Published online May 6, 2023. doi: 10.12998/wjcc.v11.i13.3076
Peer-review started: December 19, 2022
First decision: February 17, 2023
Revised: February 23, 2023
Accepted: March 27, 2023
Article in press: March 27, 2023
Published online: May 6, 2023
Processing time: 126 Days and 11.7 Hours
Moyamoya disease (MMD) is a rare cause of acute stroke and transient ischemic attacks in children. We described clinical, diagnostic features and follow-ups of a young child with acute stroke.
We report a 4-year-old girl with left hemiparesis after an acute ischemic stroke. Her history was also significant for repeated left or right focal motor seizures, generalized tonic-clonic convulsions and transient ischemic attacks. Her magnetic resonance imaging and computed tomography (CT) of the brain and magnetic resonance angiography, CT angiography and venography on the cerebral vessels revealed evidence of bilateral fronto-parietal ischemic infarctions, occlusion of the right and left internal carotid arteries started at its bifurcation and non-visualization of right and left anterior and middle cerebral arteries. There was evidence of progression in angiography manifested as development of collaterals from the basal perforating vessels, increase in the extent of large intracranial arterial stenosis/occlusion and extensive collateral circulation with predominance from the posterior circulation. Physical and neurological evaluation and comprehensive laboratory investigations excluded an obvious comorbid disease or risk factor for the child’s condition. The diagnosis of MMD was highly suggested as a cause of the child’s steno-occlusive condition. She was treated symptomatically with levetiracetam, an antiepileptic medication. Aspirin was prescribed for secondary prevention. Her clinical manifestations were improved during the three years of follow-up. Revascularization surgery was postponed.
Up to our knowledge, this is the first report for MMD in a child in our country. The clinical improvement and the stabilization of the child’s condition over the 3 years of follow-up could be attributed to the rapid and extensive recruitment of collaterals and absence of risk factors or comorbidities. Revascularization surgery is highly recommended.
Core Tip: Stroke in children is a significant cause of long-lasting morbidity. The advances in neuroimaging and laboratory investigations serve an important role in proper evaluation of stroke in children and identification of its potential etiologies, risk factors and outcomes. Moyamoya disease (MMD) is a rare progressive non-inflammatory steno-occlusive arteriopathy of the large cerebral blood vessels. It is a rare cause of ischemic stroke and recurrent transient attacks in children. The disease is very under-recognized in different areas of the world except East Asia, predominantly Japan. MMD can be sporadic or familial. The Japanese term “moyamoya” refers to the puff of smoke morphology of the dilated basal collateral vessels within the brain tissue seen on cerebral angiography. Compared to other arteriopathies, MMD is unique as its treatment solely relies on surgical revascularization. Therefore, increasing reporting and evaluation of cases with MMD from different ethnicities may help in better understanding of its causes and proper management.
- Citation: Hamed SA, Yousef HA. Idiopathic steno-occlusive disease with bilateral internal carotid artery occlusion: A Case Report. World J Clin Cases 2023; 11(13): 3076-3085
- URL: https://www.wjgnet.com/2307-8960/full/v11/i13/3076.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i13.3076
Moyamoya disease (MMD) is an idiopathic progressive steno-occlusive arteriopathy of the large cerebral blood vessels. It is a rare and under-estimated cause of acute ischemic stroke and transient ischemic attacks (TIAs) in children and cerebral and subarachenoid hemorrhages in adults[1]. MMD usually presents between the ages of 3 to 10 years old or even earlier and in the 3rd and 4th decades of life[1,2]. Some studies from Asia and Europe reported that MMD is the cause of cerebrovascular stroke in 6% to 22% of children[3]. Familial MMD has been reported in 7% to 12% from Japan and approximately 6% from United States[4,5]. The ring finger protein 213 or the p.4810K variant in the RNF213 gene has been identified by both genome- wide association studies and whole exome sequencing in 95.0% for familial and 75.0% for sporadic cases with MMD. RNF213 gene has been suggested to be the most frequent susceptibility gene in MMD. However, there are other genes, allelic heterogeneity and ethnic specific variants that also has been thought to contribute rarely to the susceptibility, clinical characteristics and outcomes of MMD. Also some studies emphasized the effect of genetic variants on risk stratification and clinical presentation, follow-up of patients and surgical outcomes of MMD[4-6].
