Published online Jun 26, 2023. doi: 10.12998/wjcc.v11.i18.4438
Peer-review started: April 15, 2023
First decision: April 28, 2023
Revised: May 8, 2023
Accepted: May 23, 2023
Article in press: May 23, 2023
Published online: June 26, 2023
Processing time: 72 Days and 13.4 Hours
When a firm facial mass in the cheek region is associated with a high index of clinical suspicion of its being of parotid gland origin, preventive parotidectomy is invariably performed. We report a rare case of a schwannoma that was suspected to be of parotid gland origin in a patient, who underwent successful surgical management using a modified-Blair incision and superficial musculoaponeurotic system (SMAS) layer folding method.
A 27-year-old woman presented to the hospital for evaluation of a firm, fixed, non-tender mass (2.5 cm × 3.5 cm), located anterior to the right ear, of 1 year’s duration. Contrast-enhanced facial computed tomography revealed a well-encapsulated, low-density mass adherent to the superficial lobe of the right parotid gland, with a high index of clinical suspicion of an accessory parotid gland mass. The patient was scheduled to undergo resection of the mass and superficial parotidectomy. She underwent surgery using a modified-Blair incision, and the SMAS layer was folded posteriorly to reconstruct the defect. Histopathological examination confirmed the diagnosis of a schwannoma., and we observed no postoperative complications such as hematoma, infection, or abnormal facial expressions. The incision scar was unnoticeable 2 mo postoperatively, and the facial contour was maintained without any differences between the affected and unaffected sides.
We used a modified-Blair incision and SMAS layer folding method to achieve aesthetically good results following resection of a rare schwannoma with superficial parotidec
Core Tip: Schwannoma in the face region is a rare type of tumor that originates from the Schwann cells of ectodermal origin. It can develop in any cranial nerve but rarely originates from peripheral nerves. Masses in the cheek region are usually suspected to be of parotid gland origin, and preventive parotidectomy is usually performed. In our case, the mass was initially diagnosed as arising from the parotid gland, but was finally confirmed to be a schwannoma. Therefore, we should keep in mind the possibility of schwannoma, although rare.
- Citation: Nam HJ, Choi HJ, Byeon JY, Wee SY. Removal of unexpected schwannoma with superficial parotidectomy using modified-Blair incision and superficial musculoaponeurotic system folding: A case report. World J Clin Cases 2023; 11(18): 4438-4445
- URL: https://www.wjgnet.com/2307-8960/full/v11/i18/4438.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i18.4438
A firm facial mass anterior to the ear in the cheek region is associated with a high index of clinical suspicion of being derived from the parotid gland. Typically, a mass originating from the parotid gland is most commonly a pleomorphic adenoma (among benign tumors), whereas mucoepidermoid carcinoma is the most common malignant tumor; the main treatment is preventive superficial or total parotidectomy. However, parotidectomy is associated with a risk of scar formation owing to the wide surgical incision and cavity formation after parotid gland resection, which can result in a depressed facial contour postoperatively[1-3].
Accurate preoperative evaluation of facial masses, particularly those in the cheek region, is necessary; however, in most cases, the diagnosis relies on preoperative radiological assessments, and the final diagnosis is confirmed by postoperative histopathological findings. Therefore, preventive parotidec
In this report, we describe a rare case of a facial schwannoma and discuss our surgical approach for successful mass extirpation and preventive parotidectomy accompanied by a modified-Blair incision to minimize scarring, and the superficial musculoaponeurotic system (SMAS) layer folding method to restore the facial contour.
A 27-year-old woman presented for evaluation of a mass in the region of the cheek anterior to the right ear.
The mass had gradually increased in size over the year prior to presentation. The patient did not report any local symptoms such as pain or heat sensation and did not complain of any general health problems.
The patient denied any medical, medication, or surgical history, and reported no history of external trauma to the right side of the face.
There was no significant personal or family history.
