Published online Jul 6, 2023. doi: 10.12998/wjcc.v11.i19.4567
Peer-review started: May 4, 2023
First decision: May 12, 2023
Revised: May 21, 2023
Accepted: May 24, 2023
Article in press: May 24, 2023
Published online: July 6, 2023
Processing time: 57 Days and 11.4 Hours
A healthy body shape is essential to maintain athletes’ sports level. At present, little is known about the effect of athletes’ body shape on anterior cruciate ligament reconstruction (ACLR). Moreover, the relationship between body shape and variables such as knee joint function after operation and return to the field has not been well studied.
To verify the relationship between a body shape index (ABSI) and the functional prognosis of the knee after ACLR in athletes with ACL injuries.
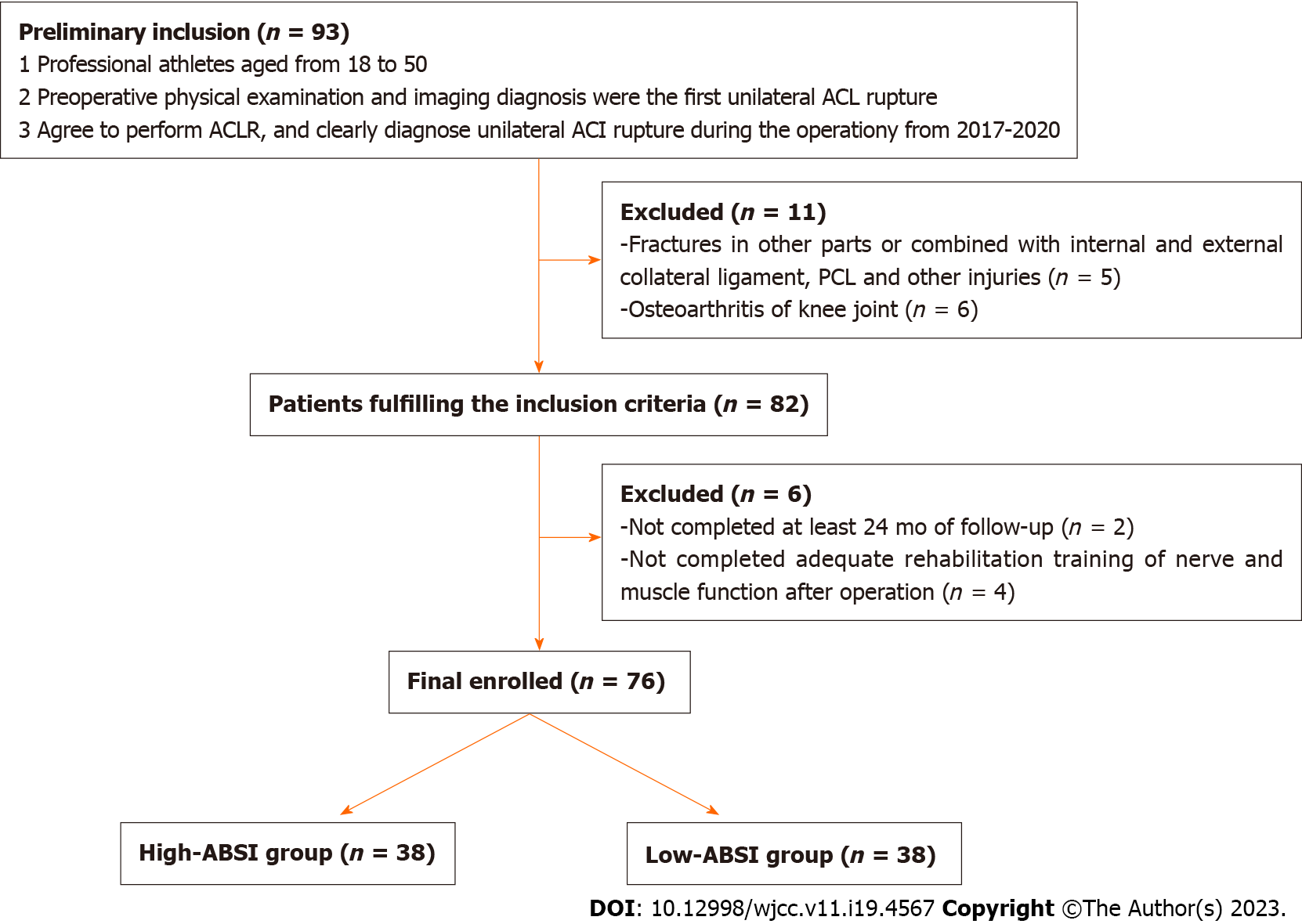
We reviewed 76 athletes with unilateral ACL ruptures who underwent ACLR surgery in the First Hospital of Shanxi Medical University between 2017 and 2020, with a follow-up period of more than 24 mo. First, all populations were divided into a High-ABSI group (ABSI > 0.835, n = 38) and a Low-ABSI group (ABSI < 0.835, n = 38) based on the arithmetic median (0.835) of ABSI values. The primary exposure factor was ABSI, and the outcome indicators were knee function scores as well as postoperative complications. The correlation between ABSI and postoperative knee function scores and postoperative complications after ACLR were analyzed using multifactorial logistic regression.
The preoperative knee function scores of the two groups were similar. The surgery and postoperative rehabilitation exercises, range of motion (ROM) compliance rate, Lysholm score, and Knee Injury and Osteoarthritis Outcome Score of the two groups gradually increased, whereas the quadriceps atrophy index gradually decreased. The knee function scores were higher in the Low-ABSI group than in the High-ABSI group at the 24-mo postoperative follow-up (P < 0.05). In multifactorial logistic regression, ABSI was a risk factor of low knee joint function score after surgery, specifically low ROM scores (odds ratio [OR] = 1.31, 95% confidence interval [CI] [1.10-1.44]; P < 0.001), low quadriceps atrophy index (OR = 1.11, 95%CI [0.97-1.29]; P < 0.05), low Lysholm scores (OR = 2.34, 95%CI [1.78-2.94]; P < 0.001), low symptoms (OR = 1.14, 95%CI [1.02-1.34]; P < 0.05), low activity of daily living (OR = 1.34, 95%CI [1.18-1.65]; P < 0.05), low sports (OR = 2.47, 95%CI [1.78-2.84]; P < 0.001), and low quality of life (OR = 3.34, 95%CI [2.88-3.94]; P < 0.001). ABSI was also a risk factor for deep vein thrombosis of the lower limb (OR = 2.14, 95%CI [1.88-2.36], P < 0.05] and ACL recurrent rupture (OR = 1.24, 95%CI [0.98-1.44], P < 0.05) after ACLR.
