Published online May 6, 2023. doi: 10.12998/wjcc.v11.i13.3105
Peer-review started: February 7, 2023
First decision: February 28, 2023
Revised: March 16, 2023
Accepted: April 4, 2023
Article in press: April 4, 2023
Published online: May 6, 2023
Processing time: 76 Days and 14.6 Hours
Microsatellite instability (MSI) is a predictive biomarker for cancer immunotherapy. The tumor-agnostic nature of MSI makes it a denominator for immunotherapy in several solid tumors. It can be assessed using next-generation sequencing (NGS), fluorescent multiplex PCR, and immunohistochemistry (IHC).
Here, we report 3 cases with discordant MSI results detected using different methods. A cholangiocellular carcinoma case revealed proficient mismatch repair (MMR) by IHC but high MSI (MSI-H) by liquid NGS. A cervical cancer case revealed deficient MMR by IHC, microsatellite stable by PCR, and MSI-H by NGS. Lastly, an endometrial cancer case revealed proficient MMR by IHC but MSI-H by NGS.
IHC for MMR status is the first choice due to several advantages. However, in cases of indeterminate IHC results, molecular testing by MSI-PCR is preferred. Recently, NGS-based MSI assays are being widely used to detect MSI-H tumors. All three methods have high accuracy; however, the inconsistencies between them may lead to misdiagnosis.
Core Tip: Microsatellite instability (MSI), a predictive biomarker for cancer immunotherapy can be assessed using next-generation sequencing, fluorescent multiplex PCR, and immunohistochemistry (IHC). Even though IHC for mismatch repair status is the first choice, in cases of indeterminate IHC results, molecular testing by MSI-PCR is preferred. Recently, next-generation sequencing-based MSI assays are also being widely used. Although all methods have high accuracy, they may have inconsistent results leading to misdiagnosis.
- Citation: Şenocak Taşçı E, Yıldız İ, Erdamar S, Özer L. Discrepancy among microsatellite instability detection methodologies in non-colorectal cancer: Report of 3 cases. World J Clin Cases 2023; 11(13): 3105-3113
- URL: https://www.wjgnet.com/2307-8960/full/v11/i13/3105.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i13.3105
In the era of immunotherapy, microsatellite instability (MSI) is a key biomarker of genetic alteration. It is indicated by a high number of mutations within microsatellites, which are repeat sequences of 1-9 nucleotides[1]. While the DNA mismatch repair (MMR) system can correct DNA replication errors in normal tissues, the loss of function or lack of MMR genes in tumor cells causes MSI[2]. Thus, MSI is an important factor in tumor development, and its incidence correlates positively to survival[3].
MSI can be distinguished into three types: high (MSI-H); low; and stable (MSS)[4]. Lately, MSI has been identified in several cancer types[5]. The recent American Society of Clinical Oncology provisional guidelines on somatic mutations in metastatic and locally advanced cancer recommends the evaluation of MMR deficiency status, as MSI is accepted as a tumor-agnostic factor in all patients who are potential candidates for immunotherapy[6].
The most widely used methods for MSI assessment are next-generation sequencing (NGS), fluorescent multiplex PCR, and immunohistochemistry (IHC)[1]. IHC is the gold standard method due to its easy access, high sensitivity, and practical nature. It detects the expression of MMR proteins (MLH1, PMS2, MSH2, and MSH6) in tumor tissues[7]. NGS-based multiplex gene assay, approved for use in all solid tumors, can indirectly measure MMR status using DNA extracted from formalin-fixed paraffin-embedded tissue specimens, where deficient MMR tumors usually have a hypermutated phenotype[8]. Finally, PCR is a molecular approach that can be carried out on tumor DNA, measuring the MMR protein apparatus functionality[9]. Cases of indeterminate MSI status with IHC can occur if loss of only one heterodimer unit is present. Two reference panels of PCR, Bethesda and pentaplex, were designed for colorectal cancer (CRC) and have shown poor performance in other cancer types[10]. Despite the high accuracy of these methods (94.6%, 99.9%, and 89.0%-95.0% for PCR, NGS, and IHC, respectively), the inconsistency between them may result in misdiagnosis[11-13]. The specific guidance regarding preferred methodology is still lacking.
