Published online Jul 6, 2023. doi: 10.12998/wjcc.v11.i19.4684
Peer-review started: February 28, 2023
First decision: May 8, 2023
Revised: May 16, 2023
Accepted: May 31, 2023
Article in press: May 31, 2023
Published online: July 6, 2023
Processing time: 122 Days and 11.7 Hours
Podocyte infolding glomerulopathy (PIG) is a newly described and rare glomerular disease. To date, only approximately 40 cases have been reported globally.
A 26-year-old female patient presented to our hospital with a complaint of intermittent edema of both lower limbs over the past 2 years. The patient was diagnosed with PIG. She was prescribed corticosteroid therapy in other hospitals during the initial stage, to which she had responded poorly and had developed femoral head necrosis. Therefore, we administered immunosuppressants, renin-angiotensin system inhibitors, combined with traditional Chinese medicine. The patient was followed for 1 year, during which her clinical condition improved.
Integrated Chinese and Western medicine may be effective for PIG treatment, which requires active intervention to improve prognosis.
Core Tip: Due to the limited number of reported cases, insufficient information on the characteristics, diagnosis, and treatment of podocyte infolding glomerulopathy is available. Based on our case and those reported in PubMed, we believe that treatment with corticosteroids, immunosuppressants, and renin-angiotensin system inhibitors is effective. Some patients cannot tolerate corticosteroids and immunosuppressants. When adverse effects occur, clinicians should avoid making negative treatment. Doctors should actively intervene and offer patients treatment suggestions, among which traditional Chinese medicine may be an effective treatment method.
- Citation: Chang MY, Zhang Y, Li MX, Xuan F. Integrated Chinese and Western medicine in the treatment of a patient with podocyte infolding glomerulopathy: A case report. World J Clin Cases 2023; 11(19): 4684-4691
- URL: https://www.wjgnet.com/2307-8960/full/v11/i19/4684.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i19.4684
The concept of podocyte infolding glomerulopathy (PIG) was put forward by the Japanese Society of Nephrology in 2008[1]. PIG can be diagnosed based on histopathological findings. Its pathological feature is the presence of microspheres, microtubules, or both in the glomerular basement membrane (GBM) under electron microscopy, and podocyte infolding into the GBM[1]. Knowledge of the pathogenesis and progression of PIG is rare, due to the limited number of reported cases[1]. Thus, integrated and definitive immunological therapies are not yet available. Some case reports have su
Therefore, further clinical data need to be accumulated to gain a better understanding of this type of glomerulopathy. Herein, we report a case of PIG in a 26-year-old woman with nephrotic syndrome without renal functional impairment. Along with a literature review of previous cases, we summarize the clinical features and treatment methods for PIG.
A 26-year-old Chinese woman presented to our hospital with intermittent edema of both lower limbs of 2 years’ duration.
The patient developed pitting edema of the lower extremities in January 2020. She went to the Sixth Medical Center of Chinese PLA General Hospital. Laboratory data are presented in Table 1. A renal biopsy was performed. She was prescribed 80 mg valsartan and 60 mg prednisone daily. After 8 wk of treatment, the dose of prednisone was reduced by 10 mg every 2 wk. However, she had poor compliance and stopped the medication when the dose had been reduced to 20 mg daily in May 2020. Laboratory tests were not performed thereafter. The patient later developed intermittent edema but ignored it.