ABSI is a risk factor for the poor prognosis of knee function in ACL athletes after ACLR, and the risk of poor knee function after ACLR, deep vein thrombosis of lower limb, and ACL recurrent rupture gradually increases with the rise of ABSI.
Core Tip: A body shape index (ABSI), an excellent body mass index, was used to substitute for the traditional body mass index to more objectively assess the degree of body size/obesity in athletes. Subsequently, multivariate logistic regression analysis was used to determine whether ABSI is a risk factor for poor knee function, lower extremity deep vein thrombosis, and fractures after anterior cruciate ligament surgery. The findings of this study have the potential to narrow the gap in previous research.
- Citation: Wang YJ, Zhang JC, Zhang YZ, Liu YH. Assessment of functional prognosis of anterior cruciate ligament reconstruction in athletes based on a body shape index. World J Clin Cases 2023; 11(19): 4567-4578
- URL: https://www.wjgnet.com/2307-8960/full/v11/i19/4567.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i19.4567
Athletes are at high risk of anterior cruciate ligament (ACL) ruptures, often combined with meniscal and cartilage injuries, which can lead to knee instability and osteoarthritis (OA) if not treated promptly[1]. Due to the high demand for knee function in athletes, ACL reconstruction (ACLR) is generally recommended as early as possible to obtain knee stability after ACL rupture in athletes[2]. Given that good postoperative knee function is the foundation of an athlete's performance on the field[3], it is important to identify the factors that influence an athlete's post-ACLR knee function.
A proper body shape plays a central role in athletic performance, and the increasing obesity has a negative impact on athletes' physical performance, such as increased lower limb loading and slower movement speed[4]. As a result, it is important to regularly monitor the obesity of athletes to ensure that the mandatory physical obesity level is kept at a low level[5]. Obesity and overweight have previously been identified as high-risk variables for poor function following ACLR, and a higher body mass index (BMI) tends to predict higher knee loading and OA risk in ACLR patients, especially when the BMI reaches 25 kg/m2[6,7]. Furthermore, a high BMI can also alter post-ACLR gait biomechanics, and increase knee compression and shear forces, as well as patellofemoral pressure and knee extensor moment, which can lead to knee instability[8,9]. Therefore, an increasing number of athletes of all sizes or weights are in need of ACLR, and it is of great importance to identify risk factors that influence the outcomes and complications of ACLR in athletes.
BMI is a measurement of a person's weight and height; it is used to determine a person's fat proportion and is also a common clinical indicator for assessing overweight and obesity[10]. BMI does not directly measure body fat composition, but is defined as a positive correlation between weight and obesity at a specific height[11]. Currently, statistics on obesity are mostly based on BMI, which ranges from 18.5 to 24.9 in a healthy person, 25 to 29.9 in an overweight person, and 30+ in an obese person. However, the median distribution of "normal" BMI (approximately 22) differs considerably from the mean BMI of the current population. In addition, the correlation between BMI and the risk of various diseases is not linear[12]. Put another way, a higher BMI does not always reflect an increase in fat, as being overweight may be due to an increase in muscle tissue, especially in athletes with higher levels of musculoskeletal development[13]. ABSI was first proposed by researchers at the City University of New York in 2012[14]. ABSI is calculated from height, weight, and waist circumference (WC), and it standardizes WC with body shape (weight and height), similar to body BMI[15]. ABSI corrects the effect of height and/or body mass to better assesses abdominal fat[16].
There is a paucity of research regarding the association between ABSI and postoperative knee function and complications in athletes with ACLR. Therefore, it is of great significance to identify risk factors associated with poorer postoperative knee function or complications in athletes with ACLR and to emphasize early intervention and guided rehabilitation to allow athletes to return to competition as soon as possible.
Patients included in this retrospective cross-sectional study were professional athletes with ACL injuries who underwent arthroscopic ACLR between December 2017 and December 2020 at the Department of Orthopedics in the First Hospital of Shanxi Medical University (Shanxi, China), and all patients agreed to participate in this study. According to the Helsinki Declaration, the study was approved by the Ethics Committee of the First Hospital of Shanxi Medical University, and written informed consent was obtained from all patients. Because of the retrospective nature of our study, no additional clinical trial registration was required.
Inclusion criteria: (1) Active professional athletes aged 18-years-old to 40-years-old; (2) first-time knee arthroscopy, within 6 mo after ACL injury; (3) intraoperative definite diagnosis of unilateral ACL rupture (may be combined with meniscal or articular cartilage injury); and (4) patients who had no contraindications to surgery and agreed to undergo arthroscopic ACLR surgery and participate in this study.
Exclusion criteria: (1) Presence of lower extremity fractures or combined injuries such as medial or lateral collateral ligament or posterior cruciate ligament; (2) previous knee meniscal injury or osteoarthritis; and (3) history of previous knee surgery.
Ultimately, a total of 76 ACLR athletes were included in this study through screening.
Demographic information was collected through the medical record system and questionnaires including sex, age, type of exercise, smoking and alcohol history, preoperative physical examination (height, weight, BMI, and abdominal circumference), comorbidities (e.g., hypertension, diabetes, cancer, meniscal injury), ACL injury, site of injury, and surgical status (duration of surgery, duration of tourniquet, femoral and tibial fixation).