Here, we report a cholangiocellular carcinoma case revealing proficient MMR by IHC but MSI-H by liquid NGS. A cervical cancer case revealed deficient MMR by IHC, MSS by PCR but MSI-H by NGS. An endometrial cancer case revealed proficient MMR by IHC but MSI-H by NGS.
Case 1: A 43-year-old female patient with a history of Klatskin tumor was referred to our clinic with progressive disease.
Case 2: A 29-year-old female patient with a history of locally advanced cervical cancer was referred to our clinic for a second opinion.
Case 3: A 62-year-old female patient with a history of endometrial cancer presented with acute, intermittent mid-back pain for the 3 mo prior to admission.
Case 1: Progression of the present illness was found during treatment response evaluation 2 wk prior.
Case 2: Progression of cervical cancer was found during a screening a week prior.
Case 3: Pain had worsened for the prior 2-3 wk.
Case 1: She had presented with jaundice, epigastric pain, itching, and weakness to her doctor in 2018, and her abdominal ultrasonography revealed a mass near the liver. Magnetic resonance imaging (MRI) of the abdomen confirmed obstruction due to tumor confluence of the bile ducts. Secondary to the mechanical obstruction, there was external drainage of the bile ducts from the right anterior and posterior sections of the liver. She underwent a left hemi hepatectomy with total caudal lobectomy, cholecystectomy, and extended lymphadenectomy. The pathology revealed moderately differentiated adenocarcinoma, CK7+/CK20-/CK17+, consistent with cholangiocarcinoma, thus stage IIA disease. IHC revealed PD-L1 combined positive score of 0 and MSS disease. She received six cycles of adjuvant gemcitabine-cisplatin treatment. During follow-up in 2020, computed tomography (CT) demonstrated recurrence of the underlying disease with predominance of peritoneal carcinomatosis, after which she was again initiated on gemcitabine and cisplatin. After five treatment cycles, cisplatin intolerance developed and treatment was continued with capecitabine and gemcitabine. The response evaluation CT revealed progression of the underlying disease with an increase in the size of the known lesions, ascites, and pleural effusion.
Case 2: She was first diagnosed in 2019 and had received radical chemoradiotherapy. Local recurrence occurred in 2020. She received four cycles of carboplatin-paclitaxel and underwent total abdominal hysterectomy and bilateral salpingo-oophorectomy. Topotecan and bevacizumab were administered in June 2021 due to disease progression; however, a ureterovaginal fistula developed, for which she underwent surgery.
Case 3: She had undergone a surgery in 2010 for endometrioid adenocarcinoma with squamous differentiation. The pathology results revealed pT1bN0 with 60% estrogen receptor, 90% progesterone receptor, and 50% Ki-67 expression and was classified as stage I disease. She did not receive any adjuvant treatment.
Significant family or personal history was not detected for any of the cases.
Case 1: Vital signs were in the normal ranges. No abnormalities were found during systemic examination.
Case 2: Vital signs were as follows: body temperature, 36.0 °C; blood pressure, 100/60 mmHg; and heart rate, 90/min. She had colostomy. No other abnormalities were found during systemic examination.
Case 3: On physical examination, the vital signs were as follows: body temperature, 36.5 °C; blood pressure, 110/68 mmHg; and heart rate, 80/min. Systemic examination did not reveal any pathology.
Case 1: Carbohydrate antigen 19-9 level was elevated (1200 U/mL). Other analyses were in the normal ranges.
Case 2: Carbohydrate antigen 19-9 level was elevated (264000 U/mL). Other analyses were in the normal ranges.
Case 3: Levels of serum tumor markers were elevated (carbohydrate antigen 125, 51 U/mL; carbohydrate antigen 19-9, 175 U/mL).
Case 1: Positron emission tomography (PET/CT) was carried out for re-staging, and it revealed development of new hypermetabolic lesions in the left supraclavicular region, L2 corpus, and peritoneum.
Case 2: PET/CT was carried out for optimal staging in August 2021, which revealed increased uptake in the pelvis, more prominent in the left supra/peri vesical, left paracolic, and cutaneous regions. She was referred to our clinic with the results. We performed an MRI of the abdomen, which confirmed a recurrent mass, 60 mm × 81 mm in size, near the sigmoid colon.
Case 3: MRI of the lumbar spine performed due to back pain showed a 5 cm soft tissue mass near the left renal vein. Staging PET/CT confirmed the mass lesion without distant metastases.