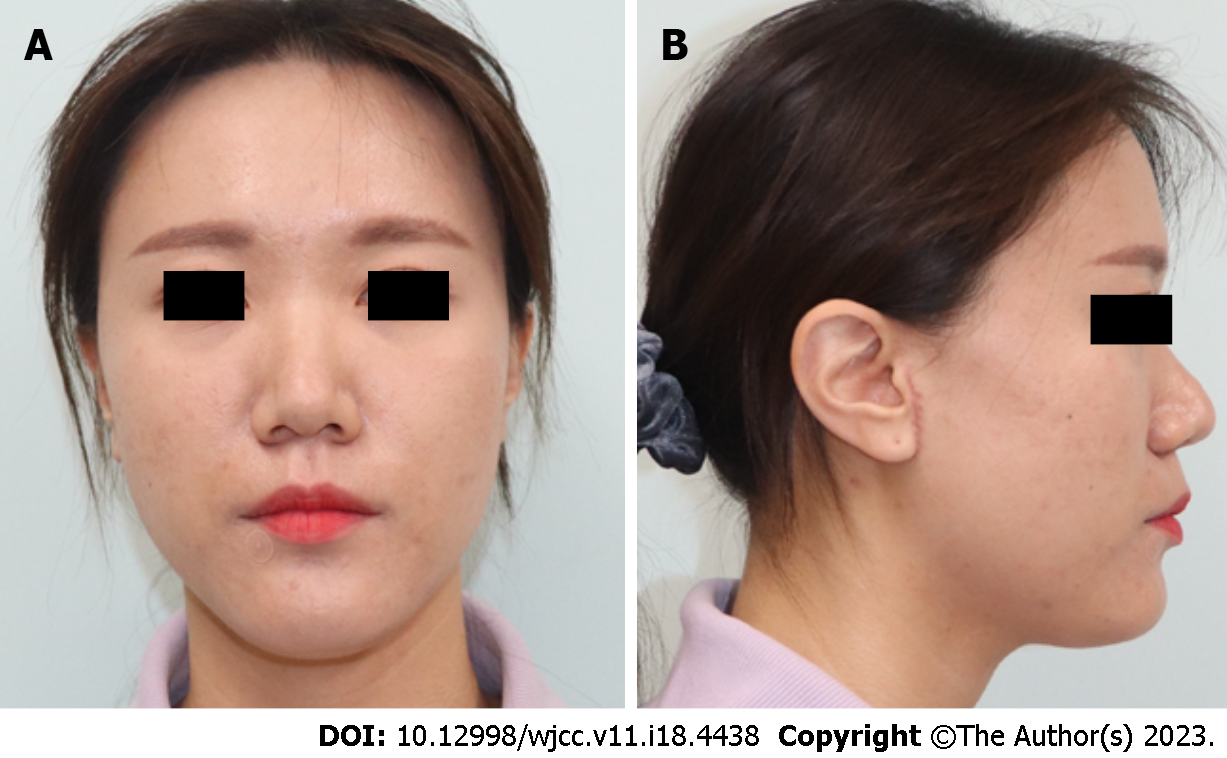
Physical examination of the mass revealed a firm and fixed mass (2.5 cm × 3.5 cm) without any signs of infection, such as tenderness, swelling, induration, or any abnormal skin surface sensation (Figure 1). We observed a local bulge secondary to a mass effect; however, the facial expressions were normal (Figure 2).
There were no abnormal findings on evaluation of laboratory samples. The blood results were within normal limits; the serum amylase level was 30 U/L (normal range is 30 to 110 U/L).
