Published online May 6, 2023. doi: 10.12998/wjcc.v11.i13.3052
Peer-review started: December 3, 2022
First decision: February 17, 2023
Revised: February 24, 2023
Accepted: March 31, 2023
Article in press: March 31, 2023
Published online: May 6, 2023
Processing time: 142 Days and 22 Hours
Vaginal myomectomy is the most common form of radical treatment for prolapsed submucosal leiomyoma and is typically performed under general anesthesia. However, an alternative treatment approach is needed for patients who cannot tolerate general anesthesia. We describe a case with such a patient who was successfully treated via a minimally invasive method under local anesthesia.
A 46-year-old female suffered from abnormal uterine bleeding, severe anemia, and a reduced quality of life attributed to a massive prolapsed submucosal leiomyoma. She could not tolerate general anesthesia due to a congenital thoracic malformation and cardiopulmonary insufficiency. A new individualized combined treatment, consisting uterine artery embolization (UAE), percutaneous microwave ablation (PMWA) of the pedicle and the endometrium, and trans
UAE combined with PMWA can be performed under local anesthesia and is a promising alternative treatment for patients who cannot tolerate general anesthesia.
Core Tip: Uterine leiomyoma is a clinically common and benign tumor. The mainstream treatment for prolapsed leiomyoma is myomectomy. General anesthesia is usually needed when resecting large lesions. However, for patients with severe systematic disease who cannot tolerate general anesthesia, radical treatment is not feasible. We report a patient who was treated successfully via a minimally invasive method under local anesthesia. A large prolapsed leiomyoma was removed after a combination of uterine artery embolism and percutaneous microwave ablation treatment. This is a good example of the use of minimally invasive interventional technology for treating special patients.
- Citation: Zhang HL, Yu SY, Cao CW, Zhu JE, Li JX, Sun LP, Xu HX. Uterine artery embolization combined with percutaneous microwave ablation for the treatment of prolapsed uterine submucosal leiomyoma: A case report. World J Clin Cases 2023; 11(13): 3052-3061
- URL: https://www.wjgnet.com/2307-8960/full/v11/i13/3052.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i13.3052
Uterine leiomyomas are the most common benign pelvic tumor in reproductive-aged women, with an incidence between 25% and 80% in the literature[1,2]. Submucosal leiomyomas can cause uterine cavity deformation, which usually leads to abnormal uterine bleeding (AUB), even if the lesion is small. Without effective treatment, leiomyomas may eventually protrude through the cervical canal and prolapse into the vagina, in which case they are classified as FIGO type 0[3]. For isolated prolapsed pedunculated submucosal leiomyomas, transvaginal myomectomy is the mainstream radical treatment, performed by twisting, ligation, or excision. For large lesions with a thick pedicle that cannot be removed by twisting alone, ligation or excision followed by hysteroscopic electrocoagulation under general or epidural anesthesia is usually indicated[4,5]. However, an alternative treatment is needed for patients with severe systemic disease who cannot tolerate hysteroscopic surgery or hysterectomy. Herein, we present a case of a large prolapsed pedunculated submucosal leiomyoma impacted in the cervical canal that was successfully treated with a new method, uterine artery embolization (UAE) combined with percutaneous microwave ablation (PMWA).
A 46-year-old female complained of prolonged menses, heavy menstrual bleeding (HMB) and severe anemia for 2 years, which led to a severely reduced quality of life.
In July 2019, the patient presented with intermenstrual bleeding and was diagnosed with a 2 cm submucosal leiomyoma in a local hospital but received no treatment. Half a year later, the patient began to experience HMB (sanitary towel changed every 1-2 h), prolonged menses (20 d), and severe anemia (minimal serum hemoglobin level 4.2 g/dL). Blood transfusion and iron supplementation were required on the heaviest days. Unfortunately, her cardiopulmonary function was not amenable to general anesthesia for hysteroscopic surgery. Therefore, the regimen was switched to medical therapy. Between July 2020 and June 2021, the patient underwent intramuscular injections of goserelin acetate 36 mg every three months, but the efficacy was unsatisfactory. Her menstrual length was 10-15 d, the pictorial blood-loss assessment chart (PBAC) scale score was 810, and the secondary anemia had not been corrected. In July 2022, the patient sought help at our clinic. The symptom severity score (SSS) and health-related quality of life (HRQOL) score were 75 and 12.07, respectively, according to the uterine fibroid symptoms and quality of life questionnaire[6]. Considering the patient’s strong willingness to undergo radical treatment, we proposed a new plan: (1) Correct the anemia with pseudomenopausal therapy with combined oral contraceptive pills (COCs); and (2) determine a way to remove the prolapsed myoma under local anesthesia to permanently eliminate the source of the AUB. After taking COCs for 3 mo, the patient’s serum hemoglobin (Hb) level increased to 8.9 g/dL. Then, she was admitted to our hospital for further treatment.