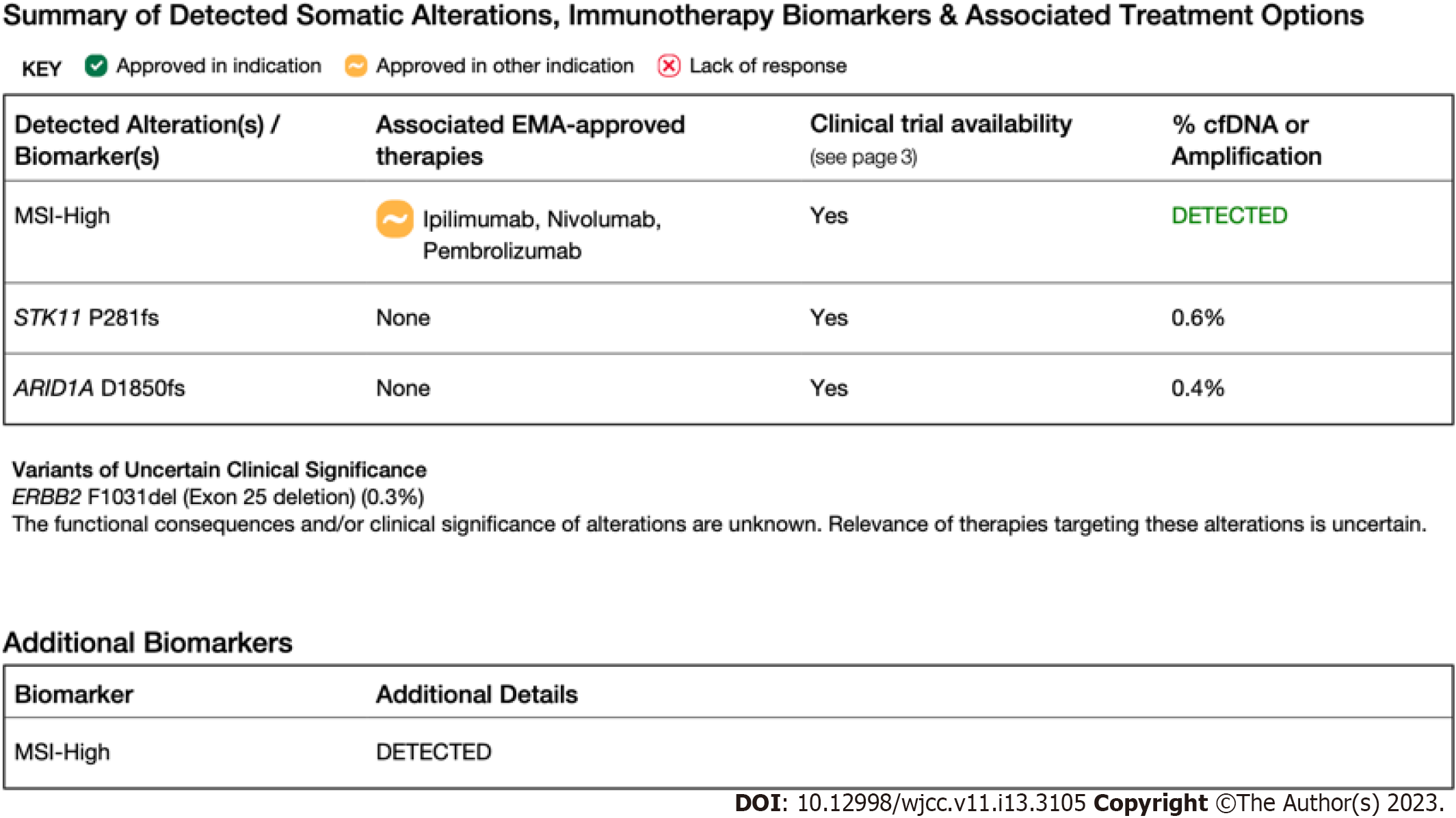
Case 1: NGS (FoundationOne CDx, 2021) was recommended for detailed molecular analysis instead of IHC and PCR. The molecular results from the surgical specimen revealed STK11 and ARID1A mutations and MSI-H disease (Figure 1).