MMD is typically characterized by endothelial hyperplasia and fibrocellular thickening of the intima, and duplication of the internal elastic lamina of the large intracranial vessels with parallel evolution of contiguous collateral anastomotic changes[7]. The anterior circulation is involved more commonly than the posterior circulation with particular involvement of the distal terminal segments of the internal carotid arteries (ICAs), M1 segment of the middle cerebral arteries (MCAs), A1 segment of the anterior cerebral arteries (ACAs) and the proximal portion of the posterior cerebral arteries (PCAs) or the basilar artery (BA)[8]. Manifestations of MMD include TIAs, ischemic strokes, intracranial hemorrhages, seizures, headaches, choreiform movements, cognitive deficits and visual deficits[1]. There are common childhood behaviors which induce cerebrovascular insults in a child with MMD, which include crying, coughing, or blowing (i.e. hyperventilation) and other activities which result in hypocapnia-induced cerebral vasoconstriction of the already maximally dilated cerebral blood vessels[9]. The definite diagnosis of MMD depends on specific angiographic criteria[8] which are: (1) Bilateral stenosis/occlusion of the distal terminal part of ICAs and/or the proximal parts of ACAs and/or MCAs; and (2) visualization of the moyamoya vessels, an abnormal arterial vascular network from deep perforating dilated arteries, in proximity to the stenosed/occluded vessels[10]. These vessels also appear as concurrent large flow voids in the basal ganglia and thalamus in magnetic resonance imaging (MRI) of the brain[11]. Suzuki et al[12] provided stages for MMD based on the angiographic finding. These stages explain the pathological changes seen over a period of time in each patient with MMD, but they do not correlate with the disease severity. The initial stages (stages 1-3) involve the occlusion of the distal parts of the ICAs with the development of moyamoya vessels, the late stages (stages 4-5) involve the ICAs-ECAs (the external carotid arteries) anastomosis followed by fading of moyamoya vessels and the last stage (stage 6) involves complete occlusion of ICAs.
The long-term outcome of MMD is poor in up to 66% of patients with the development of symptomatic neurological and cognitive deteriorations (i.e. progression) within the next five years following its diagnosis. The surgical revascularization is recommended at young age to prevent recurrence of ischemic insults[13-15]. Management of acute ischemic stroke due to MMD is symptomatic[8]. Conservative treatment can be used for secondary prevention using anti-platelet drugs and calcium channel blockers. Anti-platelet drugs (as aspirin in a dose of 1 to 5 mg/kg/d or clopidogrel in a dose of 1 mg/kg/d) are recommended for at least 3 to 5 years after the acute ischemic stroke to prevent thrombosis and thromboembolism at the sites of arterial stenosis[14] It has been reported that calcium channel blockers (as nicardipine) can be used for secondary prevention from ischemic insults. Nicardipine (in a dose of 0.25-0.5 mg/kg/d every 12 h by oral route) may improve the hemodynamics in patients with MMD by optimizing the collateral circulation[16].
A 4-year-old female (DOB: 2015) with left sided hemiparesis.
She presented in June 2019 with left sided hemiparesis and recurrent focal motor seizures.
Her history was significant since October 2017 for repeated attacks of loss of consciousness (LOC), sudden falls without LOC, convulsions or body discoloration. There were also attacks of dysarthria or speech arrest or deviation of the angle of the mouth to the left side which lasted for 20-30 min. The mother noticed that excessive crying could provoke sudden falls and hot water bath could result in drowsiness for few minutes. Her neurological examination and computed tomography (CT) of the brain were unremarkable. She was diagnosed as having epilepsy and treated with sodium valproate (VPA), an anticonvulsant drug but she discontinued treatment after few months.
In September 2018, she developed recurrent motor seizures starting in the right perioral area followed by the right upper and lower limbs. She was re-treated with VPA. In October 2018, she developed motor seizures on the left side of the body. There was no impaired awareness during the attacks and her neurological examination and repeated CT scan of the brain were normal. The treating physician prescribed VPA in a dose of 200 mg every 12 h and levetiracetam (LEV), another anticonvulsant drug, in a dose of 100 mg every 12 h. The seizures subsided with medications. In January 2019, she developed two attacks of generalized tonic-clonic convulsions (GTC) without regaining consciousness in between the attacks. She was admitted to the hospital. On admission, her oral temperature was 38.0 °C. There were no signs of meningeal irritation. Her electroencephalography showed diffuse delta slowing. Her routine laboratory investigations and chemistry analysis of the cerebrospinal fluid (CSF) were unremarkable.