| Test | Results |
| 24-h proteinuria, mg | 8730 |
| Hemoglobin, g/L | 101 (normal: 115-150) |
| White blood cells, × 109/L | 3.06 (normal: 3.5-9.5) |
| Platelets, × 109/L | 386 (normal: 125-350) |
| Cholesterol, mmol/L | 6.96 (normal: 3-5.7) |
| Triglyceride, mmol/L | 2.36 (normal: < 1.7) |
| Creatinine, μmol/L | 67.4 (normal: 53-97) |
| Albumin, g/L | 13 (normal: 40-55) |
| ANA | Negative |
| ANCA | Negative |
| Anti-double stranded DNA antibody | Negative |
| Rheumatoid factor | Negative |
| C3, mg/dL | 98.4 (normal: 90-180) |
| C4, mg/dL | 33.6 (normal: 10-40) |
| IgA, mg/dL | 292 (normal: 70-400) |
| IgM, mg/dL | 119 (normal: 40-230) |
| Anti-PLA2R antibody | Negative |
In December 2021, she was re-hospitalized due to bilateral lower extremity edema, and her 24-h proteinuria level was 4840 mg and serum albumin level was 16.8 g/L. This time, she was treated with 40 mg of intravenous methylprednisolone and 240 mg of oral allisartan isoproxil daily. Seven days later, she developed hip pain and weakness. Hip magnetic resonance imaging showed bilateral ischemic necrosis of the femoral head with joint cavity effusion; therefore, the intravenous methylprednisolone was discontinued, and oral methylprednisolone 36 mg/day was initiated and rapidly tapered (1 tablet per week, or 4 mg). Simultaneously, she was administered 100 mg cyclosporine twice a day while continuing allisartan isoproxil. Her 24-h proteinuria was 5803-7650 mg. In February 2022, her 24-h proteinuria was 6370.65 mg and she came to our hospital for treatment. At admission, the patient had mild edema of both lower limbs, hip pain, and foamy urine. The tongue was pale, with a thin white coating, and her pulse was weak.
One year earlier, the patient had developed an increase in blood pressure, reaching 170/100 mmHg. She was treated with 240 mg of oral allisartan isoproxil daily.
The patient denied any family history of kidney disease.
On physical examination, her vital signs were as follows: Body temperature, 36.8 ℃; blood pressure, 120/86 mmHg; heart rate, 90 beats per min; respiratory rate, 18 breaths per min. Furthermore, mild edema was observed in both lower limbs.
The results of laboratory examination were as follows: Urinary protein = 3+, red blood cells = 50/μL, 24-h proteinuria = 6165 mg, serum creatinine = 70.7 μmol/L, serum albumin = 24.2 g/L, cholesterol = 8.03 mmol/L, and triglyceride = 3.24 mmol/L.
