Published online May 6, 2023. doi: 10.12998/wjcc.v11.i13.3029
Peer-review started: November 15, 2022
First decision: February 17, 2023
Revised: February 26, 2023
Accepted: April 4, 2023
Article in press: April 4, 2023
Published online: May 6, 2023
Processing time: 161 Days and 6.2 Hours
The optimal treatment for heart failure (HF) is a combination of appropriate me
We report two cases in which arterial blood gas (ABG) improved and PR was possible with appropriate ventilator support. Two patients with extreme obesity complaining of worsening dyspnea–a 47-year-old woman and a 36-year-old man both diagnosed with HF–were hospitalized because of severe hypercapnia and hypoxia. Despite proper medical treatment, hypercapnia and desaturation resolved in neither case, and both patients were transferred to the rehabilitation department for PR. At the time of the first consultation, the patients were bed
Symptoms of patients with obesity and HF may improve once ABG levels are normalized through ventilator support and implementation of PR.
Core Tip: We describe two patients with heart failure (HF) and obesity who experienced respiratory failure, including hypercapnia and hypoxia. Neither patient demonstrated a significant response to pharmacological management; however, in both cases, symptoms improved with noninvasive ventilation, and they were able to return to their daily life. These findings suggest that in patients with obesity and HF who developed pulmonary hypertension and cor pulmonale may need to be treated for obesity hypoventilation and sleep apnea. The symptoms of these comorbidities may improve when arterial blood gas levels are normalized with appropriate ventilator support and pulmonary rehabilitation.
- Citation: Lim EH, Park SH, Won YH. Importance of proper ventilator support and pulmonary rehabilitation in obese patients with heart failure: Two case reports. World J Clin Cases 2023; 11(13): 3029-3037
- URL: https://www.wjgnet.com/2307-8960/full/v11/i13/3029.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i13.3029
Heart failure (HF) is a clinical syndrome characterized by shortness of breath, extreme fatigue, limb, and ankle swelling that are often accompanied by signs, including respiratory distress, gallop rhythm, and pulmonary edema. HF is most commonly caused by a structural or functional abnormality of the heart, resulting in increased intracardiac pressure and/or insufficient cardiac output at rest or during exercise[1]. HF may be classified as either acute or chronic depending on the time and speed of occurrence[2]. Acute HF refers to the rapid or gradual onset of symptoms and/or signs of HF that are sufficiently severe for the patient to seek urgent medical attention, leading to unplanned hospital admissions or emergency department visits[3,4]. Acute HF has a 1-year mortality rate of 20%–30% and an additional risk of hospitalization[5].
Pharmacological management is considered the optimal treatment for patients with acute HF[1]. However, in patients with HF and severe hypercapnia and desaturation, controlling the disease with medication alone is difficult. Appropriate ventilator support followed by pulmonary rehabilitation (PR) should be considered in such patients. Herein, we describe two patients with HF whose symptoms improved after arterial blood gas (ABG) levels normalized with the aid of noninvasive ventilation (NIV) without intubation, which was administered after medication and oxygen supply treatment proved ineffective due to severe hypercapnia.
Case 1: A 47-year-old woman with extreme obesity was admitted to the emergency department because of worsening dyspnea.
Case 2: A 36-year-old man with extreme obesity and chronic HF was admitted to the cardiology outpatient department because of worsening dyspnea.
Case 1: The patient visited the emergency room due to worsening dyspnea that had started 1 wk earlier, and systemic edema had worsened during the last 3 d. She was diagnosed with HF and admitted to the Department of Cardiology. Edema management was initiated because respiratory failure was suspected owing to the deterioration of her pulmonary edema. However, despite medical treatment, her hypercapnia and desaturation could not be reversed, and the patient was referred to the rehabilitation department for PR.
Case 2: The patient visited the emergency room because of worsening dyspnea, which had begun 2 wk earlier, and systemic edema. He was diagnosed with HF and admitted to the Department of Cardiology. He was alert at the time of hospitalization; however, he suddenly lost consciousness and was moved to the intensive care unit (ICU), where he was intubated and treated with mechanical ventilation. Therefore, edema management was initiated. However, despite medical treatment, an attempt to wean him off the ventilator failed, his hypercapnia could not be reversed, and he was referred to our department for PR.