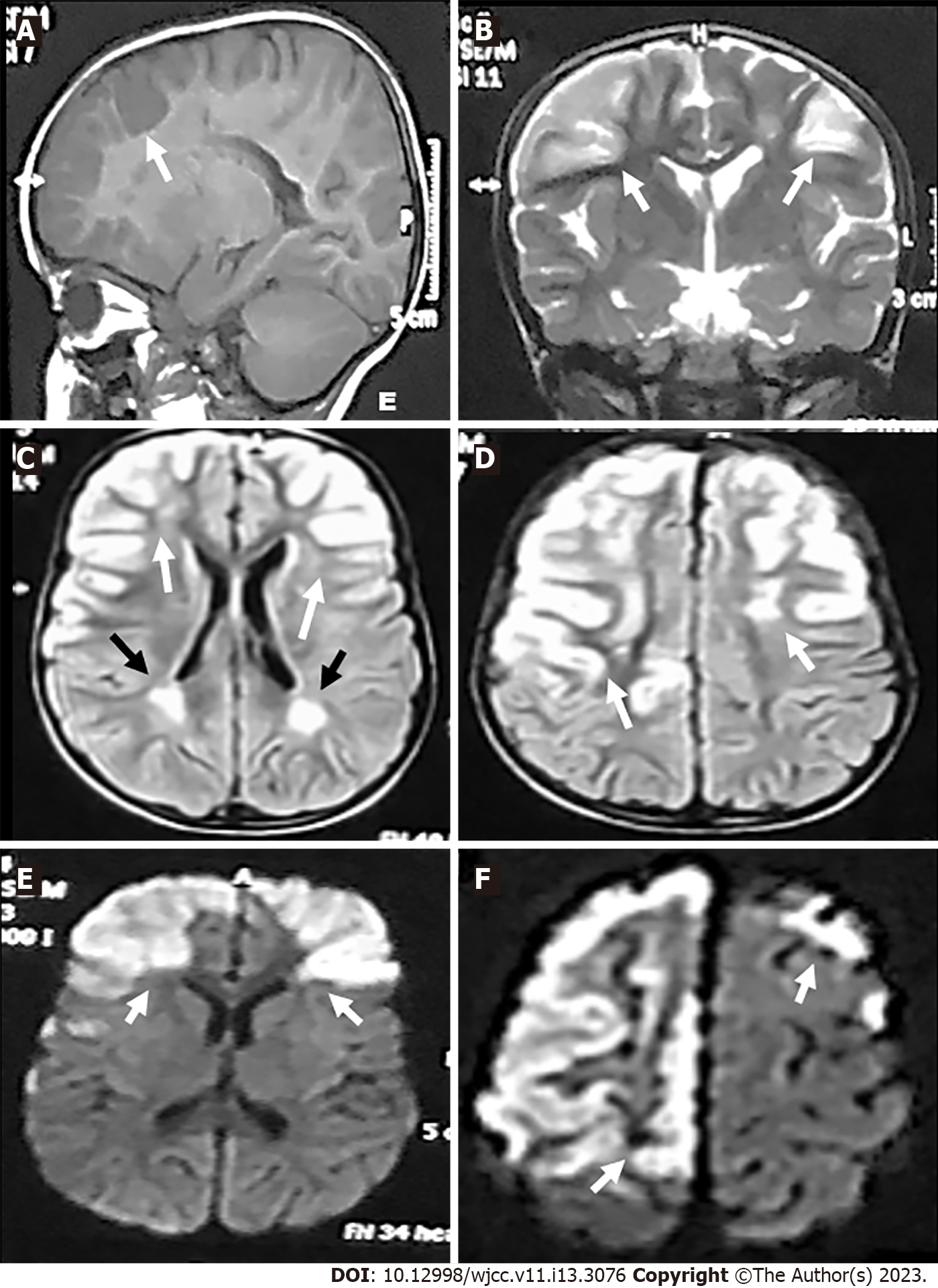
MRI (Siemens machine, 1.5 Tesla) of the brain was performed. It showed bilateral abnormal signal intensities involving both frontal lobes (with gyral pattern) being hypointense in T1-weighted imaging (T1WI) and hyperintense in T2-weighted imaging (T2WI), fluid attenuation inversion recovery (FLAIR) views and diffusion weighted imaging (DWI) (i.e. restricted diffusion). There was no mass effect or perilesional edema (Figure 1). Treatment was started with ceftriaxone, an antibiotic, and acyclovir, an antiviral drug, for concern of being an attack of encephalitis/meningoencephalitis. Intravenous LEV was also prescribed. The patient regained consciousness within days but found to have left sided hemiplegia. She was discharged after few days on oral LEV (200 mg every 12 h).
There was no history of traumatic head or neck injuries. There was no comorbid medical or surgical condition. There was no history of risk factors in temporal relation with disease onset (e.g. infection/inflammation, vaccination, etc.).
Her body weight was 18 kg. She was a product of normal pregnancy and delivery and had normal development, speech and intelligence. She was an active girl. Her detailed history, comprehensive investigations (hematological and metabolic laboratory testing) and medical and cardiac evaluations excluded an underlying diseases or predisposing factors as a cause of stroke. There was no family history of similar condition.
Neurological examination at presentation (June 2019) revealed left spastic hemiparesis (muscle power was 3/5), left upper motor neuron facial paralysis, exaggerated left deep tendon jerks and left Babinski sign. Her fundus examination was normal.
They included complete blood cell count, prothrombin time, partial thromboplastin time, erythrocyte sedimentation rate, antinuclear, anticardiolipin and antiphospholipid antibodies, protein C and S, serum lactate and pyruvate, sickle cell preparation, serum amino acids, triglycerides, cholesterol. electrocardiogram and echocardiogram. No genetic testing was done.
This will include genetic testing with special emphasis to RNF213 gene variants.