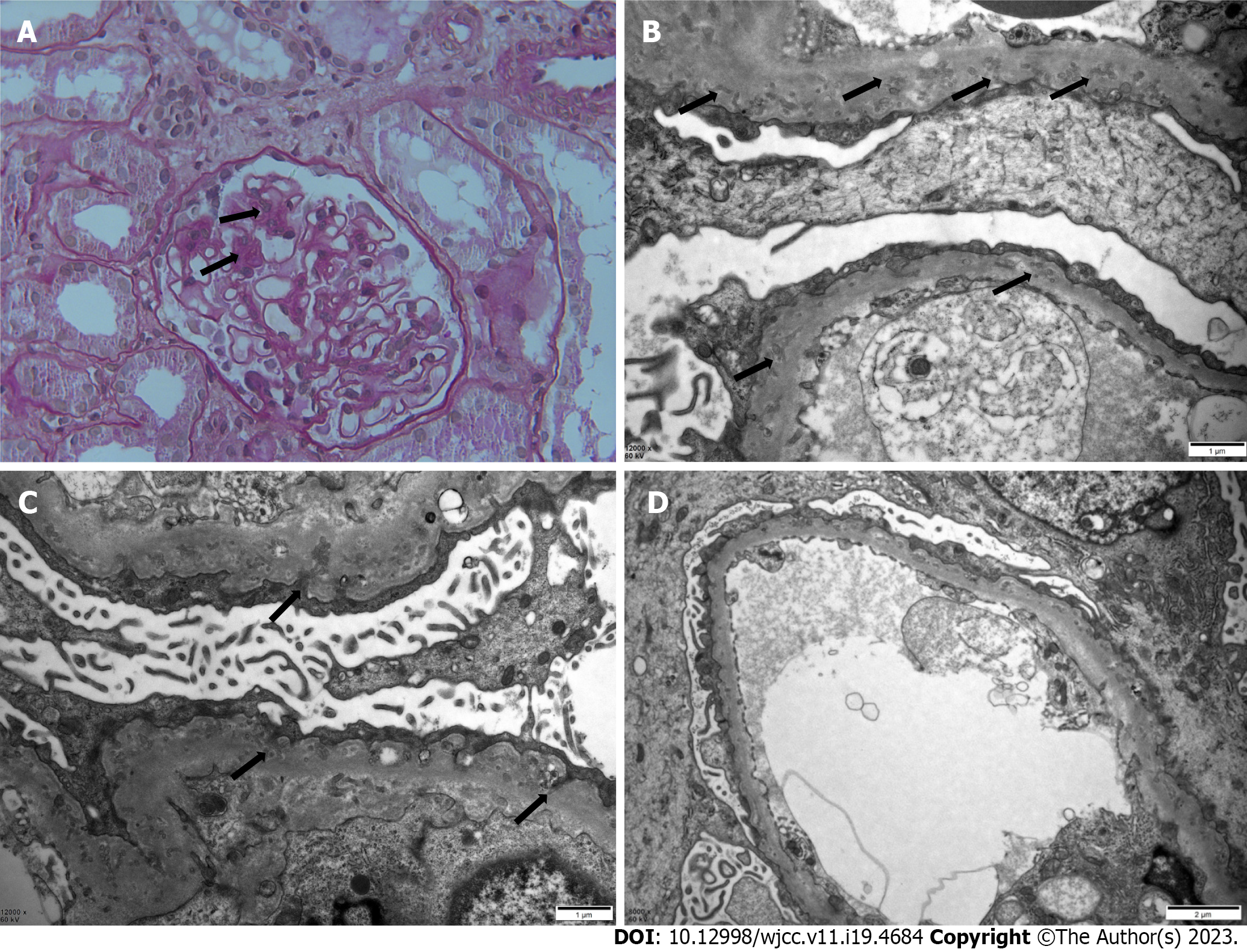
Under a light microscope, 28 glomeruli with no spherical sclerosis and three small cell crescents were visible. The mesangial area showed mild focal segmental proliferation and matrix thickening. Capillary loops were well open, and focal epithelial cell vacuolar degeneration was present. The basement membrane was mildly thickened in focal segments. No significant atrophy or inflammatory cell infiltration was seen in the renal interstitium. No significant thickening of the renal vasculature was observed (Figure 1A). Under an electron microscope, extensive podocyte foot-process effacement with infolding into the GBM was seen. The GBM was diffusely thickened with numerous microspherical and microtubular structures (Figure 1B-D). Immunofluorescent staining of the biopsy specimen showed granular deposition of immunoglobulin (Ig)G along the mesangial areas, but with no staining for C3, IgA, IgM, C1q, or fibrinogen (Supplementary Figure 1).
Combined with the patient’s medical history, a final diagnosis of PIG was made.
We continued allisartan isoproxil treatment and increased the cyclosporine dose to 150 mg in the morning and 100 mg at night. Oral mycophenolate mofetil (500 mg twice a day) was also administered.
In addition, we treated the patient with traditional Chinese medicine. First, we diagnosed the patient as having blood stasis and water stagnation, and treated her with Danggui-Shaoyao-San, a compound formula in traditional Chinese medicine, comprised of six herbs: Paeonia lactiflora Pall. 20 g, Alisma orientalis (Sam.) Juzep. 10 g, Angelica sinensis (Oliv.) Diels. 10 g, Poria cocos (Schw.) Wolf. 20 g, Atractylodes macrocephala Koidz. 20 g and Ligusticum chuanxiong Hort 10 g. Over the course of treatment, the edema of the patient was gradually reduced, and the patient’s condition was subsequently mainly characterized by deficiency of the spleen and kidney, manifested as fatigue. We differentiated the syndrome as deficiency of the spleen and kidney, combined with wind evil, and treated her with Modified Huangqi Chifeng decoction to strengthen qi further. The whole prescription was composed of seven herbs: Astragalus membranaceus Bge 30 g, Euryale ferox Salish 20 g, Rosae Laevigatae Fructus 10 g, Radix Paeoniae Rubra 10 g, Saposhnikoviae Radix 10 g, Rhizoma Dioscoreae Nipponicae 20 g, and Hedyotis Diffusae Herba 20 g.