Case 1: The patient had a history of diabetes mellitus, hypertension (HTN), and chronic kidney disease (Table 1). She was also diagnosed with asthma, chronic HF, and pulmonary HTN within the previous year.
| Case 1 | Case 2 | |
| Age | 47 | 36 |
| Gender | F | M |
| Height (cm) | 163.5 | 172 |
| Weight (kg) | 130 | 167.1 |
| Body mass index (kg/m2) | 48.93 | 56.48 |
| Past medical history | ||
| Hypertension | O | O |
| Diabetes mellitus | O | O |
| Heart failure | O | O |
| Pulmonary hypertension | O | O |
| Cor pulmonale | O | × |
| Chronic kidney disease | O | × |
| Bronchial asthma | O | × |
Case 2: The patient had a history of diabetes mellitus and HTN and had been diagnosed with HF approximately 6 mo prior to admission (Table 1).
Case 1: The patient had no remarkable family history.
Case 2: The patient had no remarkable family history.
Case 1: On admission, the patient weighed 130 kg [body mass index (BMI): 48.63 kg/m2). She was alert at the time of the first consultation, although her oxygen demand was high (15 L/min via an oxygen mask), and she was bedridden owing to dyspnea (Table 2).
| Patient 1 | Patient 2 | |||||||
| Adm | RM consult | Discharge | F/U (4 mo) | Adm | RM consult | Discharge | F/U (4 mo) | |
| Height (cm) | 163.5 | 163.5 | 163.5 | 163.5 | 172 | 172 | 172 | 172 |
| Weight (kg) | 130 | 105.5 | 98.2 | 88 | 167.1 | 138.4 | 135.7 | 138 |
| BMI | 48.63 | 39.47 | 36.73 | 32.92 | 56.48 | 46.78 | 45.87 | 46.65 |
| Noninvasive ventilation | ||||||||
| Mode | iVAPS | iVAPS | PCV | PCV | ||||
| Setting | Target Va 8 | Target Va 8 | IPAP 19 | IPAP 19 | ||||
| PS 2-14 | PS 2-14 | EPAP 5 | EPAP 5 | |||||
| EPAP 4-10 | EPAP 4-10 | |||||||
| O2 | 4 L/min | 4 L/min | 4 L/min | 4 L/min | ||||
| Apply time | 9 pm-7 am | 9 pm-7 am | 10 pm-6 am | 10 pm-6 am | ||||
| Daytime O2 | 15 L/min | 15 L/min | 1.5L/min | None | 10 L/min | 10 L/min | None | None |
| (reserve mask) | ||||||||
| ABG test1 | ||||||||
| pH | ↓7.307 | 7.354 | 7.39 | - | ↓7.148 | 7.351 | 7.406 | - |
| PCO2 (mmHg) | ↑97.1 | ↑96.7 | ↑51.7 | - | ↑110 | ↑74.9 | 37.7 | - |
| PO2 (mmHg) | ↓73.3 | ↓63.8 | ↓72.3 | - | ↓79 | 103 | 97.3 | - |
| SaO2 (%) | 93.2 | 88.6 | 94.3 | - | 91.7 | 97.5 | 98 | - |
| Pulmonary function test | ||||||||
| PCF (L/min) | NT | NT | 260 | - | NT | 340 | 370 | 510 |
| FVC (L) | NT | NT | 2.27 | 3.21 | NT | NT | 4.72 (102%) | 4.64 (101%) |
| -0.75 | -0.95 | |||||||
| FEV1 (L) | NT | NT | 1.71 | 2.39 | NT | NT | 3.46 (101%) | 2.76 |
| -0.66 | -0.93 | -0.81 | ||||||
| FEV1/FVC (%) | NT | NT | 75 | 75 | NT | NT | 73 | 59 |
| PEF (%) | NT | NT | 72 | - | NT | NT | 84 | 56 |
| 6MWT | NT | NT | 234 m | 376 m | NT | 389 m | 480 m | 549 m |
| AHI2 | Not done | 78.1/h | ||||||
Case 2: On admission, the patient’s weight was 167.1 kg (BMI: 56.48 kg/m2). The patient was alert at the time of the first consultation. He had undergone extubation 2 days earlier. His oxygen demand was high (10 L/min via a T-piece), and he was bedridden because of dyspnea (Table 2).