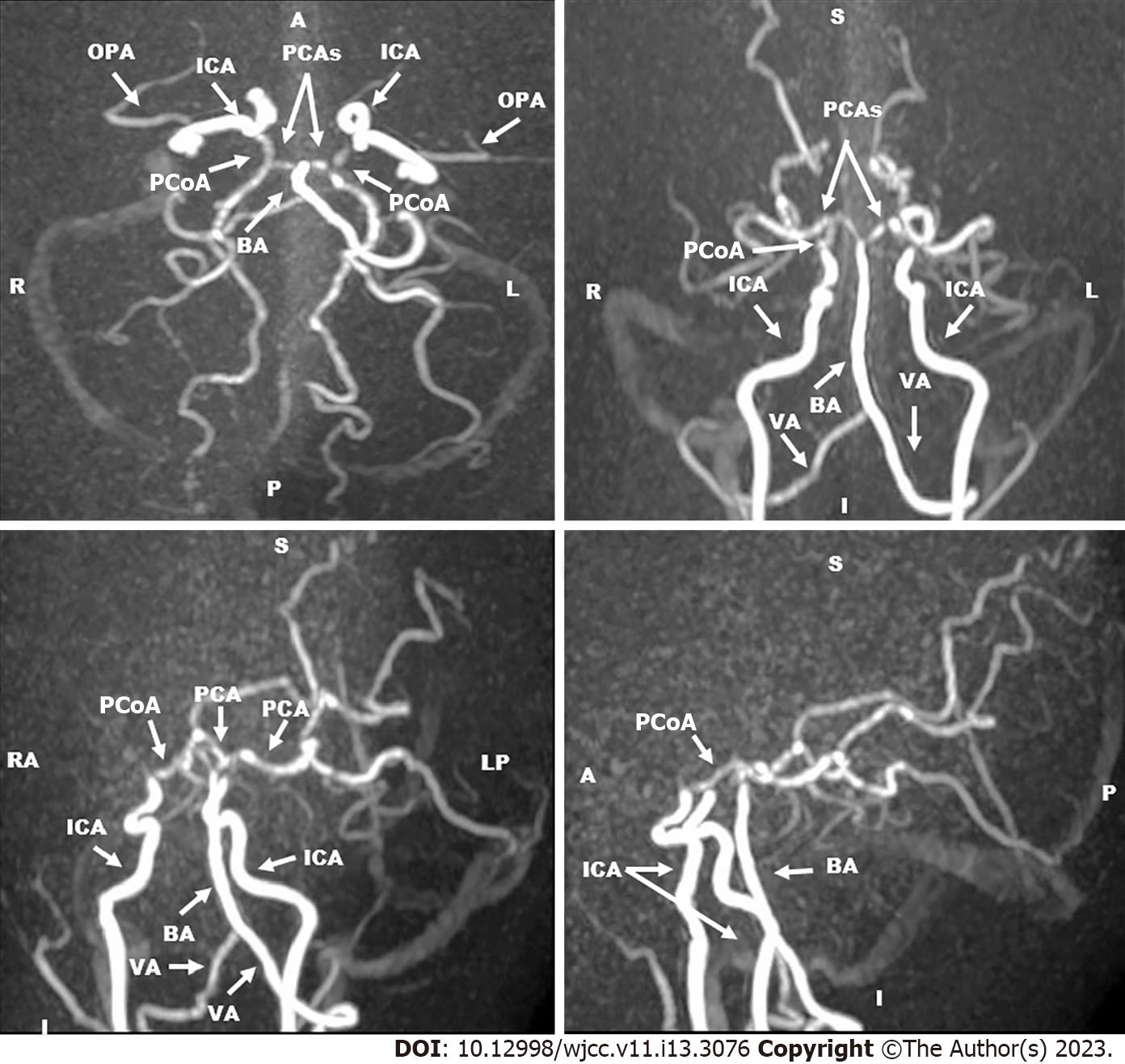
In October 2019, she did follow-up MRI of the brain and magnetic resonance angiography (MRA) of the cerebral vessels. MRA was performed using three-dimension (3D) technique and maximum intensity projection images (MIP) were obtained. MRI showed bilateral abnormal high signal intensities in the frontal and parietal gray and white matters in T2WI, FLAIR and diffusion/perfusion weighted imaging views which did not contrast enhanced. There were ex vacuo dilatation of the frontal horns and bodies of lateral ventricles with relative prominence of the overlying sulci. There were variable sized hypointense foci with near CSF intensity within the right frontal white matter indicating frontal gliotic changes and cystic encephalomalacia in brain areas supplied by ICAs/MCAs (Figure 2). MRA showed complete occlusion of the supraclinoid portion of both ICAs. The ACAs and MCAs were not visualized. The basilar artery (BA) and PCAs had normal sizes and calibers. Collaterals were predominantly from the posterior circulation (Figure 3).
