Published online May 16, 2023. doi: 10.12998/wjcc.v11.i14.3282
Peer-review started: December 21, 2022
First decision: January 20, 2023
Revised: February 2, 2023
Accepted: April 6, 2023
Article in press: April 6, 2023
Published online: May 16, 2023
Processing time: 145 Days and 18.5 Hours
Breast cancer is the most frequently diagnosed cancer worldwide. It is the leading cause of death by malignant disease in women.
A female patient, 73 years of age, sought care due to weakness, mild abdominal pain, arthralgia, and weight loss. She was taking anastrazole as maintenance therapy for localized breast cancer and had moderate anemia and elevated acute-phase markers. Upper digestive endoscopy showed isolated erosion in the gastric corpus. This lesion was compatible with signet-ring cell adenocarcinoma in anatomopathological study and was confirmed as metastasis of a breast carcinoma in immunohistochemistry, which was positive for estrogen antibody. Further imaging studies determined numerous proximal bone metastases. The patient was treated with prednisone for paraneoplastic syndrome, which improved the anemia and rheumatic disease, and with chemotherapy, which greatly improved the symptoms. She has been followed-up for 6 mo, and her anemia, arthralgias, and acute phase markers have normalized.
Systemic treatment strategies seem to be the best choice for gastric metastasis from breast cancer, resulting in disease control and relapse-free survival. Prospective studies with longer follow-up are needed to better understand the biological, pathological, and clinicopathological characteristics and outcomes of the endoscopic features associated with metastatic gastric cancer from breast carcinoma.
Core Tip: Breast cancer, the most frequently diagnosed type of cancer worldwide, is the leading cause of death due to malignant disease in women. We present the case of a female patient, 73 years of age, who sought care due to weakness, mild abdominal pain, arthralgia, and weight loss. She was taking anastrazole as a maintenance therapy for a localized breast cancer. She presented with moderate anemia and elevated acute phase markers. Upper digestive endoscopy showed isolated erosion in the gastric corpus. In anatomopathological study, the lesion was found to be compatible with signet-ring cell adenocarcinoma, while in in immunohistochemistry it was confirmed to be a metastasis of breast carcinoma, being positive for estrogen antibody. Further imaging studies determined numerous proximal bone metastases. The patient has been treated and followed up for 6 mo, and her anemia, arthralgias and acute phase markers have normalized. Systemic treatment strategies appear to be the best choice for gastric metastasis from breast cancer, providing disease control and relapse-free survival. Prospective studies with longer follow up are needed to better understand the biological, pathological, and clinicopathological characteristics and outcomes of the endoscopic features associated with metastatic gastric cancer from breast carcinoma.
- Citation: Rech MB, da-Cruz ER, Salgado K, Balbinot RA, Balbinot SS, Soldera J. Metastatic gastric cancer from breast carcinoma presenting with paraneoplastic rheumatic syndrome: A case report. World J Clin Cases 2023; 11(14): 3282-3287
- URL: https://www.wjgnet.com/2307-8960/full/v11/i14/3282.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i14.3282
Breast cancer is the most frequently diagnosed cancer worldwide. It is the leading cause of death due to malignant disease in women. Nevertheless, the lethality of this disease has been subsiding in recent decades due to the advancement of screening protocols and treatment options. It must be pointed out that breast carcinoma commonly metastasizes, generally to the lungs, bones, liver, or brain.
The aim of this report was to describe the case of a woman with metastatic gastric cancer from breast carcinoma who presented with rheumatic paraneoplastic syndrome, which responded well to steroids and chemotherapy.
A female patient, 73 years of age, sought care due to weakness, mild abdominal pain, arthralgia, and weight loss.
The patient presented moderate anemia, with a hemoglobin level of 9.9 g/dL, and elevated acute phase markers (ferritin 1657 ng/mL, C-reactive protein 16.9 mg/L, hem sedimentation rate 30 mm, and transferrin saturation 42%).
The patient was taking anastrazole as a maintenance therapy for localized breast cancer, which had been resected 4 years previously.
No previous evidence suggested that her breast cancer had metastasized.
Physical examination showed malnourishment and mild abdominal pain.