Case 1: When the patient arrived at the emergency room, the ABG analysis (ABGA) results indicated severe hypercapnia: pH 7.307; pCO2 97.1 mmHg; pO2 73.3 mmHg; and SaO2 93.2%. At the time of the consultation, her ABG levels still indicated hypercapnia: pH, 7.354; pCO2 96.7 mmHg; pO2 63.8 mmHg; and SaO2 88.6% (Table 2).
Case 2: On ICU admission, his ABGA results indicated severe hypercapnia and hypoxemia: pH 7.148; pCO2 110 mmHg; pO2 79 mmHg; and SaO2 91.7% (Table 2). At the time of the consultation, the patient’s ABG levels still indicated hypercapnia: pH 7.351; pCO2 74.9 mmHg; pO2 103 mmHg, and SaO2 97.5% (Table 2).
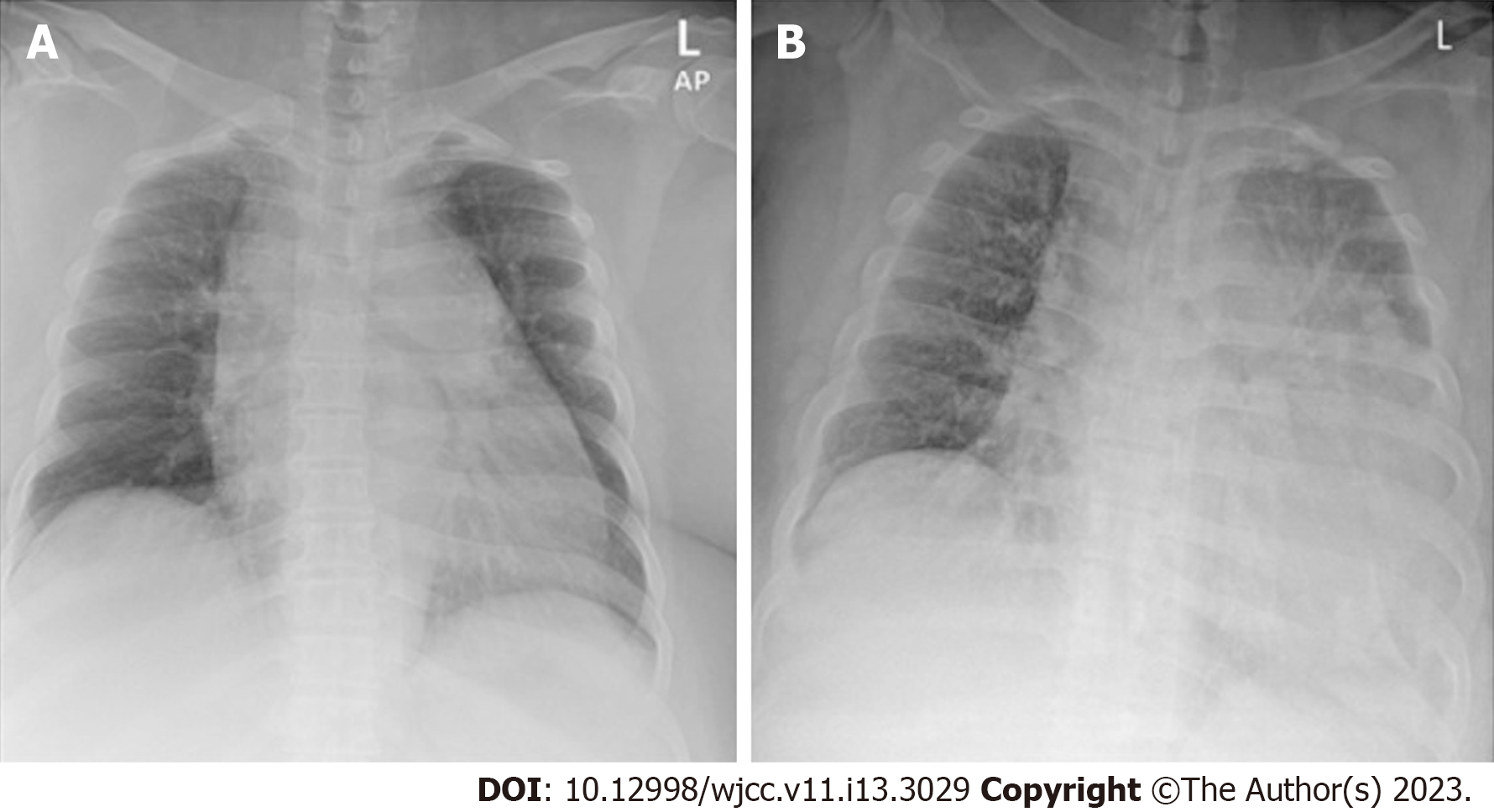
Case 1: Upon arrival at the emergency room, a chest radiograph indicated cardiomegaly (Figure 1A), and chest computed tomography (CT) revealed mosaic attenuation in both lungs and mild pericardial and pleural effusion. Transthoracic echocardiography (TTE) revealed a D-shaped left ventricle (LV) with normal LV systolic function (ejection fraction, 56%) and right ventricle (RV) dysfunction with severe tricuspid regurgitation due to coaptation failure, severe resting pulmonary HTN, RV dilatation (44 mm), right atrial enlargement, and a dilated main pulmonary artery (33 mm). These findings indicated resting pulmonary HTN deterioration compared to the TTE results that the patient had received 3 mo earlier (severe tricuspid regurgitation with moderate resting pulmonary HTN, RV dilatation (43 mm), right atrial enlargement).
Case 2: On ICU admission, chest radiography indicated cardiomegaly, pericardial effusion, and pulmonary interstitial edema with bilateral pleural effusion (Figure 1B). TTE revealed concentric LV hypertrophy, global hypokinesia with mild LV systolic dysfunction (ejection fraction, 47%), left atrial enlargement (48 mm), RV dysfunction with resting pulmonary HTN, diastolic dysfunction (grade 1), and mild pericardial effusion without hemodynamic significance. These findings indicated an aggravation of LV ejection fraction (57% → 47%), newly developed RV dysfunction with mild resting pulmonary HTN, and a decrease in LV end-diastolic pressure (15 → 11) compared