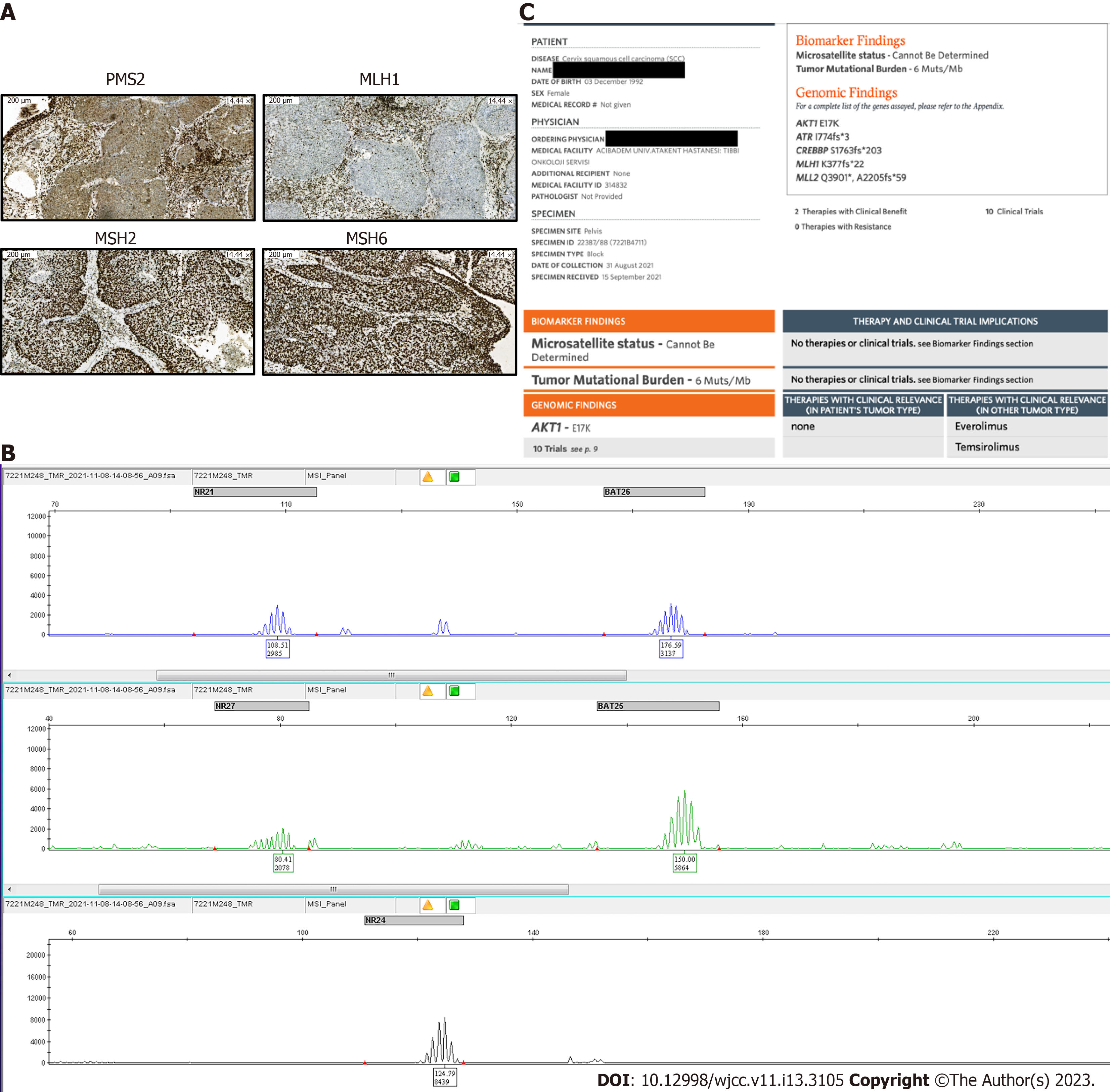
Case 2: A biopsy was performed for molecular analysis and concluded as metastasis of cervical cancer. IHC for MMR proteins showed loss of MLH-1 and PMS-2 expression, leading to a conclusion of MSI-H disease (Figure 2A). NGS (FoundationOneCDx, 2021) results from the pelvic mass revealed AKT1, ATR, CREBBP, and MLH1 mutations, as well as a tumor mutation burden (TMB) of 6 Mb (Figure 2B). Her MSI status could not be determined. PCR was performed to confirm the MSI status, and MSS disease was noted (Figure 2C).