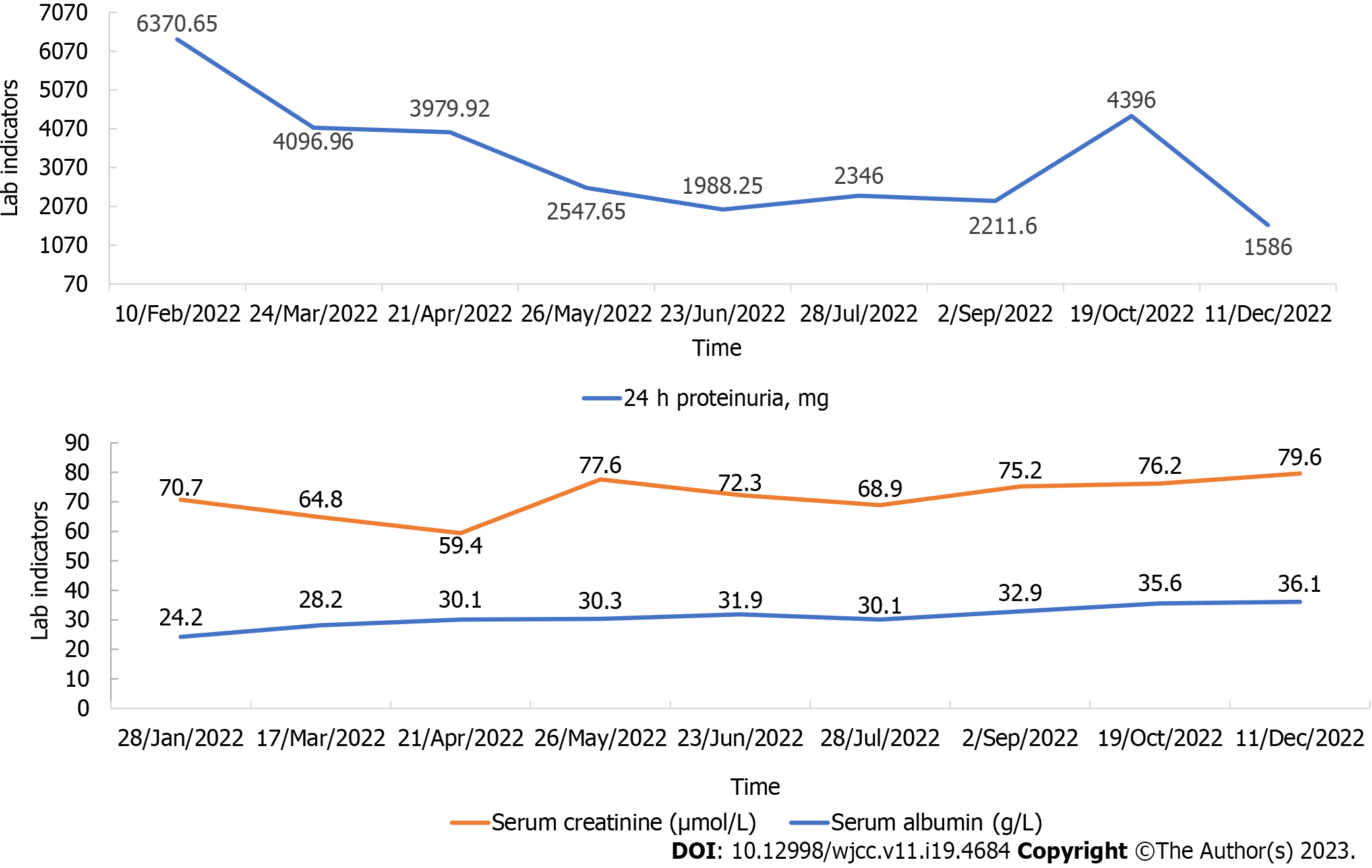
The patient was followed-up for 1 year, during which we adjusted the drugs slightly according to her symptoms. As shown in Figure 2, her serum albumin levels gradually increased and her urinary protein excretion gradually decreased, with an increase in October 2022, which was considered to be related to an upper respiratory infection. Overall, her clinical condition improved.
The concept of PIG, as a newly recognized form of glomerular disease, was first proposed by Joh et al[1] in 2008. Although PIG cases are increasing, it remains unclear whether PIG is a new disease entity or simply a transient morphological phenomenon[2,15]. Hence, it is important to discuss the salient features of this case to gain a better understanding of the nature of the disease.