The exposure variable in this study was ABSI, and it was calculated as follows: BMI = weight/height2, and ABSI = WC/ (BMI2/3 × height1/2)[17].
First, the ABSI of all ACLR patients was calculated, and then the population was divided into a High-ABSI group (ABSI > 0.835, n = 38) and Low-ABSI group (ABSI < 0.835, n = 38) according to their arithmetic median (0.835). All patients were followed up for more than 24 mo.
As previously described[18], all ACLR procedures were performed on ACL patients by the same orthopedic professor under general or combined lumbar and rigid anesthesia using autologous hamstring tendons (semitendinosus and thin femoral muscles). All patients had a thigh tourniquet. Also, all patients had a standard anteromedial femoral and tibial tunnel drilled, with a final fixation of the proximal tendon bundled to the femur with an Endobutton (Smith & Nephew Inc., Memphis, TN, United States) or rounded cannulated interference (RCI) screw (Smith & Nephew, United Kingdom) and the proximal tendon bundled to the tibia with an RCI screw (Smith & Nephew, United Kingdom) or washer (Smith & Nephew UK Ltd., Watford, England, United Kingdom). Postoperatively, all patients followed a standard rehabilitation program.
All patients received standard lower extremity nerve-muscle function training, including quadriceps (anterior thigh muscle group) isometric exercise. They also practiced thigh muscle tensing and relaxation, without significant pain, more than 500 times/d, and had posterior thigh muscle group isometric exercise more than 500 times/d. Furthermore, they had pump exercises such as forceful, slow, full-range flexion and extension of the ankle joints, more than 500 times/d. Patients were clinically reviewed by the surgeon at 3 mo and 12 and 24 mo postoperatively.
All patients completed a 24-mo postoperative follow-up to assess the functional recovery of the knees after surgeries.
Range of motion (ROM) is the arc of motion through which the joint moves, and is the most basic method for assessing the motor function of the extremities[19]. Normal knee mobility should be between 140° and 150°, and active knee flexion of 120° or more after surgery is defined as compliance.
The quadriceps muscle is not only important for the overall health of the knee joint but also plays a key role in ensuring the knee joint’s continued stability[20]. The quadriceps atrophy index is calculated as: atrophy index = (healthy thigh circumference - affected thigh circumference)/healthy thigh circumference × 100%. The smaller the value, the smaller the reduction in quadriceps strength and the better the recovery of muscle strength.
The Lysholm Scale is a condition-specific scale for evaluating ligamentous injuries of the knee, and is also a subjective rating system in the form of a percentage questionnaire[21]. The scale consists of eight items: claudication (5 points), support (5 points), strangulation (15 points), instability (25 points), pain (25 points), swelling (10 points), stair climbing and descending (10 points), and squatting (5 points). A higher score indicates better function.
Knee Injury and Osteoarthritis Outcome Score (KOOS) is an assessment of the short- and long-term outcomes of treatment after a knee injury[22]. It consists of five patient-related components: symptoms, pain, activity of daily living (ADL), sports, and knee-related quality of life (QOL). A score of 0 means very poor function of that part of the joint, and a score of 100 means perfectly normal function of that part of the joint.
According to previous studies[18], “return to sport (RTS)” is defined as an exercise that is considered to return to the same or higher level of return to pre-injury during the follow-up period.
During the follow-up period, patients in both groups were counted for complications, including knee infection, deep vein thrombosis, internal fixation failure, and ACL re-rupture. The patients were analyzed for specific complications, and given symptomatic treatment.
IBM SPSS 27.10 software and GraphPad Prism 9 software were used for statistical analyses. The measurement data are expressed as the mean ± standard deviation, and one-way analysis of variance was used for the comparison between groups. The statistical data are expressed as frequencies (percentages), and the χ2 test was used for the comparison between groups. The median method was used to divide all patients with ACLR into High-ASBI and Low-ASBI groups. The relationship between ABSI and postoperative knee function scores and complications after ACLR was evaluated by multifactorial logistic regression analysis. P < 0.05 was considered statistically significant.
First, survey data were collected from a total of 93 athletes, and 76 ACLR athletes were included in the final analysis after excluding those patients who lacked outcomes, exposure, or did not complete follow-up (Figure 1).
As shown in Table 1, there was no significant difference in age between the High-ABSI group (ABSI > 0.835) and Low-ABS group (ABSI < 0.835) in the preliminary analyses ([25.9 ± 6.3] vs [26.4 ± 7.1]; P = 0.7463). There were 57 male athletes and 19 female athletes, and there was no significant difference in sex composition between the two groups (males: 71.05% vs 78.95%; P = 0.5970), with both groups having a high percentage of males. In terms of types of athletes, soccer players had the most with 37, followed by basketball players with 28 and other athletes with 9. There were no significant differences between the High-ABSI and Low-ABS groups in terms of BMI (24.3 ± 2.2 vs 23.9 ± 2.1), percentage of smoking (10.53% vs 7.895%), and percentage of alcohol consumption (21.05% vs 18.42%) for individuals (all P > 0.05). In terms of surgery, although the High-ABSI group had a lower operative time than the Low-ABSI group, there was no significant difference between them ([88.3 ± 15.7] vs [93.4 ± 16.2]; P = 0.1676).