Her medical history mainly included congenital scoliosis and thoracic deformity, pulmonary insufficiency, and pulmonary heart disease. The heart function stabilized to New York Heart Association Cardiac Function Classification I or II while on long-term cardiotonic (ivabradine hydrochloride tablet bid 5 mg) and diuretic medication (spironolactone bid 20 mg and hydrochlorothiazide bid 25 mg).
No family history of AUB or other tumors was identified.
Vaginal examination revealed a 6 cm, dark red mass prolapsed into the vagina without significant mobility. The patient refused bimanual examination.
Laboratory tests revealed mild anemia with an Hb of 9.2 g/dL and an estradiol level below 18.35 U/L, consistent with previous hormonal therapy. Pregnancy tests, vaginal bacteriology, cervical cytology, and tumor biomarkers were all negative. Studies for systemic coagulation disorders, von Willebrand disease, and thyroid dysfunction were also performed, but the results were unremarkable.
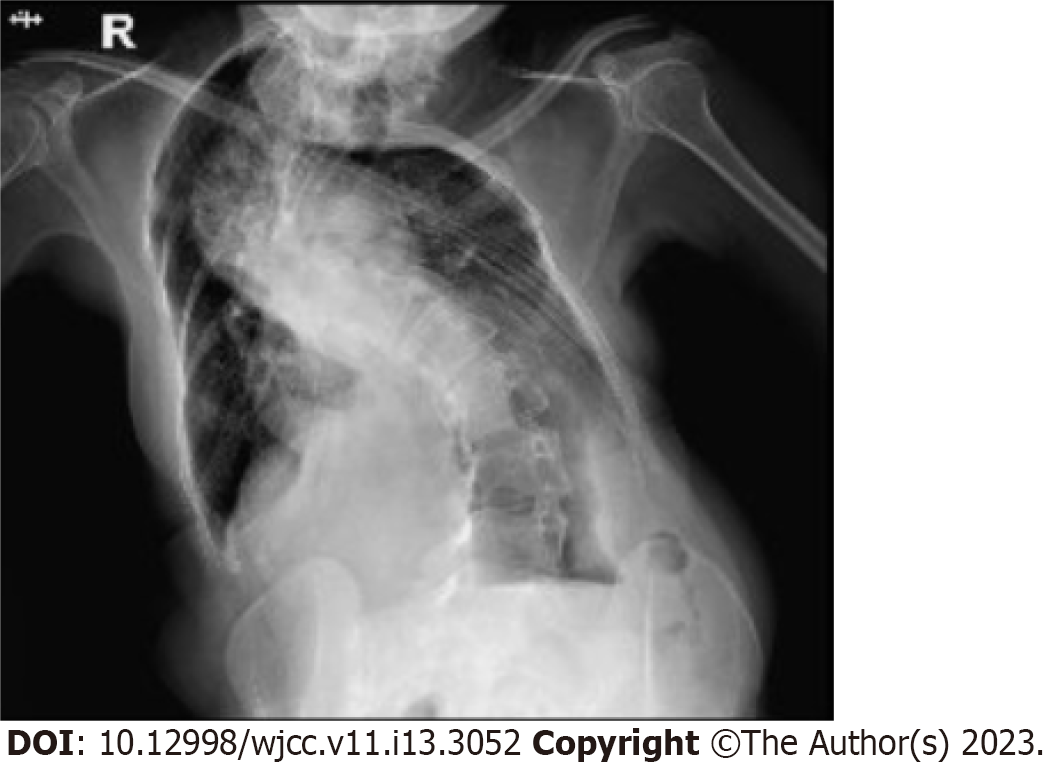
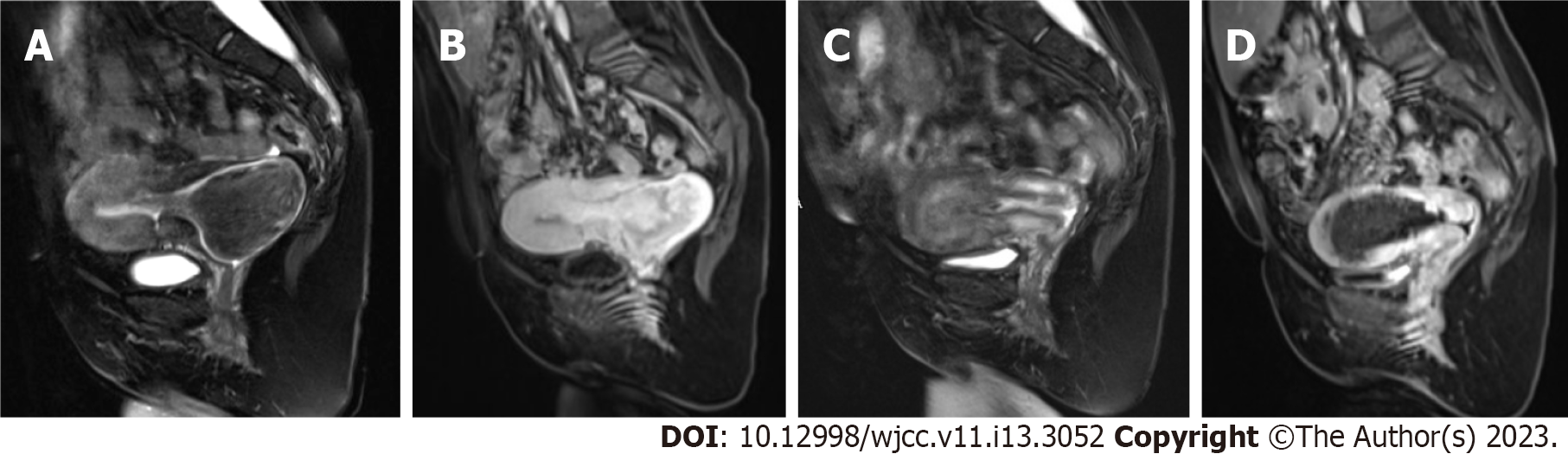
Chest X-ray showed that the patient's bilateral thorax was asymmetric, with severe scoliosis and increased and thickened bilateral lung markings (Figure 1). Electrocardiography revealed sinus tachycardia, and Doppler echocardiography showed mild pulmonary hypertension and mild regurgitation of the aortic, mitral, and tricuspid valves. Transabdominal ultrasound (TAUS) imaging revealed a mass prolapsing into the cervical canal (Figure 2A) with a large blood vessel embedded in the pedicle (Figure 2B). Contrast-enhanced ultrasound imaging (CEUS) showed that the two