to the TTE results that the patient had received 7 mo earlier. Chest CT revealed patchy consolidation with decreasing lung volume in the dependent portion of both lungs, an increase in heart size, and mild pericardial effusion. Therefore, the patient was diagnosed with aspiration pneumonia and cardiomegaly with a small pericardial effusion.
Both patients were diagnosed with HF and respiratory failure.
NIV was initiated, and oxygen demand was gradually reduced (O2 1–2 L). As the patient’s dyspnea and hypercapnia/hypoxia gradually improved to the point where she could be weaned off the ventilator during the day, she began engaging in aerobic exercise and functional training. In the early stages, evaluation using a pulmonary function test was not possible because of the patient’s severe dyspnea. When her status improved with the ventilation treatment, the pulmonary function test was finally performed and yielded a forced vital capacity (FVC) of 2.27 L (75% of predicted maximum) and a forced expiratory volume in the first second (FEV1) of 1.71 L (66% of predicted maximum), resulting to an FEV1/FVC of 75%.
We changed the patient’s treatment from mechanical ventilation to NIV because we expected long-term ventilator use after extubation. NIV was started immediately, and his oxygen demand was gradually reduced. At the time of discharge from the ICU, he was still bedridden because of dyspnea and required oxygen at a rate of 5 L/min (administered via a nasal prong) during the day; NIV was continued during the night.
At the time of discharge, the patient could move around with the aid of a walking device and only needed oxygen at a rate of 1.5 L/min during the day; ventilation continued, but only during the night. On the day of discharge, she performed a 6-minute walking test (6MWT), which yielded a 6MWT distance of 234 m; her weight at that point was 98.2 kg (BMI: 36.73 kg/m2). The ABGA results indicated hypercapnia, although her levels (pH 7.390; pCO2 51.7 mmHg; pO2 72.3 mmHg; and SaO2 94.3%) improved compared to those at the last assessment. The patient was discharged and prescribed home ventilation and O2 therapy.