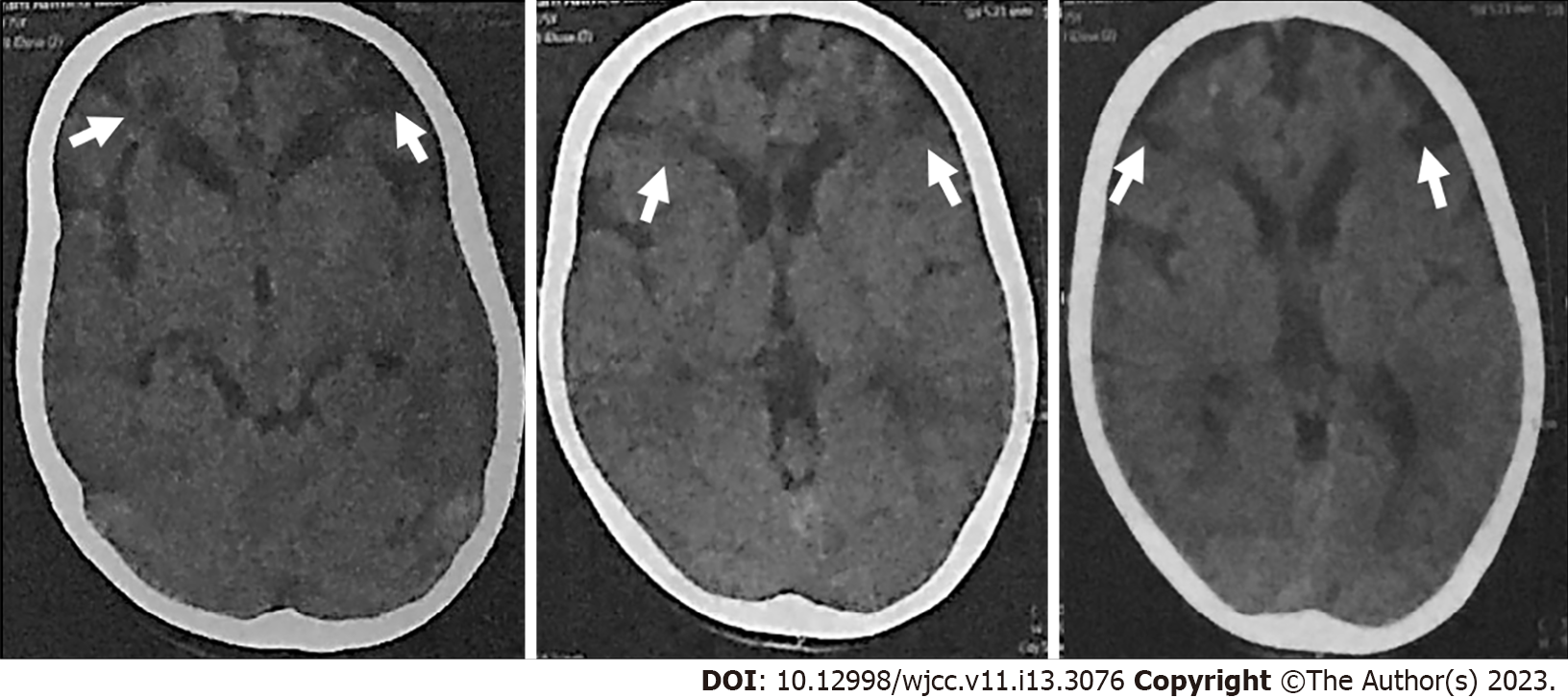
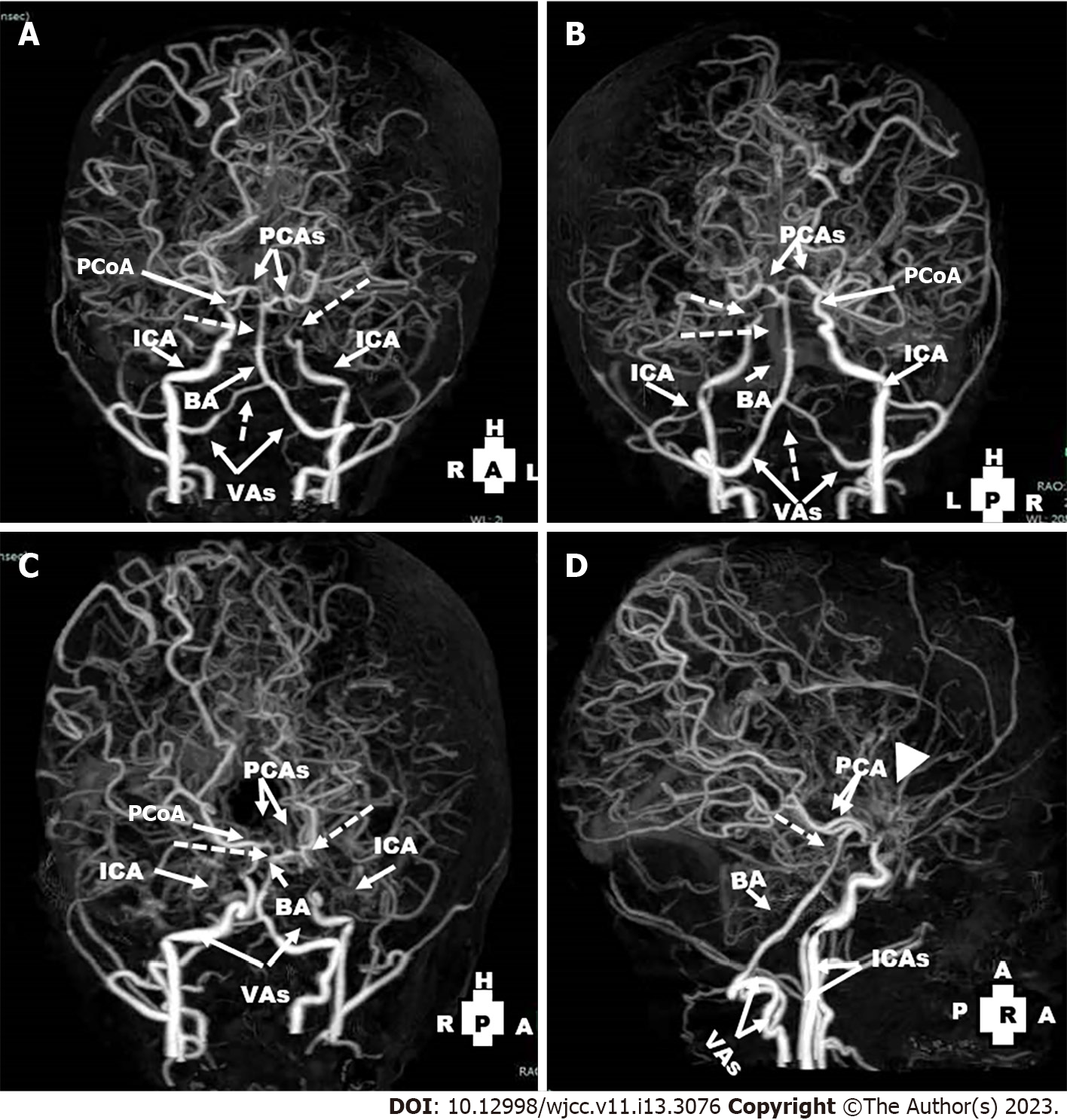
In January 2021, follow up pre- and post-contrast multi-slice CT of the brain and CT angiography (CTA) and CT venography (CTV) on the cerebral vessels were done. CT of the brain showed bilateral frontal subcortical and slightly cortical hypodense lesions with CSF density. There were dilated ventricular systems, and prominent cortical sulci and basal cisterns (Figure 4). CTA showed non-visualization of right and left ACAs and MCAs, the left ICA ended blindly before the siphon, non-visualization of the left posterior communicating artery (PCoA) and attenuation of the distal parts of the right vertebral artery (VA) (compared to its proximal part) and BA. There was dominance of the posterior circulation with extensive bilateral collaterals (compared to early MRA findings) and prominence of the dilated basal perforating vessels (moyamoya vessels) (Figure 5). CTV showed normal opacification of the dural venous sinuses with no filling defects. The superficial and deep cerebral veins were normal.
MMD.
Her treatment regimen included LEV (200 mg every 12 h) and aspirin (75 mg/d). The child was also seizure-free on LEV in a maintenance dose of 200 mg every 12 h. Recommendations included continuation of aspirin in a maintenance dose of 75 mg/d. The decision for revascularization surgery was postponed.
Clinical follow-up over at least 3 years showed no new ischemic insults or exacerbation of the existing deficits.