arteries in the pedicle were the main blood supply sources of the lesion (Figure 2C), one measuring 2.7 mm and the other 3 mm in diameter. Pelvic magnetic resonance imaging (MRI) demonstrated that the pedicle was attached to the posterior uterine inner wall (Figure 3A and B), and no evidence of malignancy was found on T2-weighted imaging or enhanced T1-weighted imaging.
A 46-year-old female complained of prolonged menses, HMB and severe anemia for 2 years, which led to a severely reduced quality of life.
After systematic evaluation of the patient, a case discussion was conducted by a multidisciplinary collaborative group, which consisted of gynecologists, radiologists, and US interventionists, to formulate an optimal radical treatment protocol. Then, a new combined sequential two-session treatment plan was developed. Session one UAE involved blocking of the feeding arteries to reduce the risk of massive intraoperative intrauterine bleeding. Session two PMWA involved ablation of the pedicle, followed by removal of the lesion by twisting; the pedicle stump (to prevent intrauterine bleeding) and the endometrium (to eliminate potential concurrent endometrial hyperplasia, which might also contribute to heavy menstrual bleeding[7]) should be ablated at the same time.
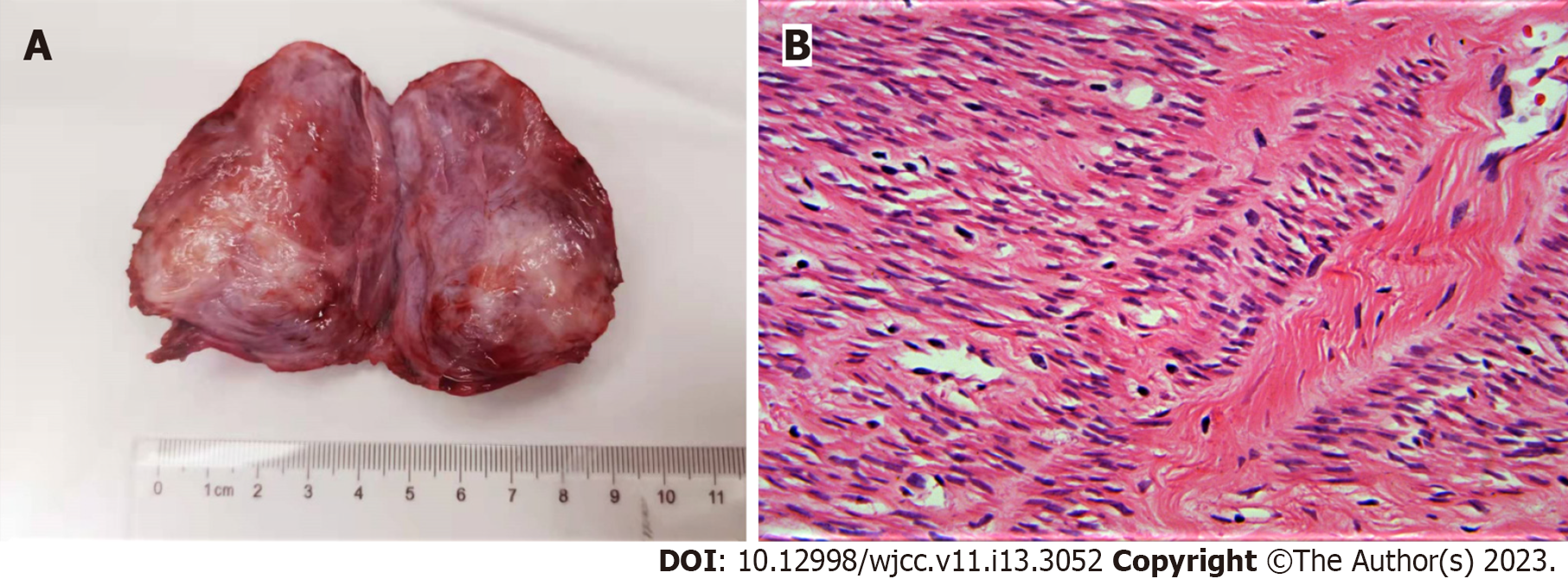
The diagnosis of this patient was defined clearly as a prolapsed pedicled submucous myoma, as its imaging manifestations were very typical. This could be proven by histopathological examination after lesion resection. The final diagnosis was uterine leiomyoma, as shown in the histopathological results (Figure 4).
