural hematoma causing transient paraparesis: Case report with a review of the literature. Neurosurg Q. 2015;25:484-487. [DOI] [Full Text] |
| 14. | Mattei TA, Rehman AA, Dinh DH. Acute Spinal Subdural Hematoma after Vertebroplasty: A Case Report Emphasizing the Possible Etiologic Role of Venous Congestion. Global Spine J. 2015;5:e52-e58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 15. | Gehri R, Zanetti M, Boos N. Subacute subdural haematoma complicating lumbar microdiscectomy. J Bone Joint Surg Br. 2000;82:1042-1045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 26] [Reference Citation Analysis (0)] |
| 16. | Choi KB, Hwang BW, Lee SH. Postoperative Acute Spinal Subdural Hematoma - Report of Two Cases. Korean J Spine. 2010;7:90-95. |
| 17. | Dawson EG, Sherman JE, Kanim LE, Nuwer MR. Spinal cord monitoring. Results of the Scoliosis Research Society and the European Spinal Deformity Society survey. Spine (Phila Pa 1976). 1991;16:S361-S364. [PubMed] |
| 18. | Scoliosis Research Society. Neuromonitoring Information Statement: SRS Information Statement, 2019. [Accessed August 2022]. Available from: https://www.srs.org/about-srs/quality-and-safety/position-statements/neuromonitoring-information-statement. |
| 19. | Schwartz DM, Auerbach JD, Dormans JP, Flynn J, Drummond DS, Bowe JA, Laufer S, Shah SA, Bowen JR, Pizzutillo PD, Jones KJ. Neurophysiological detection of impending spinal cord injury during scoliosis surgery. J Bone Joint Surg Am. 2007;89:2440-2449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 149] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 20. | Oh SH, Han IB, Koo YH, Kim OJ. Acute spinal subdural hematoma presenting with spontaneously resolving hemiplegia. J Korean Neurosurg Soc. 2009;45:390-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 21. | Kyriakides AE, Lalam RK, El Masry WS. Acute spontaneous spinal subdural hematoma presenting as paraplegia: a rare case. Spine (Phila Pa 1976). 2007;32:E619-E622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 72] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 22. | Kang HS, Chung CK, Kim HJ. Spontaneous spinal subdural hematoma with spontaneous resolution. Spinal Cord. 2000;38:192-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Orthopedics
Country/Territory of origin: Switzerland
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gao W, China; Yang D, South Korea S-Editor: Gong ZM L-Editor: Filipodia P-Editor: Yuan YY