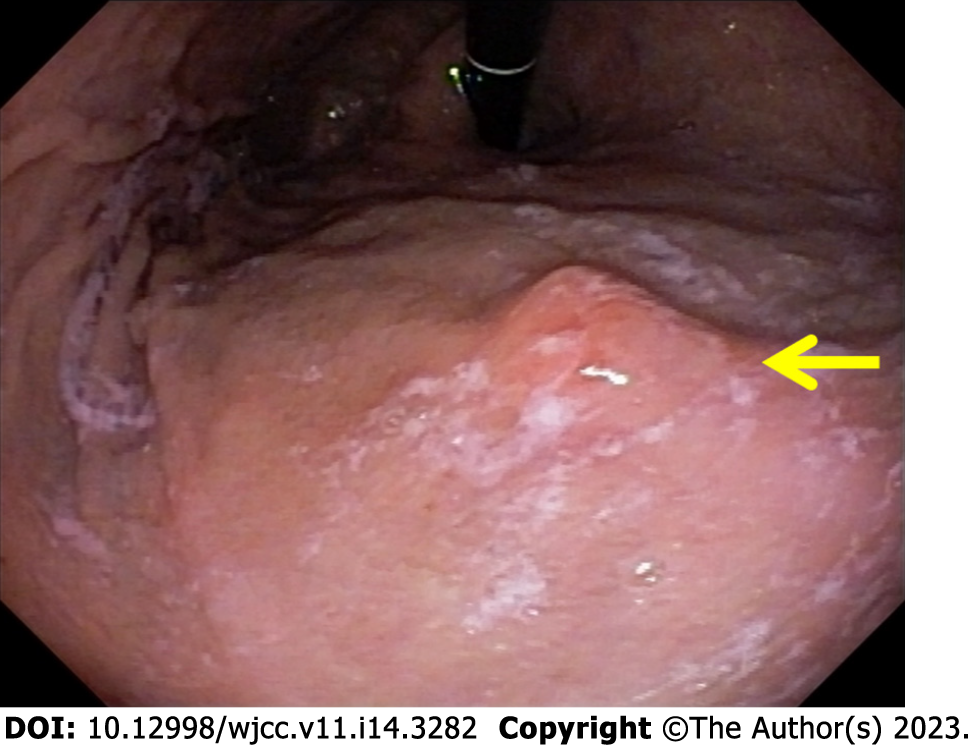
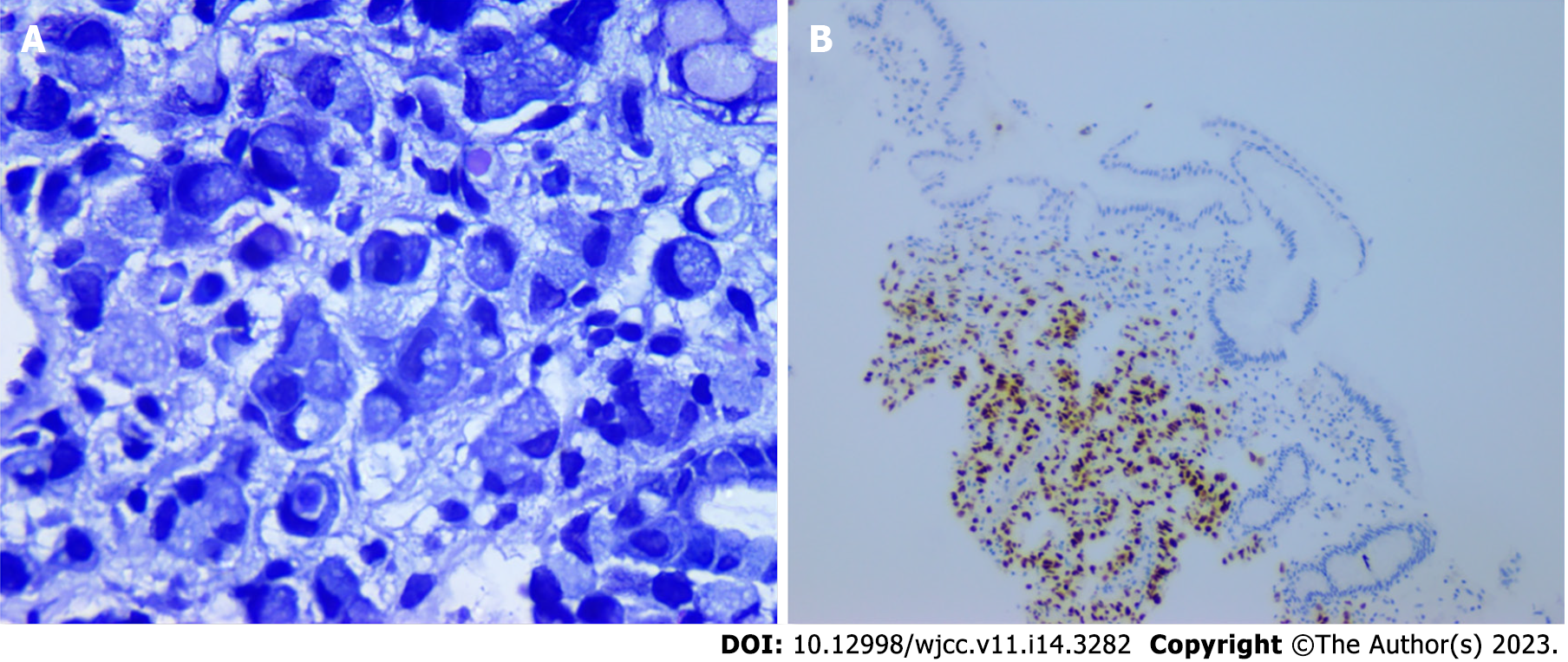
Colonoscopy revealed no remarkable findings, although upper digestive endoscopy showed isolated erosion in the gastric corpus (Figure 1). Anatomopathological study showed that the lesion was compatible with signet-ring cell adenocarcinoma, while and immunohistochemistry confirmed metastasis of the breast carcinoma, which was positive for estrogen antibody clone EP1 (Figure 2), GATA3 monoclonal antibody (L50-823), mammaglobin (23A3 + 304-1A5&31A5), equivocally positive for ERBB2/HER2 membrane receptor (Sp3), and negative for GCDFP-15 (EP1582Y).