Microstructures typical of PIG were first described in the 1970s[13], referred to as rounded extracellular particles (REPs)[16]. According to Dales and Wallace, REPs in a patient with membranous nephropathy (MN) are nuclear pore organelles based on their morphologic similarity and the presence of autoantibodies in cytoplasmic and nuclear organelles of the patient’s serum[17]. Zhang et al[8] considered that PIG was actually first identified in 1992 when Sato et al[18] described patients with collagen diseases whose renal biopsies demonstrated fine electron-dense deposits in the GBM. It is difficult to determine when PIG first appeared; however, in 2008, a new terminology for PIG was proposed by Joh et al[1], who defined it as microstructures (microspheres and/or microtubules) associated with podocytic invagination (large cytoplasmic projections from podocytes) into the GBM. In contrast to granular debris commonly seen in diseases such as MN, these microstructures are thought to originate from the primary podocyte cell membranes.
To date, 40 cases of PIG have been reported globally: 36 from Asia (28 from Japan, 6 from China, and 1 each from Korea and India), 2 from North America (1 from the United States, 1 from Canada), 1 from Argentina, and 1 from Europe[1-15]. We summarized the clinical profiles of the 40 cases, 25 of which were included in the study by Joh et al[1], while the remaining 15 cases are shown in Table 2.
| Case No. | Country of the study | Year of the study | Age (years) | Sex | Initial proteinuria (mg/day) | Hematuria | Renal function (Creatinine, μmol/L) | Blood pressure (mmHg) | Treatment | Concomitant diseases |
| Case 26[2] | Japan | 2013 | 14 | F | 3060 | 1-4 | 48.6 | PSL (40 mg/day) | First biopsy: np; Second biopsy: FSGS | |
| Case 27[3] | Japan | 2014 | 79 | M | 1426 | 113.2 | 140/67 | PSL (20 mg/day) | HTN, MM | |
| Case 28[4] | South Korea | 2016 | 44 | F | 39.8 | 100/70 | PSL (10 mg/day); ARB (15 mg/day) | SLE | ||
| Case 29[5] | India | 2018 | 45 | F | 5800 | 20 | 145.9 | 130/80 | High-dose PSL, MMF, 1 g of rituximab | UCTD |
| Case 30[6] | China | 2018 | 52 | F | pSS, HT | |||||
| Case 31[7] | Germany | 2019 | 56 | F | 35 U/L | 386.3 | High dose Pred, rituximab (1 g × 2 ) | RA | ||
| Case 32[8] | China | 2019 | 27 | F | 629 | 168.0 | PSL (48 mg/day), HCQ (0.2 g twice a day) | pSS, HT | ||
| Case 33[8] | China | 2019 | 23 | F | 16796.8 | + | 46.9 | PSL (40 mg/day), HCQ (0.2 g twice a day) tacrolimus (1 mg twice a day) | SLE | |
| Case 34[9] | Argentina | 2020 | 38 | F | SLE | |||||
| Case 35[10] | China | 2020 | 4 | M | 3670 | Given the resistance to steroids and tacrolimus, only treated him with diuretics and (ACEIs) | SIOD, hypothyroidism | |||
| Case 36[11] | China | 2020 | 33 | F | 2124 | - | 36.2 | ARB, Radix Astragali and Huangkui | UCTD | |
| Case 37[12] | Japan | 2020 | 35 | F | pSS, scleroderma | |||||
| Case 38[13] | United States | 2021 | 60 | F | 2286 | 61.9 | Rituximab (1 g × 3) | PLA2R antibody(+) | ||
| Case 39[14] | Canada | 2021 | 52 | F | 11-20 | 111.4 | 158/97 | ARB (300 mg/day); spironolactone (25 mg/day); Pred (1 mg/kg) | HBcAb(+); HBsAb(+) | |
| Case 40[15] | China | 2021 | 61 | F | 2060 | 19 | 75.1 | ARB (100 mg/day), HCQ (100 mg twice a day), PSL (40 mg/day), CTX (600 mg/month) | SLE, HTN |
As shown in Table 2, the age range of the remaining 15 cases was 4-79 years, which was greater than that reported by Joh et al[1], indicating a higher prevalence of PIG and an incidence in a broader population. Significantly more female than male patients were noted (female:male 13:2). Proteinuria reached 16798.8 mg/day, which was markedly higher than the maximum value of 7500 mg/day reported by Joh et al[1]. Three patients, with a mean age of 42.7 years, had increased serum creatinine levels exceeding 1.5 mg/dL (133 μmol/L). On the one hand, this suggests the need for early follow-up of patients and early intervention to slow down the progression of the disease. On the other hand, as PIG is not yet widespread, health education and regular medical check-ups of healthy individuals are important.