After achieving consensus with the patient in terms of the therapeutic purposes and methods, the individualized therapy was implemented step by step. In session one, a standard UAE procedure was performed by a senior radiology interventional doctor. An angiographic imaging system (Siemens, Berlin, Germany) was used to perform pelvic digital subtraction angiography. Iopromide at 300 mg iodine/mL (Ultravist 240, Bayer Schering Pharma, Brussels, Belgium) was used to image the blood supply network, and 300-500 µm diameter, nonabsorbable polyvinyl alcohol (PVA) particles (Contour; Boston Scientific, Natick, Massachusetts, United States) were used to embolize the vascular network of the myoma. Before embolism, aortography revealed that the left uterine artery and two radial arteries downstream, which delivered nutrition to the pedicle in the early phase, were dilated (Figure 5A), while the bilateral uterine arteries supplied blood to the myoma in the late phase (Figure 5B). After the location of the opening of the uterine artery was identified with iodinated contrast media injection, sufficient amounts of PVA particles were slowly injected into the feeding artery through a 3F catheter. Postembolism aortography confirmed that most of the radical arteries were blocked successfully (Figure 5C). The puncture site was locally pressurized with a pressure fixator, and the right lower extremity was immobilized for 6 h. The patient presented with fever, lower abdominal pain, and fatigue within 24 h, indicating postembolization syndrome. However, it was relieved after symptomatic treatment.
Two days after UAE, CEUS revealed significant lesion volume reduction (Figure 2D). Furthermore, significant perfusion was observed in part of the outer myometrium and the upper segment of the pedicle (Figure 2E), indicating collateral recanalization. Thus, session two of the treatment was scheduled on the same day and conducted smoothly under conscious sedation and analgesia. For preoperative analgesia, 40 µg dexmedetomidine (1 µg/kg) was diluted in normal saline to a concentration of 4 µg/mL and slowly pumped into the peripheral vein for the first 10 minutes; after that, injection of the drug was maintained at 0.2 µg/kg/h via a pump. For intraoperative analgesia, 30 mg of ketorolac tromethamine was injected as a slow bolus (> 15 s) via the peripheral vein. A monopolar water-cooling MWA system (MTI-5A; 2450 MHz, Great Wall Medical Equipment Co. Ltd., Nanjing, China) equipped with a 14-gauge, 18 cm-long monopolar MWA antenna (XR-A2018W; Great Wall Medical Equipment Co. Ltd.) with a 1 cm active tip was used to carry out the PMWA procedure. The output power was set at 50-60 W. After local infiltration anesthesia with 0.1 g lidocaine hydrochloride, the microwave antenna was inserted into the pedicle under real-time TAUS guidance (Figure 2F). Then, the pedicle was ablated from deep to shallow with the “moving-shot” technique until the entire pedicle was covered by a hyperechoic cloud. After intraoperative CEUS confirmed no enhancement throughout the pedicle, the myoma was clamped with oval forceps and removed by twisting. Finally, the pedicle stump and the endometrium of the upper and middle uterine cavity were ablated. The PMWA procedure was considered complete after B-mode US imaging revealed that the whole uterine cavity was covered by a hyperechoic cloud (Figure 2G). Then, CEUS was performed again, and the results showed no signs of intrauterine bleeding (Figure 2H).
Two days later, postoperative MRI revealed that the anatomy of the uterus had returned to normal (Figure 3C), and half of the outer myometrium had regained perfusion (Figure 3D). The postoperative course was uneventful, and the patient was discharged 3 days later.