The patient visited the outpatient department. She still relied on NIV during the night but did not need O2 supply during the day. Her O2 saturation in room air was ≥ 93%. The patient reported a subjective improvement in dyspnea, and the pulmonary function test also indicated improvements, with an FVC of 3.21 L (95% of predicted maximum), an FEV1 of 2.39 L (93% of the predicted maximum), and an FEV1/FVC ratio of 75%. The 6MWT distance measured at this visit was 376 m, and her weight was 88 kg (BMI: 32.92 kg/m2). After 4 mo of follow-up, the patient returned to work while performing activities of daily living independently, maintaining her body weight with aerobic exercises, and relying on NIV only during the night.
As the patient’s dyspnea gradually improved, he started engaging in aerobic exercises. In the early stages, a pulmonary function test could not be performed because of severe dyspnea. When his respiratory function had improved, he finally underwent the test, which yielded an FCV of 4.72 L (102% of predicted maximum), an FEV1 of 3.46 L (101% of predicted maximum), and thus an FEV1/FVC of 73%. The patient expressed an interest in active rehabilitation treatment and was therefore transferred to our department. At the time of transfer to the rehabilitation department, he did not rely on additional oxygen during the day and used NIV only at night.
Polysomnography was performed because we observed desaturation during sleep, and the use of NIV was maintained during sleep because of the patient’s severe obstructive sleep apnea (AHI 78.1/h). He engaged in aerobic exercises on an ergometer and treadmill, and gait training using a walking device. He also exercised to strengthen his muscles as his lower extremities were weak after he had been bedridden for approximately a month. When he was discharged from the hospital, the ABGA results and 6MWT demonstrated improvements, and the patient could move around independently using a walking device. The 6MWT yielded a distance of 480 m, and his weight was 135.7 kg (BMI: 45.87
The patient visited the outpatient department. He still relied on NIV at night and breathing normal room air during the day. He reported subjective improvement in his dyspnea. The pulmonary function test results were similar to those at the last assessment, and yielded an FVC of 4.64 L (101% of predicted maximum), an FEV1 of 2.76 L (81% of the predicted maximum), and an FEV1/FVC of 59%. His 6MWT distance improved compared to the previous test (510 m), and his weight was similar at 138 kg (BMI: 46.65 kg/m2). After 4 mo of follow-up, the patient returned to work while performing activities of daily living independently, maintaining his body weight with aerobic exercises, and relying on NIV only during the night.
These cases demonstrate that appropriate ventilator application and PR in patients with obesity and HF complaining of dyspnea caused by severe hypercapnia can improve symptoms and help patients return to daily life. Because dyspnea is a major barrier for patients with HF in performing activities of daily living, controlling its symptoms is especially important. HF causes complications, such as arrhythmia, thromboembolism, respiratory muscle weakness, and pulmonary HTN[6]. Obesity can also occur because of reduced physical activity[7] and is associated with mortality and various complications, including diabetes mellitus, heart problems, dementia, and cancer[8]. Therefore, treating HF is important and generally involves pharmacological management, such as diuretic therapy, in acute HF. Furthermore, whether the patient’s HF is caused by hypoventilation needs to be considered.
Previous studies have demonstrated that NIV is more effective than conventional oxygen therapy, improves dyspnea, and decreases intubation rates for acute cardiogenic pulmonary edema and acute HF associated with pulmonary disease[9,10]. By contrast, large randomized trials have reported that NIV application does not lead to a reduction in intubation rates; however, this observation might have been attributed to the relatively low intubation rates in the study patient population[11]. Additionally, NIV support is recommended as adjuvant therapy in patients with acute cardiogenic pulmonary edema with severe dyspnea or when medication treatment is ineffective because it has been proven to improve dyspnea and metabolic abnormalities in a faster and safer way than standard oxygen therapy[11,12]. Our two patients who did not significantly benefit from pharmacological management demonstrated symptom improvement and were able to return to their daily lives with the aid of appropriate NIV.