| Characteristics | High-ABSI group, n = 38 | Low-ABSI group, n = 38 | P value |
| Age in yr | 25.9 ± 6.3 | 26.4 ± 7.1 | 0.7463 |
| Age range in yr | 18-38 | 18-39 | |
| Sex | 0.5970 | ||
| Female, n = 19 | 11 (28.94) | 8 (21.05) | |
| Male, n = 57 | 27 (71.05) | 30 (78.95) | |
| Athletes | 0.8731 | ||
| Football | 18 | 19 | |
| Basketball | 16 | 14 | |
| Others | 4 | 5 | |
| Body mass index as kg/m2 | 24.3 ± 2.2 | 23.9 ± 2.1 | 0.4201 |
| Side | 0.4838 | ||
| Right | 21 | 24 | |
| Left | 17 | 14 | |
| Smoker | 4 (10.53) | 3 (7.895) | 0.4446 |
| Alcohol user | 8 (21.05) | 7 (18.42) | 0.8186 |
| Mean follow-up time in mo | 26.9 ± 5.9 | 27.5 ± 6.3 | 0.6695 |
| Operation time in min | 88.3 ± 15.7 | 93.4 ± 16.2 | 0.1676 |
| Length of stay in d | 6.9 ± 1.9 | 6.5 ± 1.6 | 0.3241 |
| Meniscus injury | 14 | 16 | 0.6388 |
| Duration of tourniquet use in min | 45.9 ± 5.9 | 43.8 ± 6.2 | 0.1347 |
| Femur fixation method | 0.5558 | ||
| EndoButton | 36 | 37 | |
| RCI screw | 2 | 1 | |
| Tibial fixation method | 0.6422 | ||
| Screw and washer | 21 | 23 | |
| RCI screw | 17 | 15 | |
| Comorbidity | 0.5488 | ||
| Diabetes | 2 | 3 | |
| Hypertension | 2 | 2 | |
| Cancers | 1 | 0 |
There was no significant difference in the duration of the tourniquet (P = 0.1347) or the number of days in the hospital (P = 0.3241) between the two groups. Also, there were no significant differences in the femur (P = 0.5558) or tibia (P = 0.6422) in terms of fixation method.
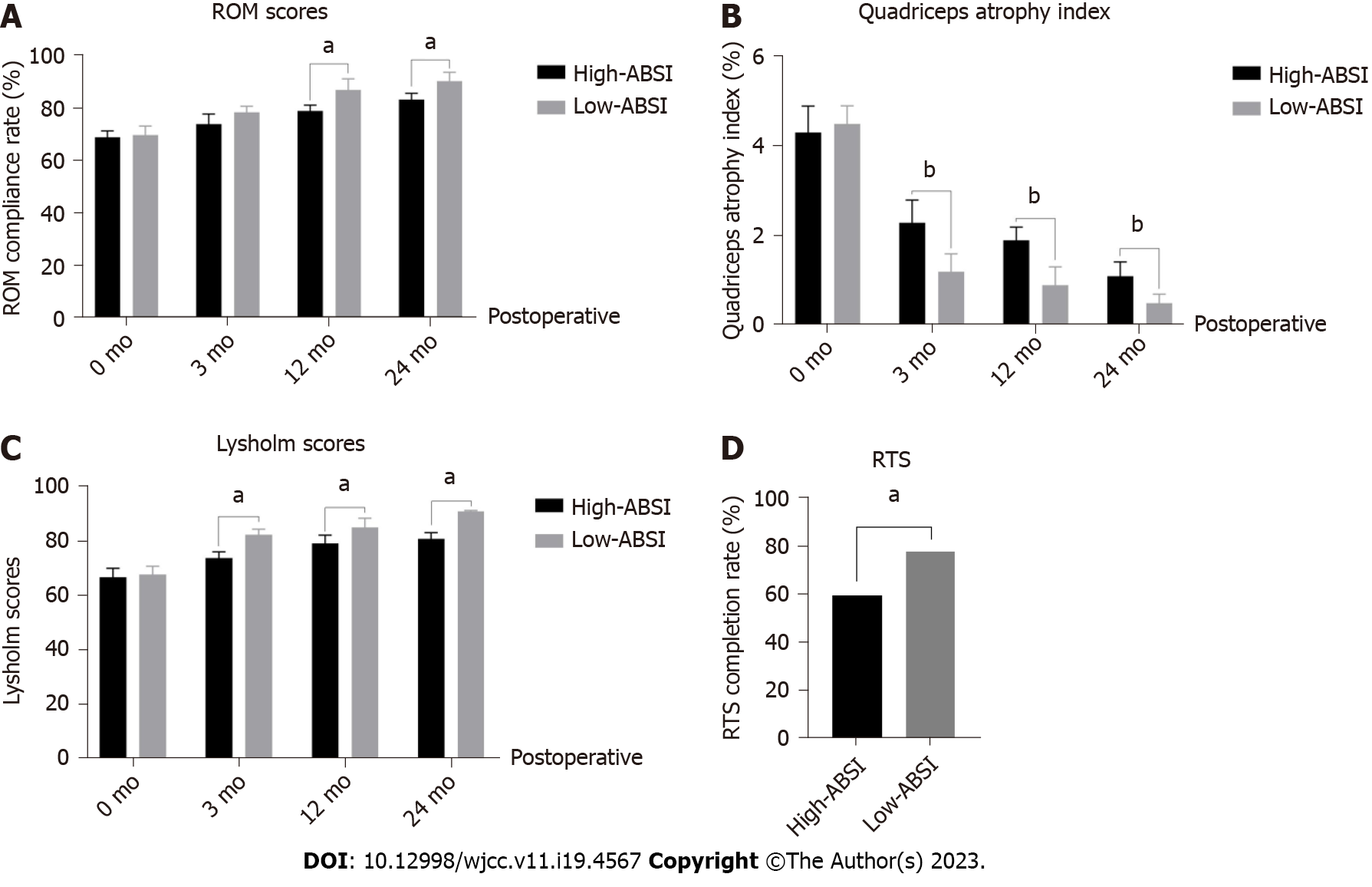
Next, the effects of ABSI level and postoperative knee function were explored. Figure 2 shows the functional recovery of the knee joint after surgery in the different ABSI groups. The results showed that the ROM scores of the two groups were similar before surgery (0 mo) and gradually improved with ongoing surgery and postoperative rehabilitation exercises (Figure 2A). Besides, the ROM scores in the Low-ABSI group were higher than those in the High-ABSI group (P < 0.05; Figure 2A), indicating that high ABSI predicted poor knee mobility. The quadriceps muscle also influenced the knee flexion and extension function. The results showed that preoperatively (0 mo), both groups had some short-term atrophy of the quadriceps muscle due to a sudden decrease in motion caused by the ACL rupture (Figure 2B). At 3 mo, 12 mo, and 24 mo postoperatively, the quadriceps atrophy index was higher in the High-ABSI group than in the Low-ABSI group (Figure 2B). This indicates that the higher the ABSI, the lower the muscle strength of the quadriceps. In addition, the Lysholm score is an excellent indicator of knee function after knee ligament injury surgery. As shown in Figure 2C, before surgery, the scores of both groups were low (P > 0.05). With ongoing surgery and rehabilitation exercises, the Lysholm scores of both groups gradually increased, and the Lysholm scores of the Low-ABSI group were consistently higher than those of the High-ABSI group (P < 0.05, Figure 2C).