Endoscopic ultrasound showed that the lesion was ineligible for endoscopic resection. Further imaging studies revealed numerous proximal bone metastases.
The patient was treated for paraneoplastic syndrome emulating polymyalgia rheumatica with prednisone at an initial dose of 1 mg/kg, with further tittering and reduction of the dose; the anemia and rheumatic disease improved.
The patient’s disease was treated with paclitaxel, pertuzumab, and trastuzumab. As it progressed, she was switched to trastuzumab deruxtecan, which greatly reduced the symptoms.
The patient has been followed up for over 6 mo, and her anemia, arthralgias, and acute phase markers have normalized (hemoglobin 11 g/dL, C-reactive protein < 5 mg/L, and hem sedimentation rate 23 mm).
We describe the case of a woman with rheumatic paraneoplastic syndrome and metastatic gastric cancer from breast carcinoma, which responded well to steroids and chemotherapy. The histological appearance was of signet-cell adenocarcinoma, like another case reported in 2015[1].
Metastatic gastric cancer is very rare, and treatment for a lesion secondary to breast carcinoma differs significantly from an adenocarcinoma of the stomach[2,3]. The prevalence of metastatic gastric cancer from breast carcinoma has been reported to be as low as 0.1%-0.5%[2,4]. Nevertheless, the prevalence in autopsies has been reported at approximately 1.7%[5]. This suggests that the prevalence of metastatic gastric cancer from breast carcinoma has been underestimated[6].
Since the most common symptoms of this metastasis are mild abdominal pain, weight loss, abdominal mass, nausea, early satiety, and melena, it is rather challenging to diagnose. Moreover, the radiological and endoscopic findings are non-specific, as was the isolated gastric erosion in the present case[7-9]. In addition, there is generally a long period of disease-free survival prior to diagnosis, which further delays diagnosis[8,10]. In a series of 37 metastatic gastric cancer cases, breast cancer was the third most common lesion (13.5%): The most common primary malignancy was melanoma (27.0%), followed by lung cancer (18.9%)[11].
In the present case, no symptoms of systemic metastasis were observed prior to those of paraneoplastic syndrome. This is not very rare: In a series of 7 patients, 6 with metastatic gastric cancer and 1 with metastatic colonic cancer from breast carcinoma, none had known systemic disease[12]. Concomitant metastasis to other organs is also possible, as in the bone metastases in the present case[13].
The largest series on metastatic gastric cancer from breast carcinoma was published in 2017 by Xu et al[14], comprising 78 cases, none with rheumatic paraneoplastic syndrome. However, after diagnosis of gastric metastasis, other organs were found to be affected in 27 patients. Correct diagnosis of these lesions requires endoscopic examination followed by biopsy and immunohistochemistry[14] for cytokeratin 20, cytokeratin 7, and estrogen receptors[15]. Although primary and metastatic gastrointestinal signet-ring cell carcinomas are generally cytokeratin 20-positive, very few metastatic lobular carcinomas are. Gastrointestinal carcinomas express estrogen receptors, as does almost every lobular breast carcinoma[15].
Early diagnosis allows early treatment of systemic disease, which generally involves hormone therapy. Surgical treatment should only be considered in cases of acute complications, such as gastrointestinal bleeding, obstruction, large lesions, or perforation[3,14,16]. In a case series of 35 patients, the chosen treatment was chemotherapy (37%), hormonotherapy (6%) or both (37%), with a 53% 2-year survival rate after diagnosis of gastric metastasis[3]. In a series of 78 patients, 56.4% received salvage chemotherapy and 51.3% received salvage hormone therapy; 41% underwent surgery, such as total gastrectomy, subtotal gastrectomy, or wedge resection; and 7.7% received radiotherapy[14]. The median survival was 10.5 mo. The best treatment choices seem to be chemotherapy or hormone therapy, both of which lead to increased survival and quality of life, as in the present case.