Obesity may occur in response to decreased physical activity in patients with HF or may cause HF by contributing to cardiac hemodynamics, endothelial dysfunction, insulin resistance, vascular changes, and metabolic disorders, including cardiac lipotoxicity[13]. Therefore, obesity should be carefully monitored in patients with HF. Previous studies have also reported independent associations between obesity and pulmonary HTN and between obesity and mortality in the presence of pulmonary HTN[14]. Obesity, insulin resistance, and sleep apnea cause pulmonary HTN, which impairs endothelial function[15]. Moreover, patients with obesity hypoventilation syndrome may experience daytime hypoventilation, chronic hypoxemia, polycythemia, pulmonary HTN, and cor pulmonale[16], which increases the likelihood that these patients will require invasive mechanical ventilation or ICU admission[17].
Dyspnea exacerbation can contribute to functional disabilities that degrade the quality of life of patients with HF, progress over time, and are associated with poor prognosis[18]. Exercise-based cardiac rehabilitation (CR) for all patients with HF has been proven to be safe and effective in improving heart and body functions, reducing readmission rates, and improving quality of life[19,20]. Chronic HF management guidelines in some countries specifically list exercise-based CR as a category I recom
Patients with obesity and HF who develop pulmonary HTN and cor pulmonale need to be assessed and potentially treated for obesity hypoventilation and sleep apnea, in addition to receiving medication for HF. The two reported cases suggest that the symptoms of these comorbidities may improve once ABG levels are normalized through appropriate ventilator support and PR.
The authors thank all the members of the Department of Physical Medicine & Rehabilitation, Jeonbuk National University Hospital.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Rehabilitation
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Hasabo EA, Sudan S-Editor: Ma YJ L-Editor: A P-Editor: Zhang YL
| 1. | Authors/Task Force Members, McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, Burri H, Butler J, Čelutkienė J, Chioncel O, Cleland JGF, Coats AJS, Crespo-Leiro MG, Farmakis D, Gilard M, Heymans S, Hoes AW, Jaarsma T, Jankowska EA, Lainscak M, Lam CSP, Lyon AR, McMurray JJV, Mebazaa A, Mindham R, Muneretto C, Francesco Piepoli M, Price S, Rosano GMC, Ruschitzka F, Kathrine Skibelund A; ESC Scientific Document Group. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2022;24:4-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 1493] [Article Influence: 373.3] [Reference Citation Analysis (2)] |
| 2. | Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, González-Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P. [2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure]. Kardiol Pol. 2016;74:1037-1147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 163] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 3. | Kurmani S, Squire I. Acute Heart Failure: Definition, Classification and Epidemiology. Curr Heart Fail Rep. 2017;14:385-392. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 129] [Cited by in RCA: 224] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 4. | Maggioni AP. Epidemiology of Heart Failure in Europe. Heart Fail Clin. 2015;11:625-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 60] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 5. | Orso D, Tavazzi G, Corradi F, Mearelli F, Federici N, Peric D, D'Andrea N, Santori G, Mojoli F, Forfori F, Vetrugno L, Bove T. Comparison of diuretic strategies in diuretic-resistant acute heart failure: a systematic review and network meta-analysis. Eur Rev Med Pharmacol Sci. 2021;25:2971-2980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (1)] |
| 6. | Watson RD, Gibbs CR, Lip GY. ABC of heart failure. Clinical features and complications. BMJ. 2000;320:236-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 111] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 7. | Reddy YNV, Rikhi A, Obokata M, Shah SJ, Lewis GD, AbouEzzedine OF, Dunlay S, McNulty S, Chakraborty H, Stevenson LW, Redfield MM, Borlaug BA. Quality of life in heart failure with preserved ejection fraction: importance of obesity, functional capacity, and physical inactivity. Eur J Heart Fail. 2020;22:1009-1018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 65] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 8. | Kinlen D, Cody D, O'Shea D. Complications of obesity. QJM. 2018;111:437-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 179] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 9. | Masip J, Peacock WF, Price S, Cullen