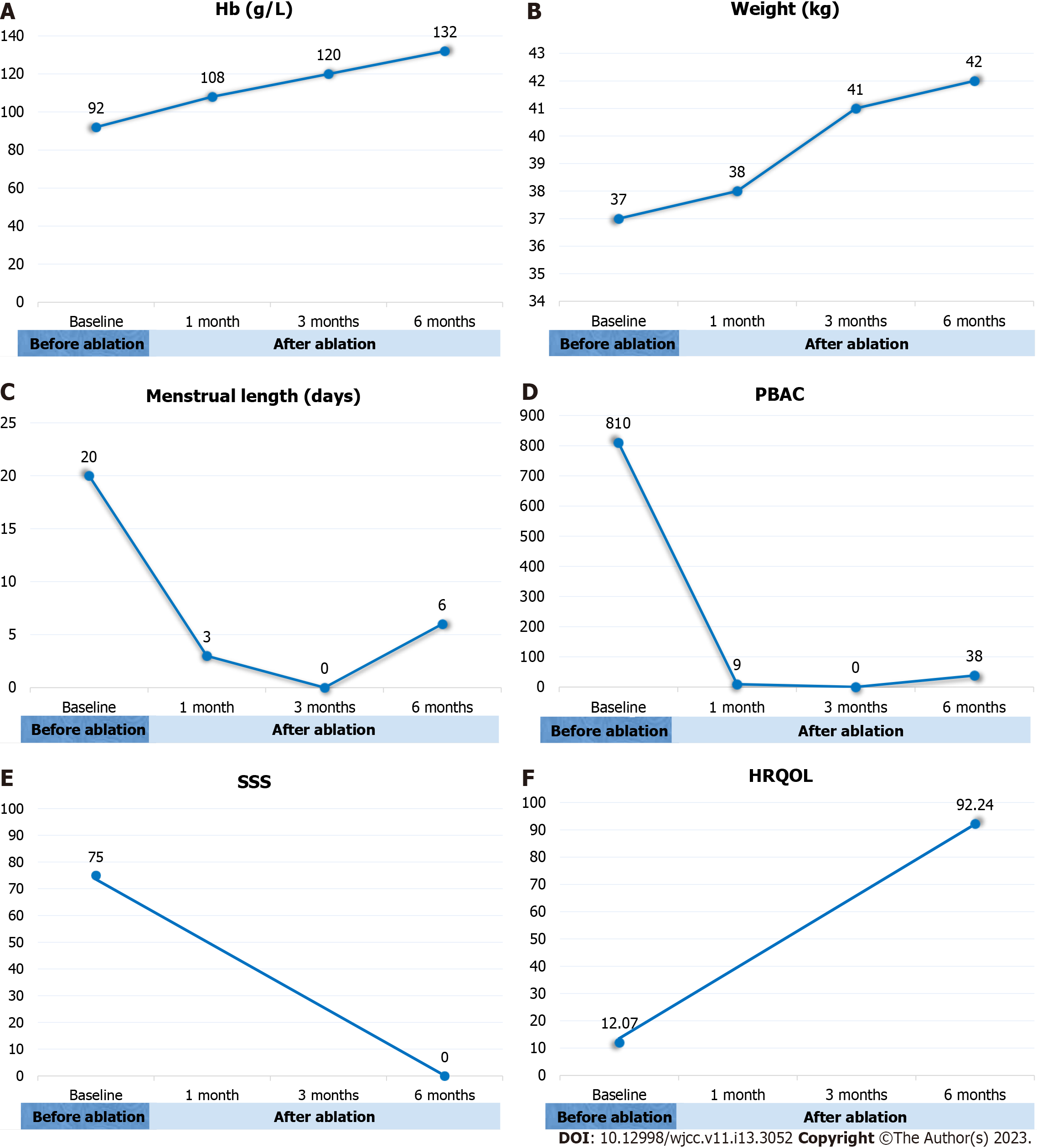
After the treatment, the patient achieved complete symptom relief. As we expected, the patient developed amenorrhea between 2 and 5 mo after treatment, and her Hb increased to normal levels at 3 mo (Figure 6A). During this period, the patient had mild lower abdominal pain for a week, which was relieved after traditional Chinese medical treatment. At the 6-month follow-up, the patient’s weight increased from 37 kg to 42 kg (Figure 6B), the menstrual length decreased to 6 days (Figure 6C), PBAC score decreased from 810 to 38 (Figure 6D), the SSS score decreased from 75 to 0 (Figure 6E), and the HRQOL score increased from 12.07 to 92.24 (Figure 6F). No major complications were recorded.