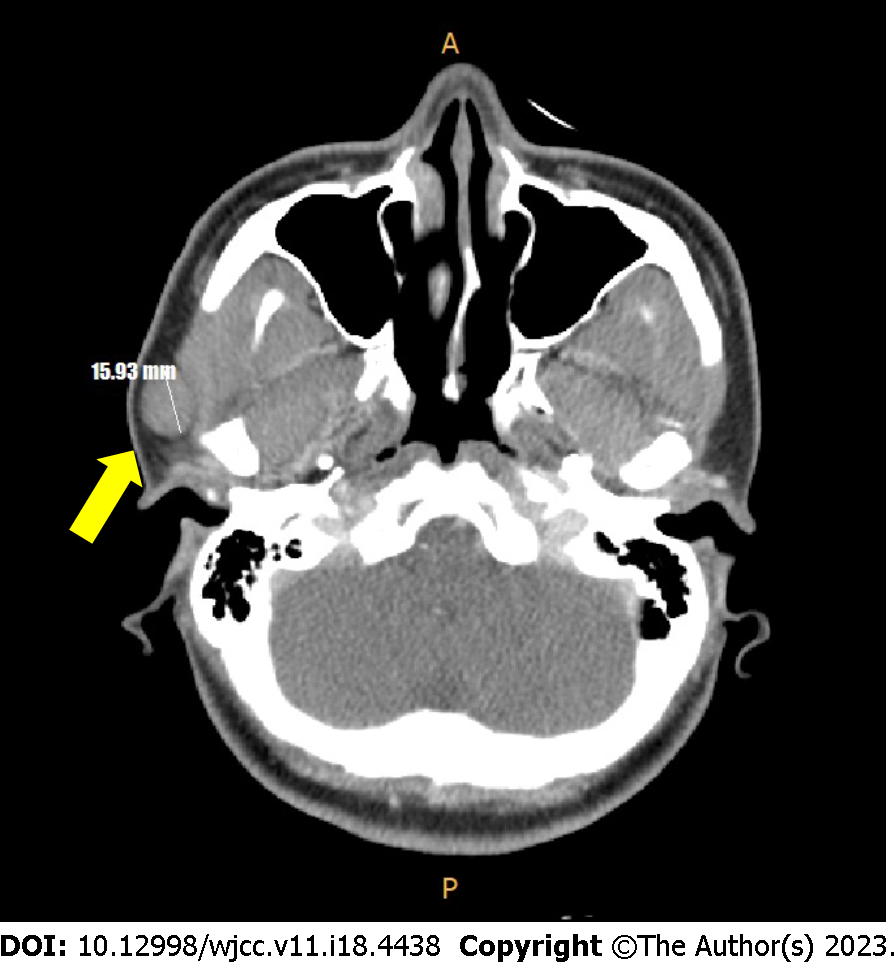
Contrast-enhanced facial computed tomography (CT) revealed a well-encapsulated low-density mass (1.6 cm × 1.3 cm) adherent to the inferior portion of the superficial lobe of the right parotid gland (Figure 3).
The patient was thoroughly informed regarding the potential for a mass originating from the parotid gland and the necessity of prophylactic parotidectomy and gave informed consent. Additionally, the definitive diagnosis was established through histological examination of the excised mass, and the patient was informed of alternative diagnoses.
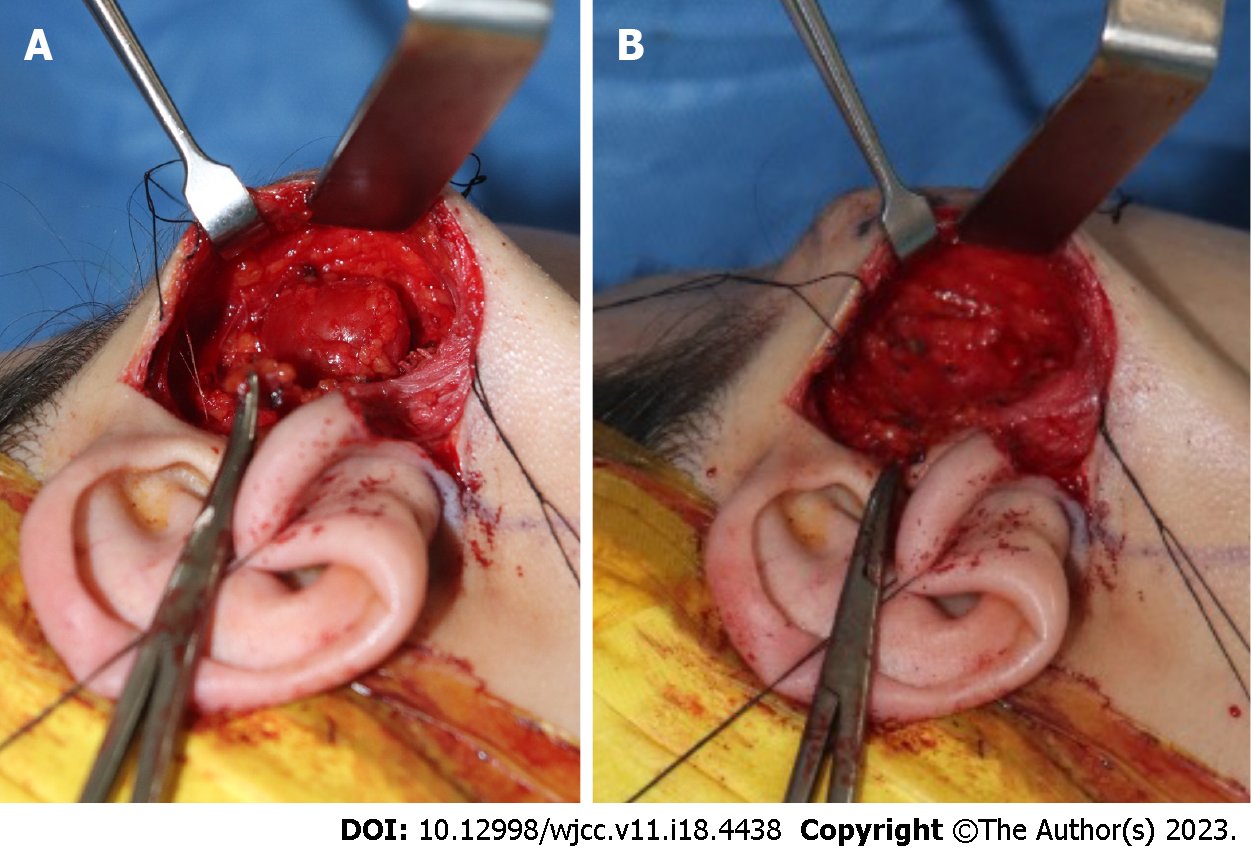
Considering the possibility of an accessory parotid gland mass, we performed complete resection with superficial parotidectomy. The surgical procedure involved the creation of a modified-Blair incision[4]. The incision line was drawn along the preauricular skin crease, commencing from the anterior and superior tragus and extending below the earlobe to the postauricular area, but not to the mandibular border of the chin (Figure 4). After creating the incision, the skin flap was dissected along the plane of the SMAS layer and elevated anteriorly, with exposure of the superficial lobe of the parotid gland, which revealed a mass in the fascia. The mass was encapsulated below the superficial lobe of the parotid gland and measured 2 cm × 2 cm. No parotid gland invasion was observed; however, the mass was firmly adherent to the gland. There was no clear evidence of feeding vessels or nerves supplying the mass (Figure 5A).