In conclusion, systemic treatment strategies appear to be the best choice for gastric metastasis from breast cancer, providing control of the disease and relapse-free survival. Prospective studies with longer follow-up are needed to better understand the biological, pathological, and clinicopathological characteristics and outcomes of the endoscopic features associated with metastatic gastric cancer from breast carcinoma.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: Federação Brasileira De Gastroenterologia; Sociedade Brasileira de Hepatologia; Sociedade Brasileira de Endoscopia Digestiva; Grupo de Estudos de Doença Inflamatória Intestinal do Brasil.
Specialty type: Medicine, research and experimental
Country/Territory of origin: Brazil
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Hou L, China; Qin Y, China; Wang Z, China S-Editor: Li L L-Editor: A P-Editor: Yu HG
| 1. | He CL, Chen P, Xia BL, Xiao Q, Cai FL. Breast metastasis of gastric signet-ring cell carcinoma: a case report and literature review. World J Surg Oncol. 2015;13:120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 2. | Abid A, Moffa C, Monga DK. Breast cancer metastasis to the GI tract may mimic primary gastric cancer. J Clin Oncol. 2013;31:e106-e107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 3. | Almubarak MM, Laé M, Cacheux W, de Cremoux P, Pierga JY, Reyal F, Bennett SP, Falcou MC, Salmon RJ, Baranger B, Mariani P. Gastric metastasis of breast cancer: a single centre retrospective study. Dig Liver Dis. 2011;43:823-827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 4. | Ambroggi M, Stroppa EM, Mordenti P, Biasini C, Zangrandi A, Michieletti E, Belloni E, Cavanna L. Metastatic breast cancer to the gastrointestinal tract: report of five cases and review of the literature. Int J Breast Cancer. 2012;2012:439023. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 69] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 5. | Taal BG, Boot H, van Heerde P, de Jong D, Hart AA, Burgers JM. Primary non-Hodgkin lymphoma of the stomach: endoscopic pattern and prognosis in low vs high grade malignancy in relation to the MALT concept. Gut. 1996;39:556-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 104] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 6. | Zelek L, Cottu PH, Mignot L, de Roquancourt A, Fizazi K, Cojean-Zelek I, Espie M, Marty M. Gastric metastases from breast cancer: a retrospective series of 12 patients. Am J Clin Oncol. 2001;24:363-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 7. | Taal BG, Peterse H, Boot H. Clinical presentation, endoscopic features, and treatment of gastric metastases from breast carcinoma. Cancer. 2000;89:2214-2221. [PubMed] |
| 8. | Takeuchi H, Hiroshige S, Yoshikawa Y, Kusumoto T, Muto Y. A case of synchronous metastasis of breast cancer to stomach and colon. Anticancer Res. 2012;32:4051-4055. [PubMed] |
| 9. | Sataloff DM, Dentchev D, Henry DH, Weese JL. Isolated Breast Metastases from Primary Gastric Adenocarcinoma. Breast J. 2000;6:62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 10. | Koike K, Kitahara K, Higaki M, Urata M, Yamazaki F, Noshiro H. Clinicopathological features of gastric metastasis from breast cancer in three cases. Breast Cancer. 2014;21:629-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 11. | Kim GH, Ahn JY, Jung HY, Park YS, Kim MJ, Choi KD, Lee JH, Choi KS, Kim DH, Lim H, Song HJ, Lee GH, Kim JH. Clinical and Endoscopic Features of Metastatic Tumors in the Stomach. Gut Liver. 2015;9:615-622. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 12. | Schwarz RE, Klimstra DS, Turnbull AD. Metastatic breast cancer masquerading as gastrointestinal primary. Am J Gastroenterol. 1998;93:111-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 123] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 13. | Fernandes GS, Corrêa TS, Carvalho EP, Katz A, Hoff PM. Gastric and endobronchial metastases in a case of lobular breast cancer. Case Rep Oncol. 2013;6:555-560. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Xu L, Liang S, Yan N, Zhang L, Gu H, Fei X, Xu Y, Zhang F. Metastatic gastric cancer from breast carcinoma: A report of 78 cases. Oncol Lett. 2017;14:4069-4077. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 15. | Tot T. The role of cytokeratins 20 and 7 and estrogen receptor analysis in separation of metastatic lobular carcinoma of the breast and metastatic signet ring cell carcinoma of the gastrointestinal tract. APMIS. 2000;108:467-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 85] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 16. | Gadde R, Tamariz L, Hanna M, Avisar E, Livingstone A, Franceschi D, Yakoub D. Metastatic gastric cancer (MGC) patients: Can we improve survival by metastasectomy? J Surg Oncol. 2015;112:38-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (1)] |