Most international guidelines agree that hysteroscopic myomectomy should be used as the first-line treatment for the management of symptomatic submucosal leiomyomas[8,9]. As surgeons accumulate sufficient skills and experience in this field, the clinical indications for hysteroscopic myomectomy are also gradually expanding to almost all submucosal leiomyomas[8,10,11], with success rates of 95% in the literature[12]. For women with a submucosal leiomyoma who have completed childbearing, endo
Several alternative, minimally invasive treatments have been developed for treating uterine leiomyomas in the past 20 years, including transcatheter UAE, MR or ultrasound (US)-guided high-intensity focused ultrasound (HIFU), US-guided MWA and radiofrequency ablation (RFA)[14-16]. With the exception of HIFU, which was not feasible in our case due to depth limitations, the other methods were all candidates for further treatment[17,18]. Their mechanisms are similar in that all can destroy the lesion blood supply network, indirectly or directly leading to coagulation and necrosis as well as tumor volume reduction several months after treatment. They are all promising methods for alleviating the symptoms of AUB, but naturally, they are associated with certain risks of complications[15]. For UAE, posttreatment complications mainly include pain, postembolism syndrome, pelvic infection, amenorrhea, and occasional embolism of the ovarian artery[19]. Minor complications after US-guided in situ thermal ablation (MWA or RFA) are similar and include pain, fever, pelvic infection, and vaginal discharge of necrotic tissue[20,21]. However, our patient had a strong desire to remove the leiomyoma during one hospitalization, which could not be achieved by applying any of the above technologies alone. Our idea to solve this problem was to leverage each technique and invent a new hybrid method mimicking the standard procedure for transcervical myoma removal.
In this new hybrid method, real-time US imaging was used to guide and monitor the surgery instead of hysteroscopy; UAE followed by PMWA was adopted to devascularize and dissect the pedicle, achieve intrauterine hemostasis, and perform endometrial ablation. To our knowledge, this method has not yet been reported.
When direct hysteroscopy guidance is not available, real-time US imaging becomes the best choice for guided treatment. Contrast-enhanced MRI and CEUS both play an important role in preoperative evaluation and local response evaluation after nonsurgical interventional treatment of uterine benign diseases[22]. In this case, with CEUS assessment before treatment, we preliminarily characterized the blood supply of the lesions, which provided a basis for assessing the bleeding risk and formulating the radical treatment plan. The second day after UAE, we observed partial vascular recanalization in the pedicle through CEUS examination, which indirectly confirmed the opening of some collateral branches of the uterine arteries, providing a basis for determining the optimal time for subsequent PMWA treatment. Finally, during the PMWA session, CEUS was used to detect potential intrauterine hemorrhage and evaluate the local response following thermal coagulation of the pedicle stump and the endometrium instead of hysteroscopy. This could inspire future PMWA treatments for patients with AUB caused by leiomyoma or adenomyosis.
The reasons why we used PMWA to assist in the dissection of the thick pedicle in this case were as follows. Electronic energy has been widely used to stop bleeding by inducing thermal coagulation and to cut tissue and seal vasculature with high power[23-25]. With different outpower settings and working durations of the electronic surgical instruments, protein denaturation, tissue necrosis, and even explosive vaporization of cells can be induced. Therefore, we used electronic surgical devices to cut tissue, achieve intraoperative hemostasis, and directly seal the vasculature. US-guided PMWA with 60 W output power can quickly increase the tissue temperature within the electromagnetic field to 60-100°C, which is sufficient to induce tissue necrosis and small vessel occlusion[20,21,26]. Therefore, if the microwave antenna was pointed for a long enough duration in the direction perpendicular to the long axis of the pedicle, it could also be used to cut tissues. Li et al[27] reported that RFA of 80-90 W output power effectively blocked the feeding artery of a liver tumor with a diameter ≤ 3 mm with a success rate of 100%. Unfortunately, there is no clinical evidence that US-guided in situ thermal ablations could be used to block the feeding artery of a prolapsed submucosal leiomyoma, and further study is needed. Therefore, there was a potential risk of massive intraoperative bleeding when using the PMWA technique alone to block the feeding artery. As UAE has unique advantages in achieving hemostasis, it is often used in combination with surgery to treat large submucosal myomas with a high risk of intraoperative bleeding[28]. UAE is recommended by several guidelines as an alternative treatment for symptomatic uterine leiomyomas, including submucosal myomas[19,29,30]. Therefore, UAE was preoperatively performed to create a safe condition for the ultimate radical treatment. The planned sequential treatments were carried out successfully, and the patient was eventually cured.
This case demonstrates a new combined minimally invasive treatment for large prolapsed submucosal leiomyomas with a thick pedicle that can be performed under local anesthesia. This new method has potential as an alternative treatment for patients who cannot tolerate general anesthesia.