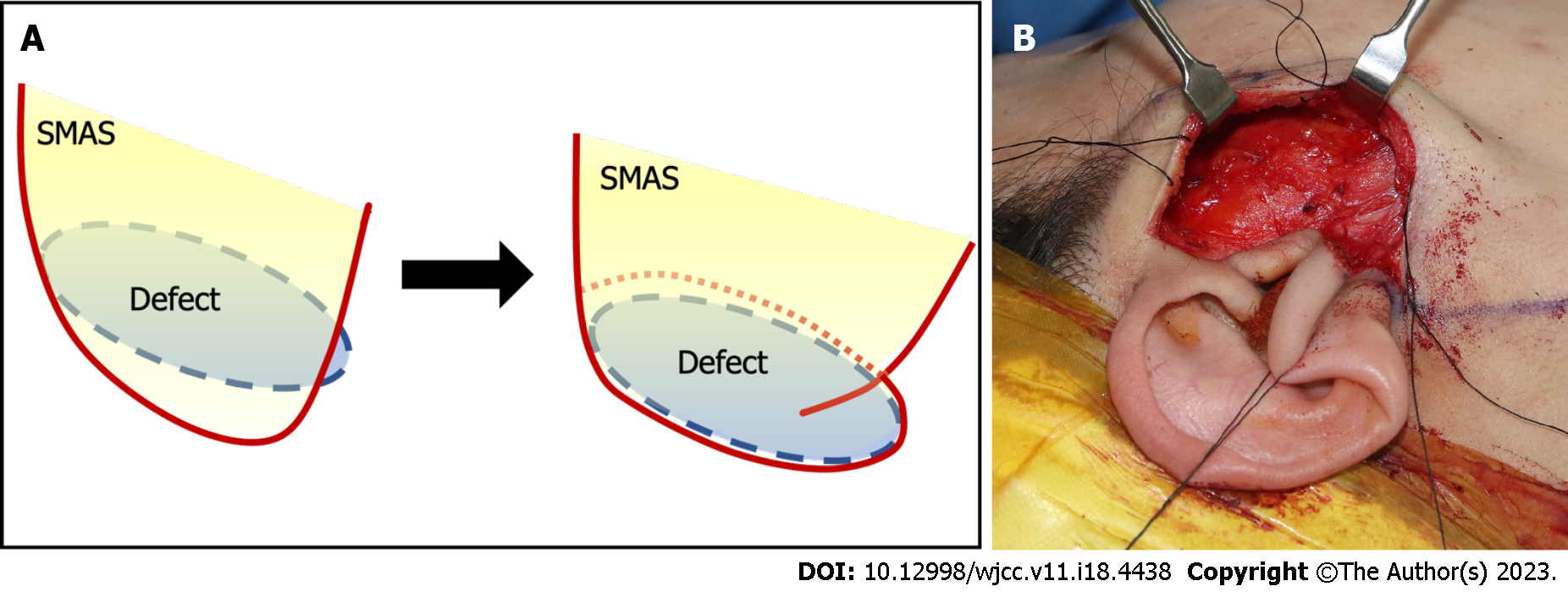
After superficial parotidectomy, we confirmed that the facial nerve branches coursing through the glands were intact (Figure 5B). The lateral portion of the elevated SMAS layer is separated by approximately 2 cm and folded to fit into the dead space. The defect was filled and closure was performed layer-by-layer (Figure 6).
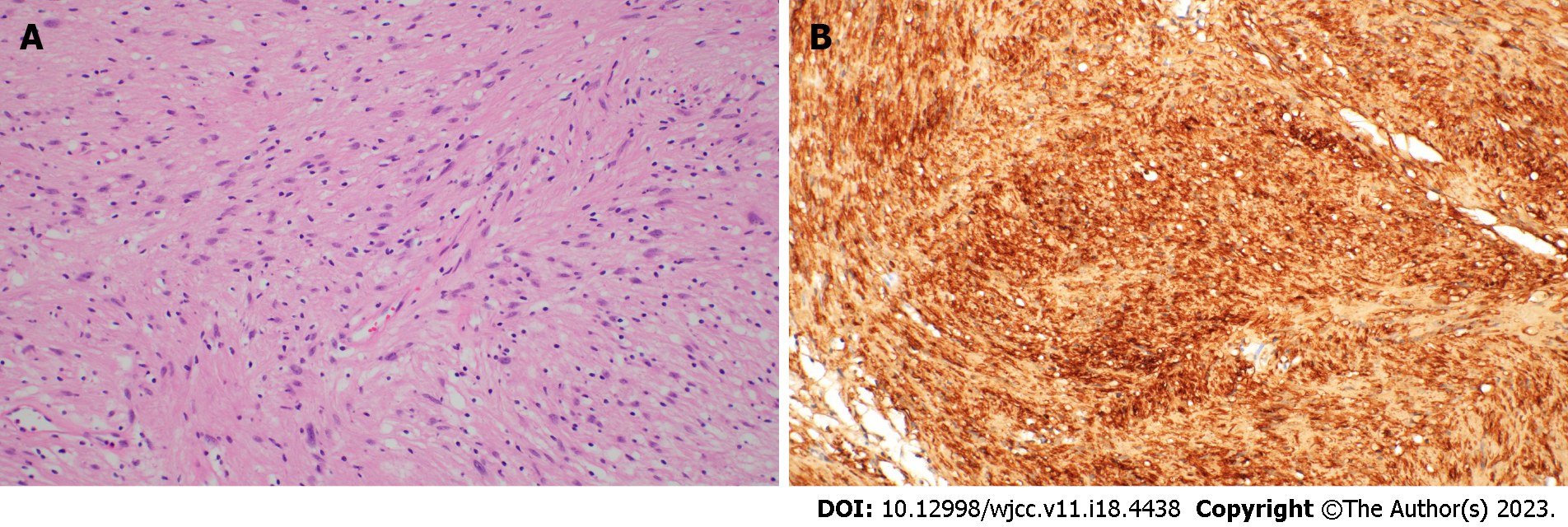
Histopathological evaluation of the resected specimen revealed a multilobular neural lesion, which was specifically diagnosed as a schwannoma (Figure 7).
The patient showed satisfactory postoperative healing without any signs of hematoma, infection, skin necrosis abnormalities in facial expression, or any neuropathies. The incision scar was unnoticeable, and no scar-induced complications were observed at the 2-mo follow-up. The facial contour was maintained without any differences compared to the contralateral face (Figure 8).
Schwannomas are benign encapsulated tumors of the peripheral nerve sheath that originate from Schwann cells of the motor or sensory nerves. Facial schwannomas are rare; fewer than 100 cases have been reported in the literature and usually originate from the peripheral facial nerve or other nerves within the parotid gland. No sex-based differences are observed in the incidence, and the peak incidence is observed in patients aged 30-60 years[5,6].
Facial schwannomas of the cheek region typically present as painless, slow-growing lumps. The lesion typically has a well-demarcated border and is hard to touch. The symptoms are usually nonspecific[7,8]. Imaging tests such as Contrast-enhanced facial CT reveal a well-defined mass containing a cystic lesion and displacement of the surrounding structures. They are typically homogeneous and may contain cystic or necrotic regions. Magnetic