Case 3: Renal mass and lymph node biopsy confirmed an adenocarcinoma, PAX8+/CK7+, consistent with primary endometrial cancer (stage IV endometrial cancer). IHC revealed no staining for human epidermal growth factor receptor 2 and did not show any losses for MMR proteins. NGS was recommended for detailed molecular analysis. However, NGS results from the metastases revealed MSI-H disease with a TMB of 54 mutations/Mb (Figure 3).
Case 1: The final diagnosis was stage IV Klatskin tumor.
Case 2: The patient was diagnosed with metastatic cervical cancer.
Case 3: The patient was diagnosed with recurrent endometrial carcinoma.
Case 1: FOLFIRINOX chemotherapy was initiated with palliative radiotherapy.
Case 2: Pembrolizumab treatment was initiated with gemcitabine-carboplatin and showed 50% metabolic regression after four treatment cycles. Secondary to the bladder and rectum invasion, pelvic sepsis developed, and pelvic exenteration was performed. The pathology revealed moderately differentiated squamous cell carcinoma infiltrating the bladder and rectum (pT3bN0). IHC findings of the surgical specimen again showed loss of MLH-1 and PMS-2 expression.
Case 3: Due to the recurrence of endometrial carcinoma, she underwent surgery for tumor removal, and the pathology results are pending.
Case 1: She was lost to follow-up months after admission to our hospital.
Case 2: Since the patient was tumor-free, pembrolizumab monotherapy was planned. After 3 mo of immunotherapy, a restaging PET/CT demonstrated marked disease progression with multiple abdominopelvic hypermetabolic lesions. She was initiated on XELOX chemotherapy but could not tolerate the treatment. Her situation deteriorated, and she died after 3 mo of palliative treatment.
Case 3: She was lost to follow-up.
The incidence of MSI differs across solid tumors. Most of the studies in this field focus on CRC, which is closely related to MSI. Our case series included three different solid tumors with discordant MSI results, which to our knowledge is the first in the literature. According to recent reports, the frequency of MSI is 0%-2.1%, 12.0%, and 25.0% for cholangiocarcinoma, cervical, and endometrial cancers, respectively[14,15]. The optimal method for detection of MSI remains unclear. In addition to sensitivity, easily accessible and cost-effective methods are required in daily practice; therefore, IHC is most frequently used. There are limited data on the concordance analysis of MSI status between IHC and NGS for CRC and gynecological cancers and a lack of data for other solid tumors.
The decision to screen for DNA MMR gene mutations using IHC and/or PCR and/or NGS for MSI involves several considerations. IHC, as a gold standard, has several advantages such as its high specificity (96.1%), accuracy (99.2%), and sensitivity[16]. In addition, it is inexpensive and easy to use. Moreover, it can be performed on small biopsy samples and can clearly suggest the affected gene (MLH1, MSH2, MSH6, or PMS2). However, there are some limitations, such as quality of tissue preparation interfering with results, an experienced pathologist needed, and non-immunoreactivity due to missing missense or frameshift/truncation mutations[17]. Studies comparing different methodologies in MSI analysis concluded that some MSI-H cases may be missed if IHC is used alone, with the incidence ranging between 11.8%-32.9%[18-20]. In addition, IHC results were prone to change after neoadjuvant and radiation therapy, which may have changed the preferred screening in some cases[21].
An alternative method, PCR, mostly covers the inadequacies of IHC, especially since it is not limited to protein expression. However, it has its disadvantages, such as the need for a specialized genetic facility, longer turnaround time, normal tissue requirement, and the fact that pre-analytic issues such as fixation, may interfere with the PCR reaction[22]. The most important limitation is the MSH6 mutation, which may cause a non-diagnostic MSI test by PCR, secondary to functional redundancy, leading to misdiagnosis as MSS.