I would like to express my gratitude to all those who helped me during the writing of this paper. I gratefully acknowledge the help of Prof. Xu for his professional instruction and Prof. Yu who offered me valuable suggestions in the exploration of new clinical treatment methods. I would also like to thank Prof. Cao for his professional help and cooperation in the sequential treatment of this patient.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Obstetrics and gynecology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Di Meglio L, Italy S-Editor: Ma YJ L-Editor: A P-Editor: Zhao S
| 1. | Giuliani E, As-Sanie S, Marsh EE. Epidemiology and management of uterine fibroids. Int J Gynaecol Obstet. 2020;149:3-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 375] [Cited by in RCA: 351] [Article Influence: 58.5] [Reference Citation Analysis (1)] |
| 2. | Stewart EA, Cookson CL, Gandolfo RA, Schulze-Rath R. Epidemiology of uterine fibroids: a systematic review. BJOG. 2017;124:1501-1512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 679] [Cited by in RCA: 634] [Article Influence: 70.4] [Reference Citation Analysis (0)] |
| 3. | Munro MG, Critchley HO, Broder MS, Fraser IS; FIGO Working Group on Menstrual Disorders. FIGO classification system (PALM-COEIN) for causes of abnormal uterine bleeding in nongravid women of reproductive age. Int J Gynaecol Obstet. 2011;113:3-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1104] [Cited by in RCA: 845] [Article Influence: 56.3] [Reference Citation Analysis (0)] |
| 4. | Keizer AL, Jacobs BL, Thurkow AL, de Lange ME, Radder CM, van Kesteren PJM, Hanstede MMF, Huirne JAF, Hehenkamp WJK. The effect of transcervical resection of submucous fibroids on menstrual blood loss: A prospective cohort study. Eur J Obstet Gynecol Reprod Biol. 2022;274:128-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 5. | van Wessel S, van Vliet HAAM, Schoot BC, Weyers S, Hamerlynck TWO. Hysteroscopic morcellation versus bipolar resection for removal of type 0 and 1 submucous myomas: A randomized trial. Eur J Obstet Gynecol Reprod Biol. 2021;259:32-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 6. | Spies JB, Coyne K, Guaou Guaou N, Boyle D, Skyrnarz-Murphy K, Gonzalves SM. The UFS-QOL, a new disease-specific symptom and health-related quality of life questionnaire for leiomyomata. Obstet Gynecol. 2002;99:290-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 290] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 7. | Stewart EA. Clinical practice. Uterine fibroids. N Engl J Med. 2015;372:1646-1655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 317] [Cited by in RCA: 279] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 8. | Amoah A, Joseph N, Reap S, Quinn SD. Appraisal of national and international uterine fibroid management guidelines: a systematic review. BJOG. 2022;129:356-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 9. | Loddo A, Djokovic D, Drizi A, De Vree BP, Sedrati A, van Herendael BJ. Hysteroscopic myomectomy: The guidelines of the International Society for Gynecologic Endoscopy (ISGE). Eur J Obstet Gynecol Reprod Biol. 2022;268:121-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 10. | Brito LG, Magnani PS, de Azevedo Trapp AE, Sabino-de-Freitas MM. Giant prolapsed submucous leiomyoma: a surgical challenge for gynecologists. Clin Exp Obstet Gynecol. 2011;38:299-300. [PubMed] |
| 11. | CRezk A, Kahn J, Singh M. Fertility sparing management in uterine fibroids. Treasure Island (FL): StatPearls Publishing, 2022. |
| 12. | Rolli R, Favilli A, Acanfora MM, Scuderi G, Di Renzo GC, Gerli S. Vaginal myomectomy is a safe and feasible procedure: a retrospective study of 46 cases. J Obstet Gynaecol Res. 2012;38:1201-1205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 13. | Brun JL, Plu-Bureau G, Huchon C, Ah-Kit X, Barral M, Chauvet P, Cornelis F, Cortet M, Crochet P, Delporte V, Dubernard G, Giraudet G, Gosset A, Graesslin O, Hugon-Rodin J, Lecointre L, Legendre G, Maitrot-Mantelet L, Marcellin L, Miquel L, Le Mitouard M, Proust C, Roquette A, Rousset P, Sangnier E, Sapoval M, Thubert T, Torre A, Trémollières F, Vernhet-Kovacsik H, Vidal F, Marret H. [Management of women with abnormal uterine bleeding: Clinical practice guidelines of the French National College of Gynecologists and Obstetricians (CNGOF)]. Gynecol Obstet Fertil Senol. 2022;50:345-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | Wang Y, Zhang S, Li C, Li B, Ouyang L. Minimally invasive surgery for uterine fibroids. Ginekol Pol. 2020;91:149-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 15. | Ierardi AM, Savasi V, Angileri SA, Petrillo M, Sbaraini S, Pinto A, Hanozet F, Marconi AM, Carrafiello G. Percutaneous High Frequency Microwave Ablation of Uterine Fibroids: Systematic Review. Biomed Res Int. 2018;2018:2360107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 16. | Yang Y, Zhang J, Han ZY, Yu MA, Ma X, Zhou HY, Hao YL, Zhao L, Dong XJ, Ge HL. Ultrasound-guided percutaneous microwave ablation for submucosal uterine fibroids. J Minim Invasive Gynecol. 