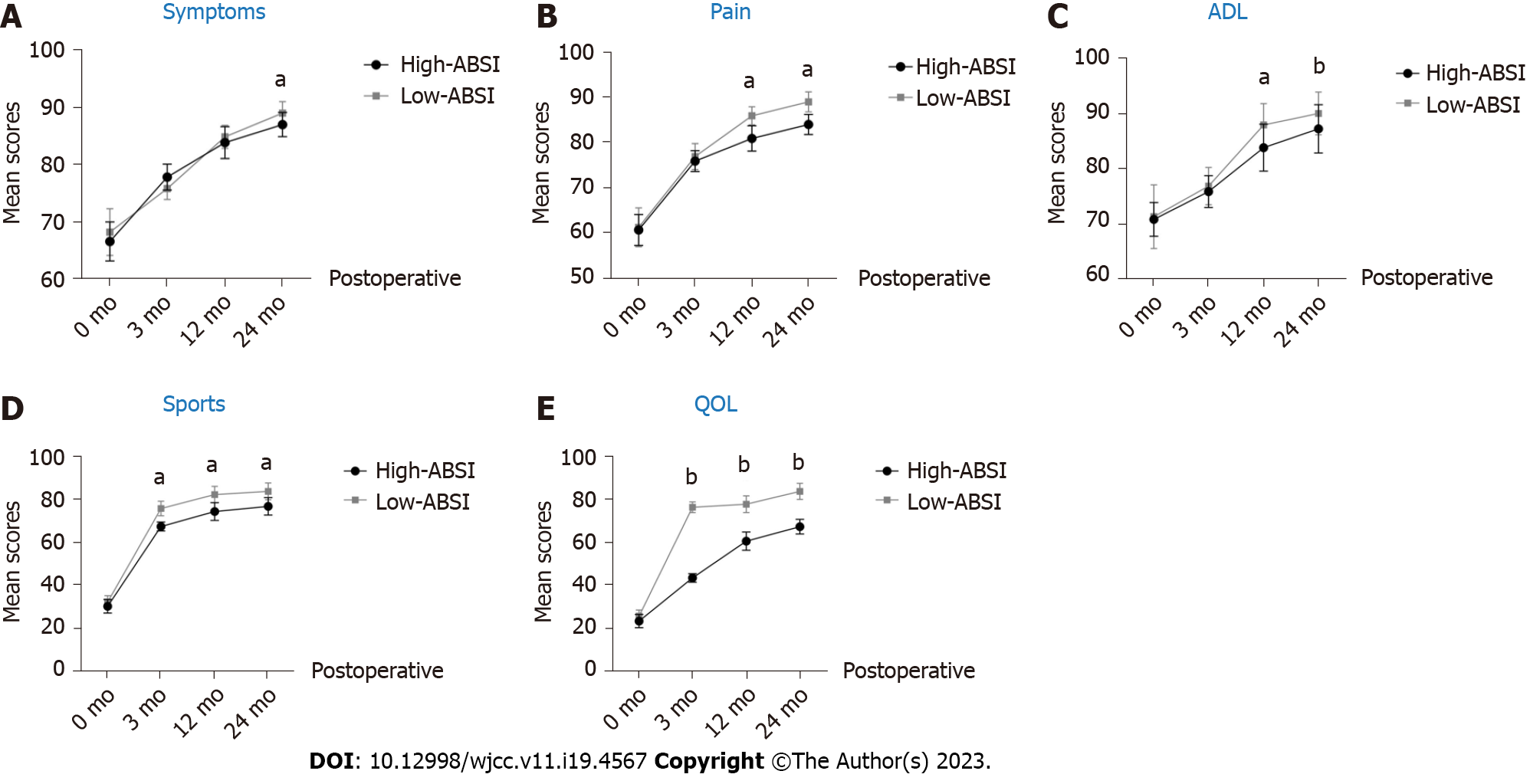
The KOOS score is a comprehensive self-rating measure of postoperative knee surgery outcomes that effectively reflects patients' perceptions of their knee health, symptoms, and function. The KOOS score consists of five subscales: symptoms, pain, ADL, sports, and QOL. As shown in Figure 3, the KOOS scores gradually increased with the ongoing surgery and rehabilitation in each of the five self-scales. At 3 mo postoperatively, the scores in both groups improved rapidly, but there was no significant difference in symptoms, pain, or ADL between the two. This might be attributed to the rapid relief of knee symptoms and pain with the implementation of surgery (P > 0.05, Figure 3). At the last follow-up (24 mo after surgery), all KOOS scores reached their highest values in both groups, and the Low-ABSI group had higher scores than the High-ABSI group (P < 0.05; Figure 3).
To determine whether a high level of ASBI is a risk factor for poorer postoperative knee function recovery in ACLR athletes, we performed multifactorial logistic regression analysis with ABSI as the independent variable and each knee function score at the last follow-up as the dependent variable. We turned these continuous variables into dichotomous variables. The median scores of ROM scores, quadriceps atrophy index, Lysholm scores, and KOOS (symptoms, pain, ADL, sports, and QOL) were taken for all groups, and scores greater than the median value were defined as low knee function, specifically low ROM scores (≤ 82 ), low quadriceps atrophy index (≥ 1.2), low Lysholm scores (≤ 83), low symptoms (≤ 88), low pain (≤ 83), low ADL (≤ 87), low sports (≤ 80), and low QOL (≤ 73).