In Joh et al's report, 17 of the 25 cases were treated with corticosteroids alone or in combination with immunosuppressants[1]. In the 15 newly reported cases of PIG summarized in Table 2, prednisolone (or prednisone) was administered to nine patients (two were treated with prednisolone or prednisone alone[2,3], five were treated with prednisolone/prednisone combined with immunosuppressants, such as mycophenolate mofetil, rituximab, hydroxychloroquine, tacrolimus, and cyclophosphamide[5,7,8,15], one was treated with prednisolone combined with the angiotensin II receptor blocker (ARB) fimasartan[4], one was treated with prednisolone combined with the ARB irbesartan and spironolactone[14]). Immunosuppressants were administered to six patients (one received rituximab alone)[13]. One patient was administered an angiotensin-converting enzyme inhibitor (ACEI) alone[10], and another was treated with the ARB olmesartan medoxomil combined with the traditional Chinese medicines Radix Astragali and Huangkui[11]. The treatments of the remaining three cases were not mentioned in the original articles[6,9,12]. Although treatment guidelines for PIG have not been established to date, a review of the literature above showed that corticosteroids are most widely used for PIG, followed by immunosuppressants and ACEI/ARB.
Analysis of concomitant diseases showed that PIG was mostly associated with collagen diseases, such as systemic lupus erythematosus (SLE) (4/15), rheumatoid arthritis (1/15), and primary Sjögren’s syndrome (2/15). In Joh et al's report[1], 13 of the 25 cases had combined SLE. It was also seen in patients with non-collagen diseases, such as undifferentiated connective tissue diseases (2/15), Schimke immuno-osseous dysplasia (1/15), hypertension (1/15), multiple myeloma (1/15), and Hashimoto’s thyroiditis (1/15). The exact relationship between collagen diseases and PIG requires further research.
In addition, in one case[2], two renal biopsies were performed; the first renal biopsy showed only PIG, but the second also suggested focal segmental glomerulosclerosis. Thus, in PIG cases, we should pay attention to follow-up, particularly when the patient’s condition does not improve for an extended period or worsens, which may indicate the need for a repeat renal biopsy.
Our patient was a 26-year-old woman without systemic autoimmune conditions, based on serological findings. The mesangial area showed mild proliferation and matrix thickening on light microscopy; however, electron microscopy confirmed the diagnosis of PIG. PIG has been classified into three groups based on electron microscopy findings: Invagination of only primary podocytes in type A, invagination and microstructures in type B, and only microstructures in type C[13]. Our patient was classified as type B (Figure 1B-D). After a period of treatment with corticosteroids, she developed femoral head necrosis. Thereafter, she was treated with immunosuppressants and renin-angiotensin system inhibitors, but the results were somewhat disappointing. She came to our hospital for treatment in February 2022. Along with Western medicine treatment, we began to treat her with Chinese herbal medicine treatment based on syndrome differentiation. Her 24-h proteinuria decreased from 6370 mg to 1586 mg over the course of a year.
Nonetheless, the treatment outcomes attained cannot be generalized as this is a case report. Future research in prospective studies and case series are required. accumulation of further case reports will yield the experience required to provide patients with better counseling.
Although PIG is a very rare glomerular disease, the combination of our case and those reported in PubMed leads us to believe that treatment with corticosteroids, immunosuppressants, and renin-angiotensin system inhibitors is effective. However, some patients cannot tolerate corticosteroids and immunosuppressants. When adverse effects occur with these treatments, clinicians should avoid making negative treatment. Doctors should actively intervene and suggest treatments to patients, among which traditional Chinese medicine is an effective treatment.