Although both methods are close to 100% sensitivity/accuracy, neither of the methods help identify all tumors with defective MMR genes. The likelihood of misdiagnosis can be overcome using both methods; however, there may be discordant results. Berardinelli et al[16] evaluated MSI with IHC and PCR and reported eight discordant results in a total of 996 patients with CRC. Thus, for these cases, they proposed the addition of a new marker as complementary analysis and suggested the use of PCR over IHC. Several other studies including CRC found discordances between IHC and molecular analysis ranging from 1%-10%[10]. The cause of the discordance was mostly related to factors like low tumor cell proportion, pre-analytical difficulties, non-expert physician, neoadjuvant treatment, tumor heterogeneity, and discordance of tumor biopsy[10]. It was also mentioned that molecular panels used during PCR analysis were principally recommended for CRC. However, they were used in all types of solid tumors and may show poor performance in other types of cancer[9].
False positive results are important to overcome since recent reports suggest that primary resistance to immune checkpoint inhibitors may be related to the misinterpretation of MMR tests[10]. The development of NGS led to the emergence of a new technique to improve MSI detection. NGS can simultaneously detect MSI and screen for MMR mutations. Although it has 100% sensitivity and specificity, the high cost limits its use[23]. In addition, the panels used in NGS show better performance in non-CRC[9]. A study evaluating the concordance analysis of MSI between PCR and NGS for solid tumors reported a concordance of 98.8%[24]. Another study investigating discrepant MMR IHC and MSI PCR test results in gynecologic cancers reported 6 out of 328 discordant results using NGS and demonstrated that NGS could help resolve discrepant MMR and MSI results[25]. The usefulness of NGS in the determination of MSI, with a sensitivity of 95.8%, specificity of 99.4%, positive predictive value of 94.5%, and negative predictive value of 99.2% in 26 cancer types, was supported by several other studies with a concordance of 99.4% compared with PCR-based testing[26].
At our clinic, we prefer screening MSI using IHC due to its fast turnaround time and using NGS as an additional method to investigate a large variety of gene alterations at once. The discordant results were interpreted as MSI-H. MSI-H status is supported by high TMB results, a finding apparent in our third case. This finding has also been conclusively reported by other studies[27]. However, the reliability of the IHC results remains uncertain when NGS shows an MSS tumor. Our second case with cervical cancer showed rapid progression after the surgery. Although, seeding during the exenteration procedure may explain the recurrence, another reason may be the loss of tumor antigenicity after the surgery, restricting the trigger in the host cell immune response since the patient was tumor-free. These facts may also explain resistance to immunotherapy rather than the discordance. More trials comparing the IHC and NGS results are needed for better assessment.
There may be two limitations to our study. The first is different pathologists performing the histological analysis. Although international guidelines exist in terms of evaluation, the experience of the pathologist may interfere with the results. Second, as seen in other studies, different samples may cause discrepancy between the results. However, it is not always easy to access the surgical/biopsy specimens when the time interval between the diagnosis and metastases is long.
The rare non-colorectal MSI cases in the literature and the lack of investigation into IHC-NGS discordance highlights the uniqueness of our cases. Today, the gold standard of MSI analysis is IHC. However, considering the defined 100% and 98.7% positive and negative predictive values, respectively[24], with reduced costs and turnaround time, NGS may be the preferred first-line option for MSI analysis to reduce the incidence of misdiagnoses in the future.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: Turkey
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Leal RF, Brazil; Wang Z, China S-Editor: Liu XF L-Editor: Filipodia P-Editor: Zhao S
| 1. | Baudrin LG, Deleuze JF, How-Kit A. Molecular and Computational Methods for the Detection of Microsatellite Instability in Cancer. Front Oncol. 2018;8:621. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 77] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 2. | Lower SS, McGurk MP, Clark AG, Barbash DA. Satellite DNA evolution: old ideas, new approaches. Curr Opin Genet Dev. 2018;49:70-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 144] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 3. | Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK, Lu S, Kemberling H, Wilt C, Luber BS, Wong F, Azad NS, Rucki AA, Laheru D, Donehower R, Zaheer A, Fisher GA, Crocenzi TS, Lee JJ, Greten TF, Duffy AG, Ciombor KK, Eyring AD, Lam BH, Joe A, Kang SP, Holdhoff M, Danilova L, Cope L, Meyer C, Zhou S, Goldberg RM, Armstrong DK, Bever KM, Fader AN, Taube J, Housseau F, Spetzler D, Xiao N, Pardoll DM, Papadopoulos N, Kinzler KW, Eshleman JR, Vogelstein B, Anders RA, Diaz LA Jr. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357:409-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3799] [Cited by in RCA: 5153] [Article Influence: 572.6] [Reference Citation Analysis (0)] |
| 4. | Bonneville R, Krook MA, Chen HZ, Smith A, Samorodnitsky E, Wing MR, Reeser JW, Roychowdhury S. Detection of Microsatellite Instability Biomarkers via Next-Generation Sequencing. Methods Mol Biol. 2020;2055:119-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 57] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 5. | Maraka S, Singh Ospina NM, Mastorakos G, O'Keeffe DT. Subclinical Hypothyroidism in Women Planning Conception and During Pregnancy: Who Should Be Treated and How? J Endocr Soc. 2018;2:533-546. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 6. | Chakravarty D, Johnson A, Sklar J, Lindeman NI, Moore K, Ganesan S, Lovly CM, Perlmutter J, Gray SW, Hwang J, Lieu C, André F, Azad N, Borad M, Tafe L, Messersmith H, Robson M, Meric-Bernstam F. Somatic Genomic Testing in Patients With Metastatic or Advanced Cancer: ASCO Provisional Clinical Opinion. J Clin Oncol. 2022;40:1231-1258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 167] [Article Influence: 41.8] [Reference Citation Analysis (0)] |
| 7. | Li K, Luo H, Huang L, Zhu X. Microsatellite instability: a review of what the oncologist should know. Cancer Cell Int. 2020;20:16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 131] [Cited by in RCA: 348] [Article Influence: 58.0] [Reference Citation Analysis (1)] |
| 8. | First Comprehensive Companion Diagnostic OK'd. Cancer Discov. 2018;8:OF4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 9. | Waalkes A, Smith N, Penewit K, Hempelmann J, Konnick EQ, Hause RJ, Pritchard CC, Salipante SJ. Accurate Pan-Cancer Molecular Diagnosis of Microsatellite Instability by Single-Molecule Molecular Inversion Probe Capture and High-Throughput Sequencing. Clin Chem. 2018;64:950-958. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 57] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 10. | Evrard C, Tachon G, Randrian V, Karayan-Tapon L, Tougeron D. Microsatellite Instability: Diagnosis, Heterogeneity, Discordance, and Clinical Impact in Colorectal Cancer. Cancers (Basel). 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 131] [Cited by in RCA: 124] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 11. | Cheah PL, Li J, Looi LM, Koh CC, Lau TP, Chang SW, Teoh KH, Mun KS, Nazarina AR. Screening for microsatellite instability in colorectal carcinoma: Practical utility of immunohistochemistry and PCR with fragment analysis in a diagnostic histopathology setting. Malays J Pathol. 2019;41:91-100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 12. | Arulananda S, Thapa B, Walkiewicz M, Zapparoli GV, Williams DS, Dobrovic A, John T. Mismatch Repair Protein Defects and Microsatellite Instability in Malignant Pleural Mesothelioma. J Thorac Oncol. 2018;13:1588-1594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 13. | Hempelmann JA, Lockwood CM, Konnick EQ, Schweizer MT, Antonarakis ES, Lotan TL, Montgomery B, Nelson PS, Klemfuss N, Salipante SJ, Pritchard CC. Microsatellite instability in prostate cancer by PCR or next-generation sequencing. J Immunother Cancer. 2018;6:29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 91] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 14. | Naganuma A, Sakuda T, Murakami T, Aihara K, Watanuki Y, Suzuki Y, Shibasaki E, Masuda T, Uehara S, Yasuoka H, Hoshino T, Kudo T, Ishihara H, Ogawa T, Kitamoto Y, Ogawa A. Microsatellite Instability-high Intrahepatic Cholangiocarcinoma with Portal Vein Tumor Thrombosis Successfully Treated with Pembrolizumab. Intern Med. 2020;59:2261-2267. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 15. | Lorenzi M, Amonkar M, Zhang J, Mehta S, Liaw KL. Epidemiology of microsatellite instability high (MSI-H) and deficient mismatch repair (dMMR) in solid tumors: A structured literature review. J Oncol. 2020;2020:1-17. [RCA] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 16. | Berardinelli GN, Scapulatempo-Neto C, Durães R, Antônio de Oliveira M, Guimarães D, Reis RM. Advantage of HSP110 (T17) marker inclusion for microsatellite instability (MSI) detection in colorectal cancer patients. Oncotarget. 2018;9:28691-28701. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 40] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 17. | McCracken J, Neff J. Discordant IHC/PCR test results for mismatch repair status in colorectal adenocarcinoma. CAP Today. 2018;1-4. [DOI] [Full Text] |