We present a younger female child with epilepsy right and left focal seizures and GTC and TIAs. She developed left sided hemiparesis after an attack of ischemic cerebrovascular stroke. A common child behavior which triggered the TIA was crying (i.e. hyperventilation or hyperpnea excitement). She had no family history of similar condition. There were no risk factors (e.g. infection, vaccination, etc.) in temporal relation to the disease onset. There were no comorbid other neurological or extra-neurological manifestations. Her comprehensive laboratory investigations did not reveal an identifiable cause for the ischemic attacks. Her MRI, MRA and CTA findings are highly supportive for the diagnosis of MMD.
For the presented child, the diagnosis of MMD was provided based on the followings: (1) The bilateral involvement (stenosis/occlusion) of the distal terminal parts of ICAs. The brain infarcts involved the fronto-parietal areas (areas supplied by the ICAs/MCAs) and appeared with “gyral pattern” which was atypical from infarcts of conventional ischemic strokes due to other steno-occlusive diseases which typically have territorial, border zone, deep lacunar or multiple-dots patterns[17-19]. The occlusion of the ICA started at its bifurcation, i.e. in the supraclinoid part distal to the ophthalmic and anterior choroidal arteries. The right and left ophthalmic arteries and PCoAs were visualized on MRA but the ACAs and MCAs were not visualized. There were no infarcts in basal ganglia, diencephalon, midbrain and temporal lobes, i.e. brain areas supplied by the anterior choroidal artery; and (2) There were imaging evidences for disease progressive overtime regardless to the apparent symptomatic improvement. They included: (1) The robustness of bilateral collaterals which was seen overtime; and (2) the visualization of the basal moyamoya vessels or “puff of smoke”.