resonance imaging (MRI) is another imaging modality that can be used to visualize schwannomas. On MRI finding, schwannomas typically appear as well-defined, encapsulated masses. The signal intensity of the mass may be heterogeneous, reflecting areas of cystic degeneration or hemorrhage. however, it is difficult to diagnose based on these findings. Schwannomas that originate from the facial nerve or its branches may manifest with the clinical symptoms of facial nerve paralysis, although the incidence of such lesions is low. They may originate from nerves other than the facial nerve, and the preoperative diagnosis is inaccurate in more than 75% of cases[9].
Our patient presented with a painless mass without any abnormalities in facial expression or symptoms during the preoperative physical examination. The contrast-enhanced facial CT scan of the patient revealed a well-defined, round-shaped mass, which is a typical pattern for schwannomas on CT imaging. The MRI was recommended; however, the patient refused owing to the cost issue, and therefore, a definitive diagnosis could not be established through imaging examinations. Additionally, no evidence of facial nerve branch impingement was observed intraoperatively, suggesting that the schwannoma did not originate from the facial nerve or its branches.
Usually, preventive parotidectomy is performed to avoid the risk of recurrence or malignant transformation in patients with a suspected parotid gland mass in cheek region, even in cases of benign lesions[2,10]. In this case, we considered the possibility of an accessory parotid gland and performed preventive superficial parotidectomy using a modified-Blair incision and the SMAS layer folding method to address the potential issues of scarring and contour deformity derived from dead space after parotid gland resection.