| 18. | Lin CH, Lin JK, Chang SC, Chang YH, Chang HM, Liu JH, Li LH, Chen YT, Tsai SF, Chen WS. Molecular profile and copy number analysis of sporadic colorectal cancer in Taiwan. J Biomed Sci. 2011;18:36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 19. | Lindor NM, Burgart LJ, Leontovich O, Goldberg RM, Cunningham JM, Sargent DJ, Walsh-Vockley C, Petersen GM, Walsh MD, Leggett BA, Young JP, Barker MA, Jass JR, Hopper J, Gallinger S, Bapat B, Redston M, Thibodeau SN. Immunohistochemistry vs microsatellite instability testing in phenotyping colorectal tumors. J Clin Oncol. 2002;20:1043-1048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 349] [Cited by in RCA: 476] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 20. | Bartley AN, Luthra R, Saraiya DS, Urbauer DL, Broaddus RR. Identification of cancer patients with Lynch syndrome: clinically significant discordances and problems in tissue-based mismatch repair testing. Cancer Prev Res (Phila). 2012;5:320-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 117] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 21. | Goldstein JB, Wu W, Borras E, Masand G, Cuddy A, Mork ME, Bannon SA, Lynch PM, Rodriguez-Bigas M, Taggart MW, Wu J, Scheet P, Kopetz S, You YN, Vilar E. Can Microsatellite Status of Colorectal Cancer Be Reliably Assessed after Neoadjuvant Therapy? Clin Cancer Res. 2017;23:5246-5254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 22. | Zhang L. Immunohistochemistry vs microsatellite instability testing for screening colorectal cancer patients at risk for hereditary nonpolyposis colorectal cancer syndrome. Part II. The utility of microsatellite instability testing. J Mol Diagn. 2008;10:301-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 165] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 23. | Stadler ZK, Battaglin F, Middha S, Hechtman JF, Tran C, Cercek A, Yaeger R, Segal NH, Varghese AM, Reidy-Lagunes DL, Kemeny NE, Salo-Mullen EE, Ashraf A, Weiser MR, Garcia-Aguilar J, Robson ME, Offit K, Arcila ME, Berger MF, Shia J, Solit DB, Saltz LB. Reliable Detection of Mismatch Repair Deficiency in Colorectal Cancers Using Mutational Load in Next-Generation Sequencing Panels. J Clin Oncol. 2016;34:2141-2147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 202] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 24. | Shimozaki K, Hayashi H, Tanishima S, Horie S, Chida A, Tsugaru K, Togasaki K, Kawasaki K, Aimono E, Hirata K, Nishihara H, Kanai T, Hamamoto Y. Concordance analysis of microsatellite instability status between polymerase chain reaction based testing and next generation sequencing for solid tumors. Sci Rep. 2021;11:20003. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 25. | Smithgall MC, Remotti H, Hsiao SJ, Mansukhani M, Liu-Jarin X, Fernandes H. Investigation of discrepant mismatch repair immunohistochemistry and microsatellite instability polymerase chain reaction test results for gynecologic cancers using next-generation sequencing. Hum Pathol. 2022;119:41-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 26. | Vanderwalde A, Spetzler D, Xiao N, Gatalica Z, Marshall J. Microsatellite instability status determined by next-generation sequencing and compared with PD-L1 and tumor mutational burden in 11,348 patients. Cancer Med. 2018;7:746-756. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 228] [Cited by in RCA: 368] [Article Influence: 46.0] [Reference Citation Analysis (0)] |
| 27. | Chalmers ZR, Connelly CF, Fabrizio D, Gay L, Ali SM, Ennis R, Schrock A, Campbell B, Shlien A, Chmielecki J, Huang F, He Y, Sun J, Tabori U, Kennedy M, Lieber DS, Roels S, White J, Otto GA, Ross JS, Garraway L, Miller VA, Stephens PJ, Frampton GM. Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutational burden. Genome Med. 2017;9:34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1661] [Cited by in RCA: 2615] [Article Influence: 290.6] [Reference Citation Analysis (0)] |