L, Martin-Sanchez FJ, Seferovic P, Maisel AS, Miro O, Filippatos G, Vrints C, Christ M, Cowie M, Platz E, McMurray J, DiSomma S, Zeymer U, Bueno H, Gale CP, Lettino M, Tavares M, Ruschitzka F, Mebazaa A, Harjola VP, Mueller C; Acute Heart Failure Study Group of the Acute Cardiovascular Care Association and the Committee on Acute Heart Failure of the Heart Failure Association of the European Society of Cardiology. Indications and practical approach to non-invasive ventilation in acute heart failure. Eur Heart J. 2018;39:17-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 126] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 10. | Masip J. Noninvasive Ventilation in Acute Heart Failure. Curr Heart Fail Rep. 2019;16:89-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 11. | Gray A, Goodacre S, Newby DE, Masson M, Sampson F, Nicholl J; 3CPO Trialists. Noninvasive ventilation in acute cardiogenic pulmonary edema. N Engl J Med. 2008;359:142-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 407] [Cited by in RCA: 385] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 12. | Weng CL, Zhao YT, Liu QH, Fu CJ, Sun F, Ma YL, Chen YW, He QY. Meta-analysis: Noninvasive ventilation in acute cardiogenic pulmonary edema. Ann Intern Med. 2010;152:590-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 143] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 13. | Ebong IA, Goff DC Jr, Rodriguez CJ, Chen H, Bertoni AG. Mechanisms of heart failure in obesity. Obes Res Clin Pract. 2014;8:e540-e548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 166] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 14. | Frank RC, Min J, Abdelghany M, Paniagua S, Bhattacharya R, Bhambhani V, Pomerantsev E, Ho JE. Obesity Is Associated With Pulmonary Hypertension and Modifies Outcomes. J Am Heart Assoc. 2020;9:e014195. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 65] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 15. | Zamanian RT, Hansmann G, Snook S, Lilienfeld D, Rappaport KM, Reaven GM, Rabinovitch M, Doyle RL. Insulin resistance in pulmonary arterial hypertension. Eur Respir J. 2009;33:318-324. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 234] [Cited by in RCA: 214] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 16. | Olson AL, Zwillich C. The obesity hypoventilation syndrome. Am J Med. 2005;118:948-956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 171] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 17. | Nowbar S, Burkart KM, Gonzales R, Fedorowicz A, Gozansky WS, Gaudio JC, Taylor MR, Zwillich CW. Obesity-associated hypoventilation in hospitalized patients: prevalence, effects, and outcome. Am J Med. 2004;116:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 337] [Cited by in RCA: 297] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 18. | Dunlay SM, Manemann SM, Chamberlain AM, Cheville AL, Jiang R, Weston SA, Roger VL. Activities of daily living and outcomes in heart failure. Circ Heart Fail. 2015;8:261-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 141] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 19. | Taylor RS, Sagar VA, Davies EJ, Briscoe S, Coats AJ, Dalal H, Lough F, Rees K, Singh S. Exercise-based rehabilitation for heart failure. Cochrane Database Syst Rev. 2014;2014:CD003331. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 172] [Cited by in RCA: 224] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 20. | Leggio M, Fusco A, Loreti C, Limongelli G, Bendini MG, Mazza A, Coraci D, Padua L. Effects of exercise training in heart failure with preserved ejection fraction: an updated systematic literature review. Heart Fail Rev. 2020;25:703-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 21. | Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Colvin MM, Drazner MH, Filippatos GS, Fonarow GC, Givertz MM, Hollenberg SM, Lindenfeld J, Masoudi FA, McBride PE, Peterson PN, Stevenson LW, Westlake C. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol. 2017;70:776-803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1130] [Cited by in RCA: 1408] [Article Influence: 156.4] [Reference Citation Analysis (0)] |
| 22. | Pelliccia A, Sharma S, Gati S, Bäck M, Börjesson M, Caselli S, Collet JP, Corrado D, Drezner JA, Halle M, Hansen D, Heidbuchel H, Myers J, Niebauer J, Papadakis M, Piepoli MF, Prescott E, Roos-Hesselink JW, Graham Stuart A, Taylor RS, Thompson PD, Tiberi M, Vanhees L, Wilhelm M; ESC Scientific Document Group. 2020 ESC Guidelines on sports cardiology and exercise in patients with cardiovascular disease. Eur Heart J. 2021;42:17-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 358] [Cited by in RCA: 1125] [Article Influence: 225.0] [Reference Citation Analysis (1)] |