Studies of large cohort of children found that the development of collaterals occurred before any significant hemodynamic impairment or development of ischemic stroke due to arterial occlusion, indicating that the neo-vascularization in MMD is a dynamic process triggered by progressive stenosis and not a passive compensation for the ischemic stroke or arterial occlusion[10]. Authors also found a development of new arterioles from pre-existing vascular structures (i.e. arteriogenesis), which developed within days after arterial occlusion in children with MMD (but not in other steno-occlusive diseases) and the reformation of small sized collaterals into large sized vessels overtime[20,21]; (2) the non-visualization of left PCoA; (3) the attenuation of the distal terminal part of the VA and the distal part of the BA (i.e. stenosis); and (4) the stenosis/occlusion of the left ICA before the siphon. The absence of visual impairment and the normal fundus examination at the time of CTA (done 15 mo after the acute stroke) confirmed that the progressive stenosis/occlusion of the left ICA was distal to the left ophthalmic artery. It also seems that the progressive left ICA pathology might also be distal to the left anterior choroidal artery, because there were no clinical or CT evidences for the presence of infarctions in brain areas supplied by the anterior choroidal artery. However, CT is less sensitive than MRI for visualization of deep brain infarcts[12]. Previous studies indicated that CTA is non-invasive technique. It captures snapshots in different contrast bolus phases and provides more relevant data regarding secondary collateral flow, which significantly correlates with the neurological outcome. They also indicated that combined MRI, MRA and MRV, non-invasive imaging tests, are enough for the diagnosis of suspected MMD in a child with sensitivity of 92% and a specificity of 100%[8]. It has been found that MRA is beneficial for diagnosis of large basal intracranial vessels and Willisian, leptomeningeal, and transdural collaterals[17,18], whereas conventional angiography is better for diagnosis of smaller moyamoya collaterals and extracranial distal collateral networks[8]. MRI and MRA also differentiate MMD from the mimicking conditions including vascular dissection, inflammatory vasculitis and cystic medial necrosis[8]. The patient did not perform conventional angiography. In practice, cerebral conventional angiography must be reserved for children with MMD in the following circumstances due to its invasiveness: (1) If diagnosis was uncertain; and (2) inconsistency between the clinical progression and the finding of MRA or CTA during follow-up, during pre-operative planning, and sometimes during follow-up evaluation after surgical re-vascularization[12].
The long-term outcome of this patient has to be followed overtime. The clinical improvement (marked reduction of TIAs and seizures) and stabilization of her clinical condition during the 3 years period of follow-up could be attributed to the rapid and extensive recruitment of collaterals and absence of risk factors or comorbidities. However, there are factors indicative for poor prognosis which included: (1) The early age at onset of symptoms[9]; (2) the large extent of infarctions seen on MRI at the time of initial presentation; (3) the presence of encephalomalacia and atrophy of the cerebral hemispheres (i.e. evidence of severe stroke); and (4) CTA evidence of progressive arteriopathy. We suggest that the marked difference in the extent of collaterals between MRA, which was done 3 mo after the cerebrovascular stroke, and the follow-up CTA, which was done 15 mo after the stroke, indicates the rapid and progressive degree of carotid stenosis/occlusion. Therefore, surgical revascularization is indicated for this child to reduce the recurrence of stroke[15]. However, experience of this type of surgery is not locally available.
Management of the patient was symptomatic treatment for epilepsy. Aspirin was prescribed in a dose of 75 mg/d for secondary prevention[8,14]. It has been indicated that the main line of treatment of MMD is surgical revascularization[15]. However, conservative treatment using the anti-platelet drugs (as aspirin or clopidogrel)[13] and the calcium channel blockers (as nicardipine)[16] may play a supportive role especially when surgical treatment is not available locally. Furthermore, because patients with MMD have deficit in cerebral hemodynamics and cerebral vascular reserve, thus advices for prevention of rapid deterioration included: Avoidance of situations which induce hyperventilation, dehydrations and hypotension. In MMD, hyperventilation causes decrease in arterial carbon dioxide tension which causes vasoconstriction, induces cerebral hypoxia and may result in steal response in brain areas which already have chronic hemodynamic stress[22].
To the best of our knowledge, this is the first case report of a child with MMD disease from our locality. Clinical and imaging follow-ups for the child was done over a period of at least 3 years after the acute ischemic stroke. Her clinical manifestations were improved and no further ischemic insults were happened which could be attributed to the massive development of bilateral collaterals. Revascularization surgery is highly recommended for optimal treatment of the patient.
I would like to thank the patient’s parents for their cooperation and approval to publish the clinical, laboratory and imaging results of their daughter.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: Egypt
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Mzhavanadze ND, Russia; Tang W, China S-Editor: Li L L-Editor: A P-Editor: Yu HG
| 1. | Lee S, Rivkin MJ, Kirton A, deVeber G, Elbers J; International Pediatric Stroke Study. Moyamoya Disease in Children: Results From the International Pediatric Stroke Study. J Child Neurol. 2017;32:924-929. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 77] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 2. | Suzuki J, Kodama N. Moyamoya disease--a review. Stroke. 1983;14:104-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 604] [Cited by in RCA: 600] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 3. | Amlie-Lefond C, Bernard TJ, Sébire G, Friedman NR, Heyer GL, Lerner NB, DeVeber G, Fullerton HJ; International Pediatric Stroke Study Group. Predictors of cerebral arteriopathy in children with arterial ischemic stroke: results of the International Pediatric Stroke Study. Circulation. 2009;119:1417-1423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 254] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 4. | Søgaard I, Jørgensen J. Familial occurrence of bilateral intracranial occlusion of the internal carotid arteries (Moya Moya). Acta Neurochir (Wien). 1975;31:245-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 27] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 5. | Kitahara T, Okumura K, Semba A, Yamaura A, Makino H. Genetic and immunologic analysis on moya-moya. J Neurol Neurosurg Psychiatry. 1982;45:1048-1052. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 49] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 6. | Shlobin NA, Hoffman SC, Clark JR, Du RY, Lam S. Clinical Usefulness of Genetic Testing For Patients with Moyamoya Disease: A Systematic Review. World Neurosurg. 2021;152:198-205.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 7. | Takagi Y, Kikuta K, Nozaki K, Hashimoto N. Histological features of middle cerebral arteries from patients treated for Moyamoya disease. Neurol Med Chir (Tokyo). 2007;47:1-4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 116] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 8. | Fukui M. Guidelines for the diagnosis and treatment of spontaneous occlusion of the circle of Willis ('moyamoya' disease). Research Committee on Spontaneous Occlusion of the Circle of Willis (Moyamoya Disease) of the Ministry of Health and Welfare, Japan. Clin Neurol Neurosurg. 1997;99 Suppl 2:S238-S240. [PubMed] |
| 9. | Tagawa T, Naritomi H, Mimaki T, Yabuuchi H, Sawada T. Regional cerebral blood flow, clinical manifestations, and age in children with moyamoya disease. Stroke. 1987;18:906-910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 44] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 10. | Takahashi M. Magnification angiography in moyamoya disease: new observations on collateral vessels. Radiology. 1980;136:379-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 34] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 11. | Yamada I, Suzuki S, Matsushima Y. Moyamoya disease: comparison of assessment with MR angiography and MR imaging vs conventional angiography. Radiology. 1995;196:211-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 83] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 12. | Suzuki J, Takaku A. Cerebrovascular "moyamoya" disease. Disease showing abnormal net-like vessels in base of brain. Arch Neurol. 1969;20:288-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1622] [Cited by in RCA: 1760] [Article Influence: 30.9] [Reference Citation Analysis (0)] |
| 13. | Scott RM, Smith JL, Robertson RL, Madsen JR, Soriano SG, Rockoff MA. Long-term outcome in children with moyamoya syndrome after cranial revascularization by pial synangiosis. J Neurosurg. 2004;100:142-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 179] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 14. | Research Committee on the Pathology and Treatment of Spontaneous Occlusion of the Circle of Willis; Health Labour Sciences Research Grant for Research on Measures for Infractable Diseases. Guidelines for diagnosis and treatment of moyamoya disease (spontaneous occlusion of the circle of Willis). Neurol Med Chir (Tokyo). 2012;52:245-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 566] [Cited by in RCA: 601] [Article Influence: 42.9] [Reference Citation Analysis (0)] |
| 15. | Wouters A, Smets I, Van den Noortgate W, Steinberg GK, Lemmens R. Cerebrovascular events after surgery vs conservative therapy for moyamoya disease: a meta-analysis. Acta Neurol Belg. 2019;119:305-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 16. | Hosain SA, Hughes JT, Forem SL, Wisoff J, Fish I. Use of a calcium channel blocker (nicardipine HCl) in the treatment of childhood moyamoya disease. J Child Neurol. 1994;9:378-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 17. | Patrick JT, Fritz JV, Adamo JM, Dandonna P. Phase-contrast magnetic resonance angiography for the determination of cerebrovascular reserve. J Neuroimaging. 1996;6:137-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 18. | Grond M, Rudolf J, Schneweis S, Terstegge K, Sobesky J, Kracht L, Neveling M, Heiss WD. Feasibility of source images of computed tomographic angiography to detect the extent of ischemia in hyperacute stroke. Cerebrovasc Dis. 2002;13:251-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 58] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 19. | Cho HJ, Jung YH, Kim YD, Nam HS, Kim DS, Heo JH. The different infarct patterns between adulthood-onset and childhood-onset moyamoya disease. J Neurol Neurosurg Psychiatry. 2011;82:38-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 20. | Kim SJ, Son TO, Kim KH, Jeon P, Hyun SH, Lee KH, Yeon JY, Kim JS, Hong SC, Shin HJ, Bang OY. Neovascularization precedes occlusion in moyamoya disease: angiographic findings in 172 pediatric patients. Eur Neurol. 2014;72:299-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 21. | van Royen N, Piek JJ, Buschmann I, Hoefer I, Voskuil M, Schaper W. Stimulation of arteriogenesis; a new concept for the treatment of arterial occlusive disease. Cardiovasc Res. 2001;49:543-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 223] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 22. | Nariai T, Senda M, Ishii K, Wakabayashi S, Yokota T, Toyama H, Matsushima Y, Hirakawa K. Posthyperventilatory steal response in chronic cerebral hemodynamic stress: a positron emission tomography study. Stroke. 1998;29:1281-1292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 0.9] [Reference Citation Analysis (0)] |