In the cheek region, the modified-Blair incision is commonly used in surgical treatment to minimize scarring compared to the conventional Blair incision and simultaneously reduce the risk of facial nerve injury[11-13]. In this case, we used the modified-Blair incision line superior to jawline, so that we could avoid facial nerve injury during parotidectomy and minimize scarring. Additionally, the SMAS layer was folded into the defect after extirpation of the mass, so we could successfully minimize facial contour deformities and achieve a more aesthetically favorable outcome postoperatively.
Our study has several limitations. First, we overlooked the possibility of a schwannoma and performed preventive parotidectomy, owing to limited preoperative radiological evaluations. In addition, we did not detect any nerve impingement intraoperatively, and consequently only the mass was excised, despite it being a basic surgical principle for schwannomas that the fascicle should be preserved. However, despite these pre-and intraoperative diagnostic limitations, the entire mass was successfully excised, and the patient did not show any evidence of surgery-induced neuropathy. For superficial parotidectomy, a modified-Blair incision was used to minimize incisional scarring, and the SMAS layer folding method was performed to fill the dead space so that surgical complications were minimal.
Facial schwannomas in the cheek region are rare and preoperative evaluation has some limitations. Despite these limitations, our surgical approach of superficial parotidectomy using a modified-Blair incision and the SMAS layer-folding method achieved aesthetically good postoperative outcomes.
| 1. | Lin CC, Tsai MH, Huang CC, Hua CH, Tseng HC, Huang ST. Parotid tumors: a 10-year experience. Am J Otolaryngol. 2008;29:94-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 76] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 2. | Sungur N, Akan IM, Ulusoy MG, Ozdemir R, Kilinç H, Ortak T. Clinicopathological evaluation of parotid gland tumors: a retrospective study. J Craniofac Surg. 2002;13:26-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 3. | Maahs GS, Oppermann Pde O, Maahs LG, Machado Filho G, Ronchi AD. Parotid gland tumors: a retrospective study of 154 patients. Braz J Otorhinolaryngol. 2015;81:301-306. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 4. | Bailey H. The tumors of the parotid gland with special reference to total parotidectomy. Br J Surg. 1941;28:337-346. [RCA] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 80] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 5. | Simone M, Vesperini E, Viti C, Camaioni A, Lepanto L, Raso F. Intraparotid facial nerve schwannoma: two case reports and a review of the literature. Acta Otorhinolaryngol Ital. 2018;38:73-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 6. | Bhaker P, Chatterjee D, Gochhait D, Radotra BD, Dey P. Schwannoma of the parotid gland: Diagnosis by fine-needle aspiration cytology. J Cytol. 2014;31:196-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 7. | Neely JG, Alford BR. Facial nerve neuromas. Arch Otolaryngol. 1974;100:298-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 33] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 8. | Avery AP, Sprinkle PM. Benign intraparotid schwannomas. Laryngoscope. 1972;82:199-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 20] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 9. | Guzzo M, Ferraro L, Ibba T, Quattrone P, Bianchi R, Rezzonico S, Scaramellini G. Schwannoma in the parotid gland. Experience at our institute and review of the literature. Tumori. 2009;95:846-851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 10. | Shashinder S, Tang IP, Velayutham P, Prepageran N, Gopala KG, Kuljit S, Anura MM, Chong SY. A review of parotid tumours and their management: a ten-year-experience. Med J Malaysia. 2009;64:31-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 11. | Hwang JH, Lee DG, Sim HS, Kim KS, Lee SY. Intramasseteric schwannoma treated with facelift incision and retrograde facial nerve dissection. Arch Craniofac Surg. 2019;20:388-391. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 12. | Baliga M, Uppal N, Ramanathan A. Schwannomas of the head and neck: a case series. J Maxillofac Oral Surg. 2009;8:283-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 13. | Rigante M, Petrelli L, DE Corso E, Paludetti G. Intracapsular microenucleation technique in a case of intraparotid facial nerve schwannoma. Technical notes for a conservative approach. Acta Otorhinolaryngol Ital. 2015;35:49-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Surgery
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Pisani P, Italy; Tajiri K, Japan S-Editor: Yan JP L-Editor: A P-Editor: Yan JP