As shown in Table 2, the results revealed that high ABSI was a risk factor for patients with low postoperative knee function scores, specifically low ROM scores (odds ratio [OR] = 1.31, 95% confidence interval [CI] [1.10-1.44]; P < 0.001), low quadriceps atrophy index (OR = 1.11, 95%CI [0.97- 1.29]; P < 0.05), low Lysholm scores (OR = 2.34, 95%CI [1.78-2.94]; P < 0.001), low symptoms (OR = 1.14, 95%CI [1.02-1.34]; P < 0.05), low ADL (OR = 1.34, 95%CI [1.18-1.65]; P < 0.05), low sports (OR = 2.47, 95%CI [1.78-2.84]; P < 0.001), and low QOL (OR = 3.34, 95%CI [2.88-3.94]; P < 0.001). Interestingly, while pain scores (higher scores were associated with less pain) were higher in the High-ABSI group than in the Low-ABSI group (P < 0.05; Figure 2C), multifactorial logistic regression showed that high ABSI was not a risk factor for pain (OR = 1.04, 95%CI [0.78-1.44]; P = 0.06) (Table 2). Ultimately, at the final follow-up, the percentage of RTS was significantly higher in the Low-ABSI group (78.95% vs 60.53%; P < 0.05) than in the High-ABSI group (Figure 2D).
| Items | OR (95%CI) | P value |
| Low ROM scores | 1.31 (1.10-1.44)b | < 0.001 |
| Low quadriceps atrophy index | 1.11 (0.97-1.29)a | < 0.05 |
| Low Lysholm scores | 2.34 (1.78-2.94)b | < 0.001 |
| Low KOOS scores | ||
| Low symptoms | 1.14 (1.02-1.34)a | < 0.05 |
| Low pain | 1.04 (0.78-1.44) | 0.06 |
| Low ADL | 1.34 (1.18-1.65)a | < 0.05 |
| Low sports | 2.47 (1.78-2.84)b | < 0.001 |
| Low QOL | 3.34 (2.88-3.94)b | < 0.001 |
As shown in Table 3, with the exception that the incidence of venous thrombosis of lower limbs (21.04% vs 2.631%; P = 0.0284) in the High-ABSI group was higher than that in the Low-ABSI group, there was no significant difference in the incidence of knee joint infection (5.262% vs 5.262%; P > 0.9999), internal fixation failure (2.631% vs 0.000%; P > 0.9999), and ACL current failure (7.893% vs 2.631%; P = 0.6148) between the two groups. Further multifactorial logistic regression analysis also showed that ABSI was a risk factor for deep vein thrombosis of the lower limb (OR = 2.14, 95%CI [1.88-2.36]; P < 0.05) as well as ACL recurrent rupture (OR = 1.24, 95%CI [0.98- 1.44]; P < 0.05) (Table 4).
| Characteristic | High-ABSI group, n = 38 | Low-ABSI group, n = 38 | P value |
| Knee joint infection | 2 (5.262) | 2 (5.262) | > 0.9999 |
| Internal fixation failure | 1 (2.631) | 0 (0.000) | > 0.9999 |
| Deep vein thrombosis of lower limb | 8 (21.04) | 1 (2.631)a | 0.0284 |
| ACL recurrent rupture | 3 (7.893) | 1(2.631) | 0.6148 |
| Total | 14 (36.84) | 4 (10.53) | 0.0571 |
The current standardized treatment for ACL injury is ACLR, which aims to restore the function and stability of the knee joint, thus promoting RTS[23]. Although most ACL reconstructions restore the mechanical stability of the injured knee joint, the incidence of RTS is different. In 85%-90% of ACLR patients, the prognosis of knee joint function returns to normal or close to normal within 6 mo after surgery[24]. There are also some research reports that only 63% of the people restore their exercise level before the injury at the last follow-up[25].
Our study also showed that the knee joint function scores of most patients with ACLR remained stable from 1 year to 2 years after surgery. In the last follow-up 24 mo after the operation, athletes in the High-ABSI and Low-ABSI groups had 60.53% (23/38) and 78.95% (30/38) RTS completion rates, respectively. This confirms that ACLR is undoubtedly the standard operation to reconstruct the integrity of the anterior cruciate ligament and restore the function of the knee joint.
Obesity is also a risk factor for poor function after various knee surgeries[26]. Li et al[27] found that obesity and medial cartilage injury are strong risk factors for osteoarthritis after the first single-beam ACLR. Another prospective study involving 92 patients with ACLR also pointed out that the incidence of postoperative complications in patients with high BMI (BMI > 25 kg/m2) was slightly higher than that in patients with normal BMI[21]. For obese patients with ACLR with BMI higher than 25 kg/m2, the score of the International Knee Documentation Committee was lower after the operation[28]. However, there were also some different results. In addition, another study also confirmed that the preoperative BMI of patients had no significant adverse impact on the KOOS and Lysholm scores, and that there was no significant difference in the postoperative clinical results of these patients[21]. Therefore, the relationship between obesity based on BMI content and the postoperative function of ACLR is complex, because BMI may not be able to effectively measure the content of individual fat and identify muscle and obesity. ABSI has a positive correlation with visceral fat and is not affected by muscle. Thus, it can better display the content of fat than the traditional BMI.
The study also confirmed that the knee joint function scores of the High-ABSI population were lower than those of the low-ABSI population. Multivariate logistic analysis also demonstrated that high ABSI was the influencing factor of poor function after ACLR. Similar to a previous study[24], there was no significant difference between the two groups in various functional scores (ROM compliance rate, quadriceps femoris atrophy index, Lysholm score, etc) before surgery (P > 0.05). With the progress of postoperative rehabilitation, the ROM scores, quadriceps femoris atrophy index, and Lysholm score of the two groups were significantly improved compared with those before surgery (P < 0.05). Subsequently, it was gradually revealed that the knee joint function of the High-ABSI group was still lower than that of the Low-ABSI group. At the 6th mo and 12th mo after operation, the knee joint function score of the Low-ABSI group was significantly lower than that of the Low-ABSI group. These results also confirmed the conclusion of previous research[4] that obese people would experience increased load on the lower limbs, thus affecting their recovery of knee joints. Besides, the risk of lower limb deep venous thrombosis after operation in the High-ABSI population was also higher than that in the Low-ABSI population, since obesity is a risk factor for deep venous thrombosis (OR = 1.67, 95%CI: 1.16-2.40; P = 0.006), and Mendel randomization verified the causal relationship between BMI and deep vein thrombosis[29].
Fat plays an important role in the formation of deep venous thrombosis of lower limbs. Studies have shown that lipid metabolism in adipocytes participates in the process of thrombosis, and adipocytes can promote thrombosis by releasing a variety of thrombogenic factors, such as coagulation factors, platelet activating factors, platelet-derived active substances, etc[30]. Therefore, in the process of athletes' rehabilitation, it is necessary to take measures to reduce obesity, thus reducing the incidence rate of deep vein thrombosis.
Out investigation had some limitations. This was a retrospective study with a limited number of participants and results that only covered a brief period of time. In addition, this study is part of a larger correlation investigation. To investigate the possible causal connection between ABSI and the postoperative function of ACLR in the future, additional randomized controlled trials or Mendelian randomization will be required.
ABSI is a risk factor for poor prognosis of knee function in ACL athletes after ACLR, and the risk of poor knee function after ACLR, deep vein thrombosis of the lower limb, and ACL recurrent rupture gradually increases with the rise of ABSI.
At present, the indicators of individual obesity only rely on body mass index (BMI) index, but BMI is often not linearly related to body fat content, which limits the research on the association between obesity and other diseases.
This study introduced a body shape index (ABSI), which is a body type indicator to replace traditional BMI to objectively evaluate the association between athletes' body size/obesity and anterior cruciate ligament reconstruction (ACLR).
Explore the relationship between knee joint function in athletes with ABSI and anterior cruciate ligament injuries after ACLR.
Multiple logistic regression analysis was used to investigate the relationship between knee joint function scores and postoperative complications after ABSI and ACLR surgery.
The knee joint function score of the Low-ABSI group was higher than that of the High-ABSI group (P < 0.05). High ABSI is a risk factor for low score of knee joint function after operation, and also a risk factor for deep vein thrombosis of lower limbs.
ABSI is closely related to the prognosis of knee joint function after ACLR. The rise of ABSI is likely to lead to poor knee function after ACLR and deep vein thrombosis of lower limbs.
In the future, randomized controlled trials or Mendelian randomization are needed to verify the possible causal relationship between ABSI and postoperative function of ACLR.
| 1. | van Yperen DT, Reijman M, van Es EM, Bierma-Zeinstra SMA, Meuffels DE. Twenty-Year Follow-up Study Comparing Operative Versus Nonoperative Treatment of Anterior Cruciate Ligament Ruptures in High-Level Athletes. Am J Sports Med. 2018;46:1129-1136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 94] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 2. | Wiggins AJ, Grandhi RK, Schneider DK, Stanfield D, Webster KE, Myer GD. Risk of Secondary Injury in Younger Athletes After Anterior Cruciate Ligament Reconstruction: A Systematic Review and Meta-analysis. Am J Sports Med. 2016;44:1861-1876. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 996] [Cited by in RCA: 956] [Article Influence: 95.6] [Reference Citation Analysis (0)] |
| 3. | Wilk KE, Arrigo CA. Rehabilitation Principles of the Anterior Cruciate Ligament Reconstructed Knee: Twelve Steps for Successful Progression and Return to Play. Clin Sports Med. 2017;36:189-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 93] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 4. | Meyer NL, Sundgot-Borgen J, Lohman TG, Ackland TR, Stewart AD, Maughan RJ, Smith S, Müller W. Body composition for health and performance: a survey of body composition assessment practice carried out by the Ad Hoc Research Working Group on Body Composition, Health and Performance under the auspices of the IOC Medical Commission. Br J Sports Med. 2013;47:1044-1053. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 93] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 5. | Ackland TR, Lohman TG, Sundgot-Borgen J, Maughan RJ, Meyer NL, Stewart AD, Müller W. Current status of body composition assessment in sport: review and position statement on behalf of the ad hoc research working group on body composition health and performance, under the auspices of the I.O.C. Medical Commission. Sports Med. 2012;42:227-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 308] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 6. | Luc B, Gribble PA, Pietrosimone BG. Osteoarthritis prevalence following anterior cruciate ligament reconstruction: a systematic review and numbers-needed-to-treat analysis. J Athl Train. 2014;49:806-819. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 310] [Cited by in RCA: 295] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 7. | Barenius B, Ponzer S, Shalabi A, Bujak R, Norlén L, Eriksson K. Increased risk of osteoarthritis after anterior cruciate ligament reconstruction: a 14-year follow-up study of a randomized controlled trial. Am J Sports Med. 2014;42:1049-1057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 338] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 8. | Messier SP, Beavers DP, Loeser RF, Carr JJ, Khajanchi S, Legault C, Nicklas BJ, Hunter DJ, Devita P. Knee joint loading in knee osteoarthritis: influence of abdominal and thigh fat. Med Sci Sports Exerc. 2014;46:1677-1683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 9. | Brambilla L, Pulici L, Carimati G, Quaglia A, Prospero E, Bait C, Morenghi E, Portinaro N, Denti M, Volpi P. Prevalence of Associated Lesions in Anterior Cruciate Ligament Reconstruction: Correlation With Surgical Timing and With Patient Age, Sex, and Body Mass Index. Am J Sports Med. 2015;43:2966-2973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 141] [Article Influence: 12.8] [Reference Citation Analysis (1)] |
| 10. | Caballero B. Humans against Obesity: Who Will Win? Adv Nutr. 2019;10:S4-S9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 417] [Article Influence: 59.6] [Reference Citation Analysis (0)] |
| 11. | Roche AF, Sievogel RM, Chumlea WC, Webb P. Grading body fatness from limited anthropometric data. Am J Clin Nutr. 1981;34:2831-2838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 214] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 12. | Chen J, Guan Z, Wang L, Song G, Ma B, Wang Y. Meta-analysis: overweight, obesity, and Parkinson's disease. Int J Endocrinol. 2014;2014:203930. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 13. | Prentice AM, Jebb SA. Beyond body mass index. Obes Rev. 2001;2:141-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 769] [Cited by in RCA: 782] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 14. | Krakauer NY, Krakauer JC. A new body shape index predicts mortality hazard independently of body mass index. PLoS One. 2012;7:e39504. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 462] [Cited by in RCA: 876] [Article Influence: 62.6] [Reference Citation Analysis (0)] |
| 15. | Christakoudi S, Tsilidis KK, Muller DC, Freisling H, Weiderpass E, Overvad K, Söderberg S, Häggström C, Pischon T, Dahm CC, Zhang J, Tjønneland A, Halkjær J, MacDonald C, Boutron-Ruault MC, Mancini FR, Kühn T, Kaaks R, Schulze MB, Trichopoulou A, Karakatsani A, Peppa E, Masala G, Pala V, Panico S, Tumino R, Sacerdote C, Quirós JR, Agudo A, Sánchez MJ, Cirera L, Barricarte-Gurrea A, Amiano P, Memarian E, Sonestedt E, Bueno-de-Mesquita B, May AM, Khaw KT, Wareham NJ, Tong TYN, Huybrechts I, Noh H, Aglago EK, Ellingjord-Dale M, Ward HA, Aune D, Riboli E. A Body Shape Index (ABSI) achieves better mortality risk stratification than alternative indices of abdominal obesity: results from a large European cohort. Sci Rep. 2020;10:14541. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 146] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 16. | Gažarová M, Galšneiderová M, Mečiarová L. Obesity diagnosis and mortality risk based on a body shape index (ABSI) and other indices and anthropometric parameters in university students. Rocz Panstw Zakl Hig. 2019;70:267-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 17. | Christakoudi S, Tsilidis KK, Evangelou E, Riboli E. A Body Shape Index (ABSI), hip index, and risk of cancer in the UK Biobank cohort. Cancer Med. 2021;10:5614-5628. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 51] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 18. | Patel NK, Sabharwal S, Hadley C, Blanchard E, Church S. Factors affecting return to sport following hamstrings anterior cruciate ligament reconstruction in non-elite athletes. Eur J Orthop Surg Traumatol. 2019;29:1771-1779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 19. | Mizuno T. Effects of Dynamic Stretching Velocity on Joint Range of Motion, Muscle Strength, and Subjective Fatigue. J Strength Cond Res. 2022;36:2440-2447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 20. | Peck BD, Brightwell CR, Johnson DL, Ireland ML, Noehren B, Fry CS. Anterior Cruciate Ligament Tear Promotes Skeletal Muscle Myostatin Expression, Fibrogenic Cell Expansion, and a Decline in Muscle Quality. Am J Sports Med. 2019;47:1385-1395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 21. | Ballal MS, Khan Y, Hastie G, Hatcher A, Coogan S, McNicholas MJ. Functional outcome of primary hamstring anterior cruciate ligament reconstruction in patients with different body mass index classes. Arthroscopy. 2013;29:1314-1321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 22. | Collins NJ, Prinsen CA, Christensen R, Bartels EM, Terwee CB, Roos EM. Knee Injury and Osteoarthritis Outcome Score (KOOS): systematic review and meta-analysis of measurement properties. Osteoarthritis Cartilage. 2016;24:1317-1329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 519] [Cited by in RCA: 525] [Article Influence: 52.5] [Reference Citation Analysis (0)] |
| 23. | Czuppon S, Racette BA, Klein SE, Harris-Hayes M. Variables associated with return to sport following anterior cruciate ligament reconstruction: a systematic review. Br J Sports Med. 2014;48:356-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 229] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 24. | Zaffagnini S, Grassi A, Marcheggiani Muccioli GM, Tsapralis K, Ricci M, Bragonzoni L, Della Villa S, Marcacci M. Return to sport after anterior cruciate ligament reconstruction in professional soccer players. Knee. 2014;21:731-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 99] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 25. | Ardern CL, Webster KE, Taylor NF, Feller JA. Return to sport following anterior cruciate ligament reconstruction surgery: a systematic review and meta-analysis of the state of play. Br J Sports Med. 2011;45:596-606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1016] [Cited by in RCA: 879] [Article Influence: 58.6] [Reference Citation Analysis (0)] |
| 26. | Mellion KM, Grover BT. Obesity, Bariatric Surgery, and Hip/Knee Arthroplasty Outcomes. Surg Clin North Am. 2021;101:295-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 27. | Li RT, Lorenz S, Xu Y, Harner CD, Fu FH, Irrgang JJ. Predictors of radiographic knee osteoarthritis after anterior cruciate ligament reconstruction. Am J Sports Med. 2011;39:2595-2603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 191] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 28. | DiSilvestro KJ, Jauregui JJ, Glazier E, Cherkalin D, Bennett CH, Packer JD, Henn RF 3rd. Outcomes of Anterior Cruciate Ligament Reconstruction in Obese and Overweight Patients: A Systematic Review. Clin J Sport Med. 2019;29:257-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 29. | Tan JS, Liu NN, Guo TT, Hu S, Hua L. Genetically predicted obesity and risk of deep vein thrombosis. Thromb Res. 2021;207:16-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 70] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 30. | Rothberg DL, Makarewich CA. Fat Embolism and Fat Embolism Syndrome. J Am Acad Orthop Surg. 2019;27:e346-e355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 87] [Article Influence: 12.4] [Reference Citation Analysis (1)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Orthopedics
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Baird PN, Australia; Mahmud N, United States S-Editor: Liu JH L-Editor: Filipodia P-Editor: Liu JH