We thank Ya-Li Ren from Peking University First Hospital for technical assistance with electron microscopy.
| 1. | Joh K, Taguchi T, Shigematsu H, Kobayashi Y, Sato H, Nishi S, Katafuchi R, Nomura S, Fujigaki Y, Utsunomiya Y, Sugiyama H, Saito T, Makino H. Proposal of podocytic infolding glomerulopathy as a new disease entity: a review of 25 cases from nationwide research in Japan. Clin Exp Nephrol. 2008;12:421-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 36] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 2. | Iguchi A, Sohma A, Yamazaki H, Ito T, Saeki T, Ito Y, Imai N, Osawa Y, Narita I. A case of podocytic infolding glomerulopathy with focal segmental glomerulosclerosis. Case Rep Nephrol Urol. 2013;3:110-116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 3. | Harada M, Kamijo Y, Ehara T, Shimojo H, Shigematsu H, Higuchi M. A case of podocytic infolding glomerulopathy with multiple myeloma. BMC Nephrol. 2014;15:32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 4. | Kwon KW, Jeong HJ, Lee JH. Podocytic infolding glomerulopathy: A case report. Ultrastruct Pathol. 2016;40:374-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Matthai SM, Mohapatra A, Mathew AJ, Roy S, Varughese S, Danda D, Tamilarasi V. Podocyte Infolding Glomerulopathy (PIG) in a Patient With Undifferentiated Connective Tissue Disease: A Case Report. Am J Kidney Dis. 2018;72:149-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 6. | Fang JY, Song AH, Shen B, Liu YL. A Case of Podocytic Infolding Glomerulopathy with Primary Sjögren's Syndrome and Hashimoto's Thyroiditis. Chin Med J (Engl). 2018;131:2747-2748. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 7. | Wöstmann F, Müller RU, Göbel H, Benzing T, Becker JU, Bartram MP. Case report: a peculiar glomerulopathy in a patient suffering from nephrotic syndrome. BMC Nephrol. 2019;20:326. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 8. | Zhang T, Sun W, Xue J, Chen J, Jiang Q, Mou L, Du H. Podocytic infolding glomerulopathy: two new cases with connective tissue disease and literature review. Clin Rheumatol. 2019;38:1521-1528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 9. | Malvar A, Davila P, Ferrari M, Delgado P, Iscoff P, Lococo B, Alberton V. Podocyte infolding glomerulopathy; report of the first case in Latin America and review of the literature. Nefrologia (Engl Ed). 2020;40:469-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Xiong S, Shuai L, Li X, Dang X, Wu X, He Q. Podocytic infolding in Schimke immuno-osseous dysplasia with novel SMARCAL1 mutations: a case report. BMC Nephrol. 2020;21:170. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 11. | Shi J, Zheng R, Gao H, Zhao Z, Wu H, Zhang Z. Podocyte infolding glomerulopathy with undifferentiated connective tissue disease: a case report. Ultrastruct Pathol. 2020;44:245-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Manabe S, Sato M, Kataoka H, Taneda S, Mochizuki T, Nitta K. Cell invasion in glomerular basement membrane: infolding glomerulopathy. Kidney Int. 2020;98:1623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 13. | Pandit AP, Rennke HG, Denker BM. Podocytic Infolding Glomerulopathy in a Patient with Phospholipase A2 Receptor-Positive Membranous Nephropathy and Review of the Literature. Nephron. 2021;145:496-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 14. | Ting JA, Hung W, McRae SA, Barbour SJ, Copland M, Riazy M. Podocyte Infolding Glomerulopathy, First Case Report From North America. Can J Kidney Health Dis. 2021;8:20543581211048357. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 15. | Liu X, Huang J, Zhang K, Niu Y, Liu Y, Cui C, Yu C. A case of Podocytic Infolding Glomerulopathy with SLE and literature review. BMC Nephrol. 2021;22:410. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 16. | Burkholder PM, Hyman LR, Barber TA. Extracellular clusters of spherical microparticles in glomeruli in human renal glomerular diseases. Lab Invest. 1973;28:415-425. [PubMed] |
| 17. | Dales S, Wallace AC. Nuclear pore complexes deposited in the glomerular basement membrane are associated with autoantibodies in a case of membranous nephritis. J Immunol. 1985;134:1588-1593. [PubMed] |
| 18. | Sato H, Saito T, Yoshinaga K. Intramembranous fine deposit disease associated with collagen disorders: a new morphological entity? Virchows Arch A Pathol Anat Histopathol. 1992;420:447-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Al-Ani RM, Iraq; Pezeshgi A, Iran S-Editor: Fan JR L-Editor: A P-Editor: Zhao S