2014;21:436-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 17. | Fan HJ, Cun JP, Zhao W, Huang JQ, Yi GF, Yao RH, Gao BL, Li XH. Factors affecting effects of ultrasound guided high intensity focused ultrasound for single uterine fibroids: a retrospective analysis. Int J Hyperthermia. 2018;35:534-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 40] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 18. | Anneveldt KJ, Verpalen IM, Nijholt IM, Dijkstra JR, van den Hoed RD, Van't Veer-Ten Kate M, de Boer E, van Osch JAC, Heijman E, Naber HR, Ista E, Franx A, Veersema S, Huirne JAF, Schutte JM, Boomsma MF. Lessons learned during implementation of MR-guided High-Intensity Focused Ultrasound treatment of uterine fibroids. Insights Imaging. 2021;12:188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 19. | Lang JH, Chen CL, Xiang Y. [Expert consensus of uterine artery embolization in the management of uterine fibroids and adenomysis]. Zhonghua Fu Chan Ke Za Zhi. 2018;53:289-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 20. | Zhang J, Guan Z, Qian LX, Sheng L, Zhang W, Li XH, Li CG, Jiang H. [The guideline and recommendations of the clinical application of ultrasound-guided percutaneous microwave/radiofrequency ablation in the treatment of uterine fibroids]. Zhonghua Yixue Chaosheng Zazhi. 2015;12 (5):353-356. [DOI] [Full Text] |
| 21. | Li QY, Li XL, Deng EY, Yu SY, Sun LP, Zhang HL, Zhu JE, Li JX, Xu HX. Ultrasound-guided percutaneous microwave ablation for uterine fibroids: mid-term local treatment efficiency and associated influencing factors. Br J Radiol. 2022;95:20220039. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 22. | Sidhu PS, Cantisani V, Dietrich CF, Gilja OH, Saftoiu A, Bartels E, Bertolotto M, Calliada F, Clevert DA, Cosgrove D, Deganello A, D'Onofrio M, Drudi FM, Freeman S, Harvey C, Jenssen C, Jung EM, Klauser AS, Lassau N, Meloni MF, Leen E, Nicolau C, Nolsoe C, Piscaglia F, Prada F, Prosch H, Radzina M, Savelli L, Weskott HP, Wijkstra H. The EFSUMB Guidelines and Recommendations for the Clinical Practice of Contrast-Enhanced Ultrasound (CEUS) in Non-Hepatic Applications: Update 2017 (Long Version). Ultraschall Med. 2018;39:e2-e44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 653] [Cited by in RCA: 621] [Article Influence: 77.6] [Reference Citation Analysis (1)] |
| 23. | Tani T, Naka S, Tani S, Shiomi H, Murakami K, Yamada A, Khiem DT. The invention of microwave surgical scissors for seamless coagulation and cutting. Surg Today. 2018;48:856-864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 24. | Dang KT, Naka S, Nguyen VQ, Yamada A, Tani T. Functional Evaluation of a Novel Microwave Surgical Device in a Canine Splenectomy Model. J Invest Surg. 2021;34:164-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 25. | Beisenova A, Issatayeva A, Ashikbayeva Z, Jelbuldina M, Aitkulov A, Inglezakis V, Blanc W, Saccomandi P, Molardi C, Tosi D. Distributed Sensing Network Enabled by High-Scattering MgO-Doped Optical Fibers for 3D Temperature Monitoring of Thermal Ablation in Liver Phantom. Sensors (Basel). 2021;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 26. | Zhang H, Yu S, Xu H. Ultrasound-guided microwave ablation for symptomatic adenomyosis: More areas of concern for more uniform and promising outcomes. J Interv Med. 2022;5:122-126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 27. | Li X, Xu M, Liu M, Tan Y, Zhuang B, Lin M, Kuang M, Xie X. Contrast-enhanced ultrasound-guided feeding artery ablation as add-on to percutaneous radiofrequency ablation for hypervascular hepatocellular carcinoma with a modified ablative technique and tumor perfusion evaluation. Int J Hyperthermia. 2020;37:1016-1026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 28. | Namkung J, Kang SY, Chung YJ, Cho HH, Kim JH, Kim MR. Multidisciplinary Approach in Large-sized Submucosal Myoma: Hysteroscopic Myomectomy after Uterine Artery Embolization. J Minim Invasive Gynecol. 2019;26:643-647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 29. | American College of Obstetricians and Gynecologists. ACOG practice bulletin. Alternatives to hysterectomy in the management of leiomyomas. Obstet Gynecol. 2008;112:387-400. [PubMed] |
| 30. | Kim MD. Uterine Artery Embolization for Leiomyomas and Adenomyosis: A Pictorial Essay Based on Our Experience from 1300 Cases. Korean J Radiol. 2019;20:1462-1473. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |