Published online May 6, 2023. doi: 10.12998/wjcc.v11.i13.2890
Peer-review started: December 17, 2022
First decision: February 17, 2023
Revised: February 25, 2023
Accepted: March 30, 2023
Article in press: March 30, 2023
Published online: May 6, 2023
Processing time: 128 Days and 15.3 Hours
Diabetes care is often difficult without a proper collaboration between the patient and the care provider as the disease is mostly self-managed by patients through adjustments in their lifestyles, and medication doses to optimise glycaemic control. Most clinical guidelines on the management of diabetes mellitus (DM) provide only broad principles on diabetes care, and the blind follow-up of such principles without a proper review and consideration of patient characteristics often results in inadequate glycaemic control and diabetes complications consequently. Therefore, a proper understanding of the pathobiology, clinical situation, and comorbidities of the individual case is of paramount importance to tailoring the most appropriate management strategy in real-world diabetes care. With the aid of five unique cases of DM [(1) Medically managed type 2 diabetes mellitus (T2DM) with severe obesity; (2) Management of T2DM with unreliable glycated haemoglobin (HbA1c); (3) Obesity in a patient with type 1 diabetes mellitus (T1DM); and (4) Late diagnosis and subsequent management of monogenic diabetes and 5. Sudden worsening of well-controlled T2DM)] we elaborate on the importance of individualised diabetes care and the practicalities in these situations. The review also provides an evidence update on the management of different forms of DM to guide physicians in optimising the care of their patients in day-to-day clinical practice.
Core Tip: Diabetes mellitus (DM) is a chronic disease mostly self-managed by patients as glucose control is largely related to lifestyle adjustments with appropriate dietary habits and physical activities. A proper understanding of the pathobiology of DM, associated comorbidities, the clinical situation, and the socio-cultural background of each patient is of paramount importance in planning the optimal management strategies for diabetes care. With the aid of 5 interesting real-world case scenarios, we elaborate on the importance of individualised diabetes care in this evidence-based review to empower physicians in optimising the care of their diabetes patients in day-to-day clinical practice.
- Citation: Khor XY, Pappachan JM, Jeeyavudeen MS. Individualized diabetes care: Lessons from the real-world experience. World J Clin Cases 2023; 11(13): 2890-2902
- URL: https://www.wjgnet.com/2307-8960/full/v11/i13/2890.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i13.2890
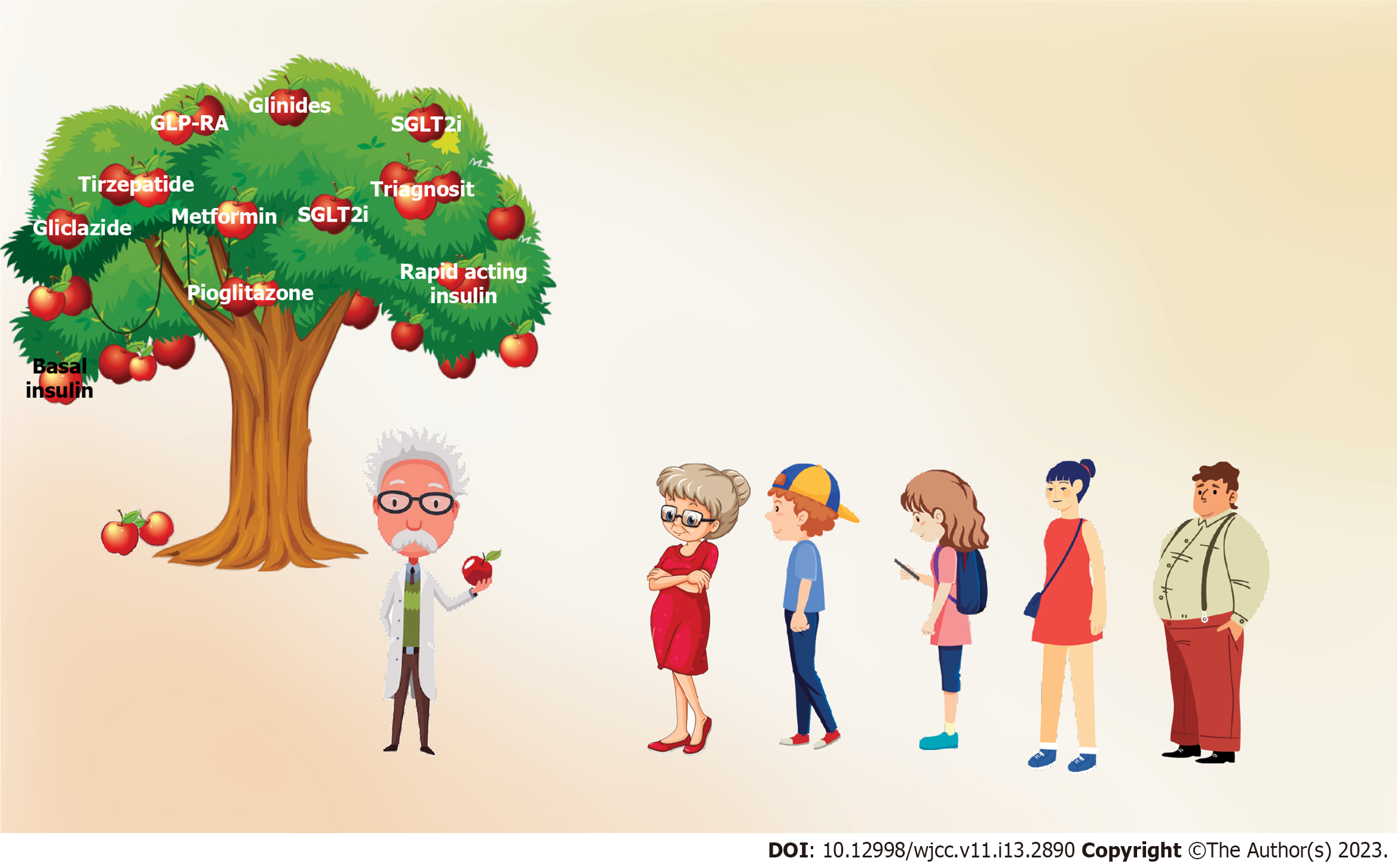
According to the most recent update of the International Diabetes Federation (IDF) in the year 2021, DM has affected 537 million adults across the globe[1]. Patients with DM are often not well-informed about the pathophysiology, natural course, potential complications, and the plan for optimal management of their illness from the outset which is the most important reason for poor disease outcomes. Although diabetes professional bodies such as the American Diabetes Association (ADA), the European Association for the Study of Diabetes (EASD), the Diabetes United Kingdom (DUK), and IDF commonly involve patient support groups and produce publications on structured diabetes education programs, empowerment of diabetes patients by the healthcare professionals during clinic reviews is often inadequate at an individual level. Figure 1 shows a graphical summary of the importance of individualised diabetes care.
As DM is a chronic disease mostly self-managed by patients, individualised diabetes care plans and self-management support (SMS) systems are expected to improve adherence to therapy and thereby, the disease outcomes. Multiple studies have shown that SMS interventions and personalised care plans result in an improvement in clinical outcomes, quality of life, knowledge about the disease, and the efficiency of self-care among patients with DM[2-4]. However, this approach is often overlooked in the day-to-day clinical practice in most healthcare systems which can contribute to adverse disease outcomes in DM cases. With the aid of 5 unique real-world clinical case scenarios, we outline the importance of individualised diabetes care in this evidence-based review.
A 58-year-old woman with a 10-year history of T2DM, initially managed with Metformin 1 g twice a day (BID), Gliclazide 160 mg BID, and insulin Glargine 60 units at night, was referred to the diabetes specialist clinic for improvement of her metabolic control due to inadequate glycaemic control despite a gradual increase in the insulin doses. Her body weight was 118 kg with a body mass index (BMI) of 41 kg/m2, and her blood pressure (BP) was 156/88 mmHg. Her biochemical profile showed: HbA1c 76 mmol/mol, creatinine 86 mmol/L, total cholesterol 5.6 mmol/L, high-density lipoprotein (HDL) 0.88 mmol/L, low-density lipoprotein (LDL) 3.1 mmol/L, triglycerides (TG) 2.3 mmol/L, gamma-glutamyl transferase (GGT) 168 U/L, and alkaline phosphatase (ALP) 146 U/L. Other biochemical tests such as thyroid functions and urine microalbumin were normal.
After a discussion about the importance of managing her gradually increasing body weight which was getting worse after the initiation of insulin 2 years ago (when her body weight was 102 kg), she agreed to start an injection of Semaglutide 0.25 mg subcutaneously weekly after stopping the Gliclazide. She tolerated the Semaglutide well with good suppression of her appetite and the dose was escalated to 0.5 mg weekly after 4 wk, and then to 1 mg weekly after 8 weeks. She started losing weight steadily and managed to reduce the dose of her insulin Glargine by 4-6 units periodically and discontinued the insulin completely in 9 mo following the initiation of Semaglutide. She achieved a total weight loss of 36 kg and her HbA1c dropped to 58 mmol/moL at the 9-mo follow-up visit. After another discussion about the potential for further improvements in her diabetes and body weight, she has been commenced on Canagliflozin 100 mg daily, the dose of which was increased to 300 mg daily after a month.
At her subsequent review 6 months later, she had a further weight loss of 20 kg but had been feeling depressed as people have not been recognizing her because of her massive weight loss and demanded to stop her antidiabetic medications completely. Her biochemical profile revealed: HbA1c 42 mmol/moL, creatinine 64 mmol/L, total cholesterol 3.8 mmol/L, HDL 0.98 mmol/L, LDL 2.8 mmol/L, TG 2.3 mmol/L, GGT 168 U/L, and ALP 146 U/L. Although her T2DM was in remission, she was cautioned about the high risk of relapse when the medications that resulted in massive weight loss (combination of Semaglutide and Canagliflozin) were discontinued. She agreed to continue Metformin 1 g BID on a long-term basis.
A 64-year-old man was referred to the diabetes clinic by his general practitioner as he could not explain the disproportionately low HbA1c of 52 mmol/moL while the patient was showing capillary blood glucose (CBG) readings of 18–24 mmol/L regularly on his home glucose monitor. He had been on mixed Isophane human insulin/regular insulin (70/30) BID (20 units before breakfast and 15 units before evening meals). He was getting monthly iron transfusions and occasional infusions of packed red blood cells for chronic anaemia related to angiodysplasia of the small intestine.
Evaluation from the hospital clinic revealed haemoglobin of 105 g/L and a fructosamine level of 564 µmol/L (reference range: 215-310). The falsely low HbA1c levels in the patient were considered a reflection of rapid red cell turnover from chronic intestinal blood loss and accelerated erythropoiesis. He was advised to up-titrate the insulin doses gradually to bring down the CBGs to < 10 mmol/L consistently. After three months of treatment under close supervision, his fructosamine levels came down to 384 µmol/L, acceptable for his age and co-morbidities. The referring physician and the patient were advised to rely upon the CBGs for optimal management of diabetes without periodic monitoring of HbA1c because of its unreliability.
A 72-year-old woman was referred to the diabetes clinic for consideration of adding an antidiabetic medication with weight loss potential. She had a background of longstanding inadequately controlled T1DM of 15 years duration. She was on treatment with insulin Aspart 36 units with main meals three times a day (TID), insulin Glargine 90 units at night, and Metformin 1 g BID for her diabetes management. Her HbA1c level was 68 mmol/moL without other major abnormalities in her biochemical tests. Her body weight was 94 kg with a BMI of 36 kg/m2 and was quite motivated to try any medications to improve her body weight and high insulin dose requirements.
After counselling about the potential gastrointestinal side effects and the lack of license for routine use in patients with T1DM, the patient agreed to try an injection of liraglutide as an add-on therapy for her diabetes management at a dose of 0.6 mg subcutaneously daily. The daily dose was up-titrated by 0.6 mg every month to a maximum of 1.8 mg daily in 2 months. She tolerated the medication well and started losing weight with her CBG readings consistently < 10 mmol/L without significant hypoglycemic episodes. She managed to reduce the dose of her insulins gradually, and at the end of one year, she was only taking insulin Aspart 10 units TID with her main meals and insulin Glargine 40 units at night. Her final body weight was 72 kg and her HbA1c was 57 mmol/moL. She also reported significant improvement in her mobility which was previously restricted by knee joint osteoarthritis.
A 42-year-old woman was reviewed in the diabetes clinic as requested by her primary care physician for the optimisation of diabetes control. She had a 12-year history of diabetes which was initially managed by oral hypoglycaemic agents (OHA). Metformin was stopped because of severe diarrhoea. She did not tolerate Gliclazide because of hypoglycaemic episodes. Subsequently, she was managed with mixed Isophane human insulin/regular insulin (70/30) BID- 30 units before breakfast and 42 units before evening meals. The CBG readings ranged between 6–16 mmol/L with occasional mild symptomatic hypoglycaemic episodes. Her latest HbA1c was 72 mmol/moL, indicating poor glycaemic control for her age although she had no established microvascular complications of diabetes.
When she reported a recent diagnosis of diabetes in her 22-year-old son and a previous history of hypoglycaemic episodes with Gliclazide, the probability of monogenic diabetes was considered. She was unwilling to undergo genetic testing for monogenic diabetes initially. Therefore, a sulphonylurea challenge test was performed. She responded very well to the sulphonylurea challenge with a marked reduction in her plasma glucose. This was suggestive of monogenic diabetes. Subsequently, she had a genetic test that showed an HNF-1A mutation. Her glycemic control improved significantly with her HbA1c level reaching 49 mmol/moL within three months of treatment with oral Gliclazide 40 mg BID only.
A 66-year-old woman with an 8-year history of well-controlled T2DM on diet and lifestyle modifications presented with a one-month duration of osmotic symptoms and weight loss of 6 kg. Her CBGs were consistently between 16–22 mmol/L for nearly three weeks before her evaluation. Her HbA1c rose to 66 mmol/moL from 51 mmol/moL three months earlier indicating that her hyperglycaemia was of very recent onset. She weighed 66 kg with a BMI of 28 kg/m2. Her liver function tests (LFT) showed elevated GGT of 240 U/L, ALP of 196 U/L, and alanine transaminase level of 68 U/L with normal bilirubin and albumin levels. She has commenced on Metformin 500 mg BID and mixed Isophane human insulin/regular insulin (70/30) 10 units BID with a plan to increase the dose every 2–3 d to reduce the glucose readings to mostly single figures without hypoglycaemia.
Blood samples for screening of T1DM antibodies [antibodies against islet cells, insulinoma antigen 2 (IA2), glutamic acid decarboxylase (GAD), and zinc transporter 8 [ZnT8)], and an outpatient ultrasound scan of the liver for investigating the presumed metabolic-associated fatty liver disease as a cause of abnormal LFT were ordered. Although her antibody screening for T1DM was reported as negative 2 d later, the liver USS showed numerous nodular lesions suggestive of metastatic cancer. A subsequent computed tomography scan of the chest, abdomen, and pelvis revealed advanced metastatic pancreatic cancer. The patient and family understood that her prognosis was very poor and declined further evaluation and interventions for the cancer. She was discharged to a palliative care facility for symptom control and end-of-life support where she died a few weeks later.
Diabetes is a unique disease entity with very different clinical presentations and courses of illness largely influenced by the patient’s biological characteristics, environmental factors, and comorbid illnesses. Therefore, formulating a management plan requires consideration of all these factors at a personal level to individualise treatment options through the SMS mentioned above. The case snippets discussed in this paper are some unique examples of this paradigm. The scientific rationale for this individualised DM care and the importance of SMS systems are discussed below.
Obesity, defined by the World Health Organisation (WHO) as having a BMI of ≥ 30 kg/m², is an excessive accumulation of fat that poses a risk to health. The prevalence of obesity worldwide has almost tripled since 1975 with > 650 million adults being obese in 2016[5]. More concerningly, the percentage of children and adolescents aged 5-19 who are overweight, and obese has increased significantly from just 4% in 1975 to a whopping > 18% in 2016. This obesity epidemic, which is a major risk factor for noncommunicable diseases such as diabetes, cardiovascular diseases, musculoskeletal disorders, and some forms of cancers, is largely preventable through lifestyle changes with the consumption of a low-energy diet and regular physical activities.
“Diabesity” is a terminology used to describe the pathophysiological interlink between obesity/overweight and T2DM. This phenomenon is a complex molecular cascade, involving lifestyle factors, abnormal genetic heterogenicity background as well as biochemical and hormonal mechanisms.
The development of T2DM in an overweight or obese individual can be annotated by the presence of visceral adiposity leading to the development of peripheral insulin resistance. There are three hypotheses proposed in the pathophysiology of diabesity[6]:
The “inflammatory hypothesis” advocates a causal link between the pro-inflammatory cytokines produced by the excess adipose tissues and insulin resistance.
The “lipid overflow hypothesis” suggests that lipid metabolites released from the adipose tissues cause inhibition of insulin signal transduction and increased insulin resistance.
The “adipokine hypothesis” suggests that the adipokines secreted from the adipose tissues made up of several hormones and chemical substances, induce inflammatory and metabolic cascades, resulting in insulin resistance.
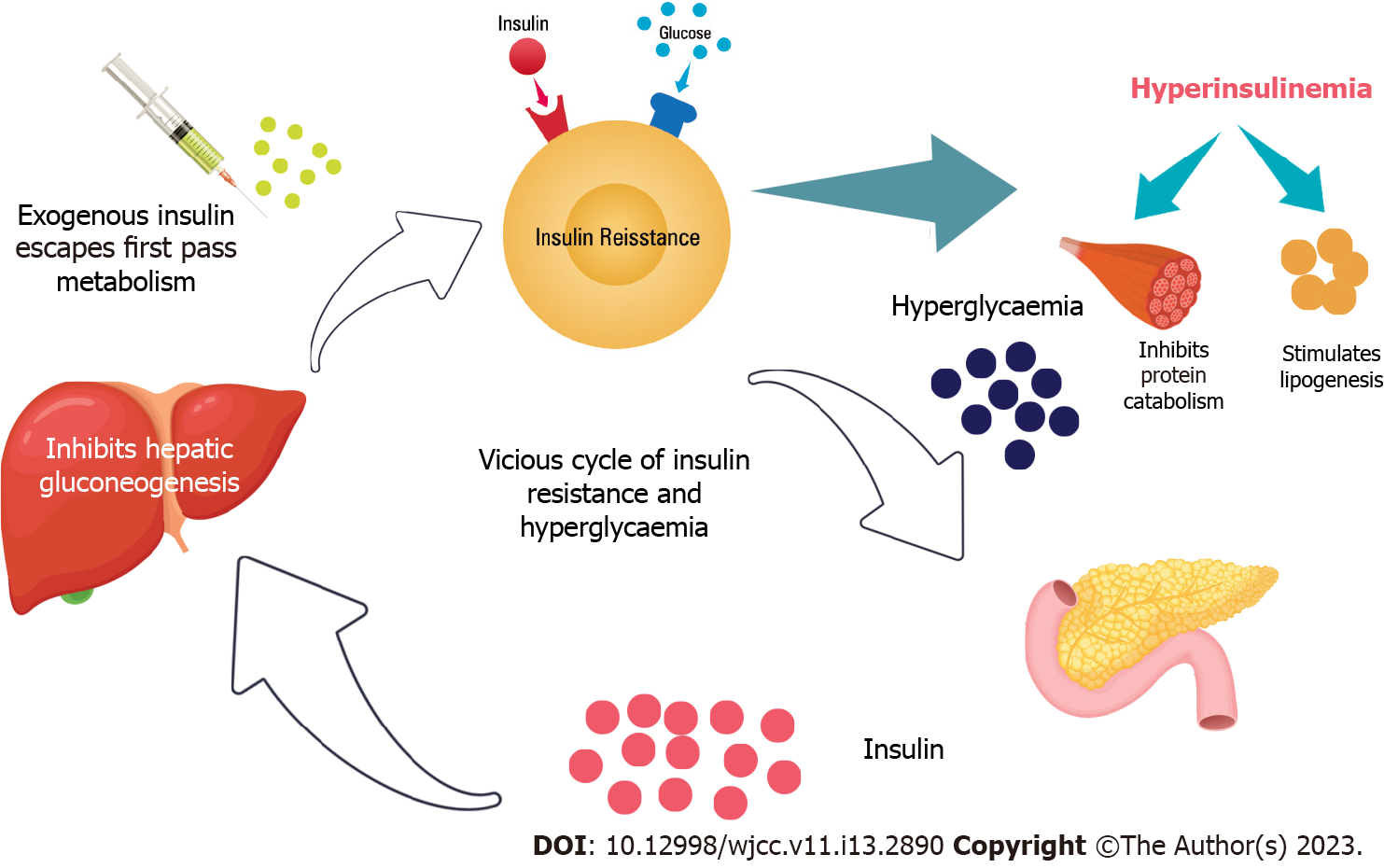
As insulin resistance progresses, an individual will require a higher amount of plasma insulin to achieve the same insulin effect due to a downregulation of the insulin receptors on cell membranes. However, insulin is an anabolic hormone that slows basal metabolism, inhibits protein catabolism, stimulates lipogenesis, and increases the accumulation of adiposity. In addition, the exogenous insulin does not have the first pass hepatic metabolism through the portal vein to suppress hepatic gluconeogenesis. This results in further weight gain associated with exogenous insulin use. The vicious cycle due to hyperinsulinemia can cause worsening of the diabesity[6,7]. Figure 2 shows the schematic diagram of the mechanism of insulin resistance and the development of diabesity.
In case 1, the patient presented with a classical picture of diabesity and metabolic syndrome (dyslipidaemia and hypertension). Her initial treatment which consisted of Metformin, sulphonylurea, and insulin had resulted in the worsening of her diabesity. Metformin, which remained as the first-line treatment in T2DM, is thought to be a weight-neutral antidiabetic medication. Although its exact mechanism of action is yet to be fully understood, it is known to inhibit hepatic glucose production and gluconeogenesis, improve peripheral insulin resistance, and it has an anorexiant effect which is beneficial in the context of managing diabesity. On the other hand, the risks and benefits of using sulphonylurea and exogenous insulin need to be carefully considered owing to their side effect of weight gain. On balance, exogenous insulin remained an important treatment in patients with uncontrolled hyperglycaemia when oral antidiabetic medications alone would not have worked sufficiently. Insulin, in addition to Metformin, is especially useful when the HbA1c level is ≥ 75 mmol/moL to achieve a rapid improvement in glycaemic control. Combination therapy is preferred as less insulin is required compared to insulin monotherapy[6,7].
The clinicians should review and rationalise antidiabetic regimes from time to time. For example, in the case we described, the insulin regime was gradually titrated down following the addition of antidiabetic medications to prevent adverse effects such as hypoglycaemia.
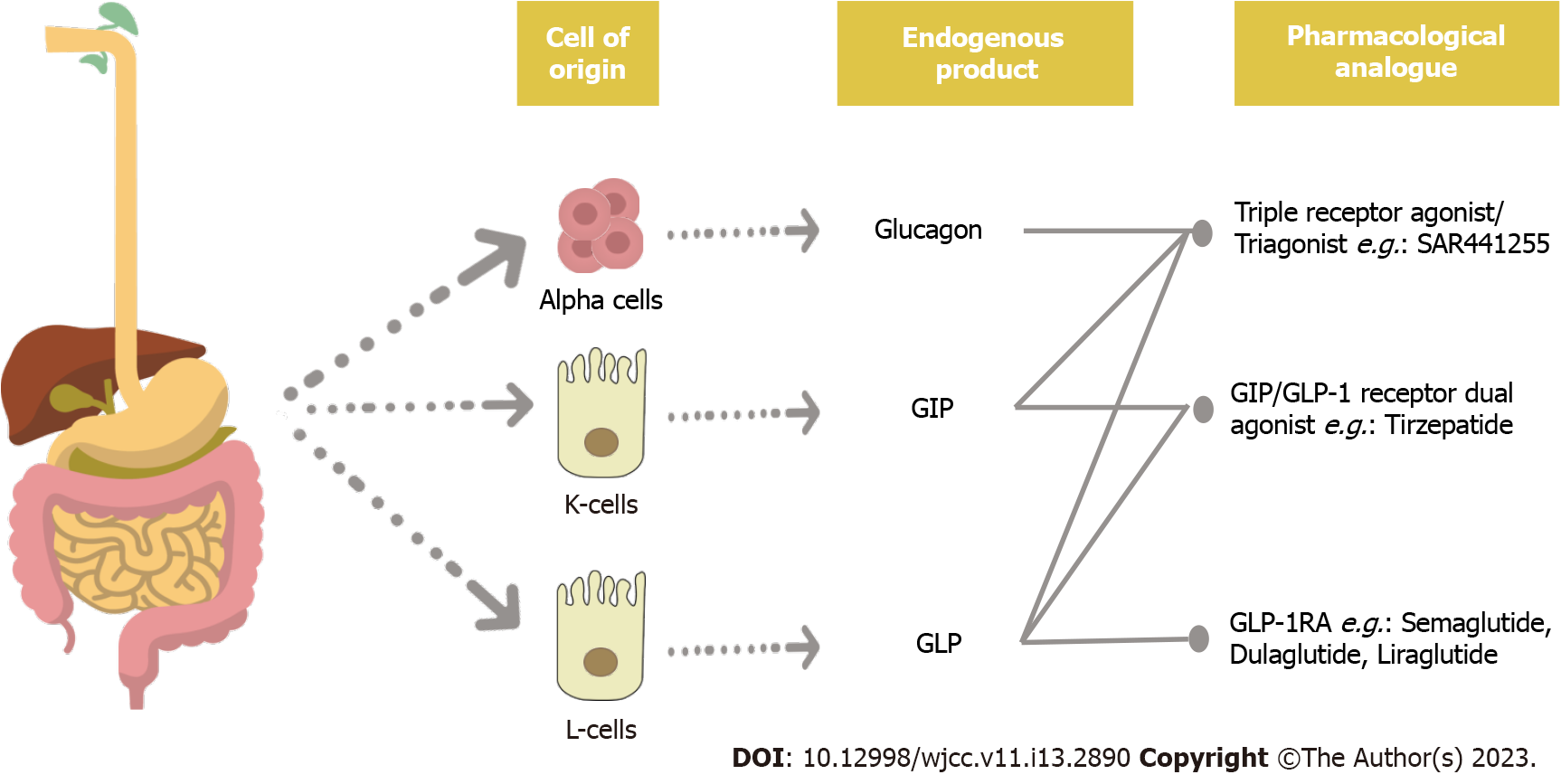
Glucagon-like peptide-1 receptor agonists: GLP-1 is a short-lived incretin hormone that is produced in response to food intake, and it controls feeding behaviour and glucose homeostasis[6,7]. The binding of GLP-1 to its receptor stimulates the release of insulin from beta-cells and suppresses the release of glucagon from alpha cells in the pancreas. Therefore, the glucagon-like peptide-1 receptor agonists (GLP-1RA), which mimics the action of endogenous GLP-1 but escapes the degradation by the dipeptidyl peptidase-4 (DPP-4) enzyme, acts by stimulating the insulin secretion, inhibiting gastric emptying in a dose-dependent manner, suppressing the appetite and altering the feeding behaviour[8].
Several studies reviewed that various preparations of GLP-1RA such as Liraglutide, Dulaglutide, Semaglutide, and Exenatide demonstrated a significant reduction in HbA1c, at least if not more than when basal insulin was used, and with significant weight reduction[9-13]. Liraglutide has been shown to achieve a significantly greater mean HbA1c reduction when compared to the placebo: 11.9 mmol/moL vs 6.0 mmol/moL[14]. A mean weight change of -5.8% was observed at the end of this 56-wk SCALE Insulin study with 51.8% of patients on Liraglutide achieving ≥ 5% weight loss. In addition, 22% of the patients had managed to lose ≥ 10% of their weight. It was thought that the delay in gastric emptying, satiety, and reduced dose of insulin or sulfonylurea may have contributed to this. Figure 3 shows a schematic diagram of the physiology of incretins and glucagon and the pharmacological manipulation for the management of diabesity.
A greater mean weight loss of 15.8% in the Semaglutide group compared to only 6.4% in the Liraglutide group was observed in a head-to-head study to compare the efficacy of GLP-1RA in weight loss[15]. The proportion of patients achieving ≥ 10%, ≥ 15%, and ≥ 20% of weight loss was significantly larger in the Semaglutide group. The study on Semaglutide also showed the benefit of continuing treatment following weight loss can result in further weight loss, with improvement in waist circumference, lipid profile, and glucose metabolism.
Moreover, the GLP-1RA has also been shown to offer cardiorenal protection, which is particularly relevant to this group of patients who are already at high risk of developing micro- and macrovascular diabetic complications. A meta-analysis demonstrated a significant 14% reduction in the risk of major cardiovascular events among the diabetic population, with the effect noted to be greater in patients with established cardiovascular disease[16].
The most common side effect from the GLP-1RA was gastrointestinal events of mild to moderate severity, which were more frequently observed following the dose escalation[10,11]. Table 1 shows the landmark clinical trials with the GLP-1RA group of drugs currently in worldwide use for the management of diabesity.
| Study name | Year | Drug molecule | Mean weight reduction (95%CI/SD) | Mean HbA1c reduction in % (95%CI/SD) | Ref. |
| Exenatide-113 Clinical Study | 2004 | Exenatide 10 mcg twice daily | -1.6 kg (SD +/-0.3) | -0.86 (SD +/- 0.11) | [10] |
| EXSCEL study | 2017 | Exenatide (ER) Once weekly | -1.27 kg (-1.40 to -1.13) | -0.53 (-0.57 to -0.50) | [11] |
| LEAD 3 (Mono) Trial | 2009 | Liraglutide 1.2 mg daily | -1.6 kg (-2.43 to -0.88) | -1.21 (-1.35 to -1.06) | [12] |
| SUSTAIN 7 Trial | 2018 | Dulaglutide 1.5 mg weekly | -3.0 kg (SD: 0.27) | -1.4 (SD: -0.06) | [12] |
| SUSTAIN 7 Trial | 2018 | Semaglutide 1 mg weekly | -6.5 kg (SD: -0.28) | -1.8 (SD: -0.06) | [12] |
Sodium-glucose cotransporter 2 inhibitors: Sodium-glucose cotransporter 2 (SGLT2) accounts for about 90% of glucose reabsorption in the kidney with its inhibition leading to a significant amount of glucose (50-100 g daily) filtered by the renal glomeruli being excreted through the urinary system[6,17]. SGLT2 inhibitors group of drugs, therefore, helps to improve diabetes with an insulin-independent glucose-lowering mechanism. SGLT2 inhibitors also act by reducing leptin and increasing the adiponectin level, resulting in lipolysis, weight loss, and reduced accumulation of adipose tissue in the myocardium[6,17,18].
Four commonly available SGLT2 inhibitors worldwide, namely Canagliflozin, Dapagliflozin, Empagliflozin, and Ertugliflozin have all demonstrated significant efficacy in lowering the HbA1c and promoting weight loss which is crucial in tackling diabesity. In addition, SGLT2 inhibitors have also been shown to confer a benefit against high blood pressure, cardiovascular diseases, heart failure, and renal disease[7,17-21]. Especially prominent benefits on cardiovascular profile with SGLT2 inhibitors therapy include the reduced risk of hospitalisation from heart failure, myocardial infarction, and cardiovascular deaths among patients with T2DM.
Studies have further demonstrated the increased efficacy in glycaemic control and weight loss but not blood pressure control when SGLT2 inhibitors and metformin are used as combination therapy[15]. On the other hand, a greater reduction in HbA1c, weight, systolic blood pressure, and total and LDL cholesterol were observed with the combined use of the SGLT2 inhibitors and GLP-1RA[16]. Potential drug synergism of this combination therapy was proposed because of the differences in the mechanism of action at different sites of the body’s glucose regulation[6,21].
The current National Institute for Health and Clinical Care Excellence (NICE) guidance of the United Kingdom (UK) has recommended the SGLT2 inhibitors (Canagliflozin, Dapagliflozin, or Empagliflozin) to be used as first-line monotherapy in circumstances where Metformin is contraindicated or not tolerated while lifestyle modification alone has not provided the adequate glycaemic control[22]. It can also be used as an add-on therapy in diabetic patients who has a history of chronic heart failure or established atherosclerotic cardiovascular disease.
The common adverse effects encountered with the use of SGLT2 inhibitors are genital thrush and urinary tract infections which can result in treatment discontinuation. Augmented glycosuria resulting from drug therapy may be the reason for these infections[23]. Table 2 shows the landmark clinical trials showing the benefits of managing diabesity with the SGLT2 inhibitors group of drugs commonly used in clinical practice across the world[24-26].
| Study name | Year | Drug molecule | Mean weight reduction (95%CI) | Mean % reduction of HbA1c (95%CI) | Ref. |
| EMPA-REG Trial | 2015 | Empagliflozin 25 mg | -1.9 kg (-2.1 to -1.7) | -0.60 (-0.64 to -0.55) | [24] |
| CANVAS Trial | 2017 | Canagliflozin 300 mg | -2.8 kg (-3.21 to -2.39) | -0.80 (-0.62 to -0.98) | [25] |
| DECLARE-TIMI-58 Trial | 2019 | Dapagliflozin 10 mg | -1.51 kg (-1.81 to -1.21) | -0.55 (-0.62 to -0.53) | [26] |
HbA1c has been widely used as a biomarker for long-term glucose monitoring in patients with T2DM as it reflects glycaemic control over a period of approximately 90-120 d[27,28]. It is convenient to perform, and fasting is not required. The availability of standardised assays and calibration of measurement according to the International Federation of Clinical Chemistry allow comparisons of results between the laboratories. In addition, the HbA1c level has been shown to correlate with diabetic-related complications[27,29]. As a result, the practice nowadays is still very much reliant on HbA1c in titrating antidiabetic treatment. Its use has been supported by several guidelines such as the NICE guidance in the UK and the guidelines from the ADA and the EASD, which recommended the utilisation of individualised HbA1c targets for monitoring control and antidiabetic treatment optimisation[30].
However, the accuracy of HbA1c can be affected in several conditions, resulting in falsely low or high readings which do not correlate with the clinical picture of glycaemic control in real life, as demonstrated in our case 2 described above. For instance, disorders that lead to a reduction in the lifespan of red blood cells such as haemolytic anaemia and chronic kidney disease, haemodilution in the context of blood transfusion or pregnancy, and decreased glycation of haemoglobin due to alcohol use, high doses of vitamins C and E, certain antiviral agents, and antibiotics use can lead to falsely low HbA1c level[27,29]. On the other hand, HbA1c can be falsely elevated due to increased lifespan of the red blood cells, decreased percentage of reticulocytes and increased glycation rates such as in the context of iron deficiency. Therefore, a patient’s clinical history needs to be carefully sought to identify any underlying conditions which can interfere with the HbA1c readings.
There are several methods for glycaemic monitoring to use in patients whose HbA1c cannot be reliably interpreted.
CBG profiling was used in our case for example. Post-meal hyperglycaemia normally precedes significant basal hyperglycaemia contributing to overall hyperglycaemia in individuals with T2DM[28]. Therefore, measuring the post-meal glucose concentrations is a helpful tool in addition to HbA1c results when there is a discrepancy between the premeal glucose profile and predicted HbA1c level. In the UK, self-monitoring of CBG device is not routinely offered to all T2DM patients. It is only available for T2DM patients who are on insulin therapy, suspected or confirmed hypoglycaemia, on oral medications that carry an increased risk of hypoglycaemia, who are pregnant or planning to conceive, or when started on glucocorticoid treatment[22]. This could potentially miss detecting T2DM patients who have poor glycaemic control but a falsely low HbA1c. The quality-controlled plasma glucose profile is recommended by the NICE, UK where HbA1c monitoring is invalid due to abnormal haemoglobin variants or disturbed erythrocyte turnover[22]. The guidelines from the ADA and the EASD recommended combining the use of plasma glucose measurement with HbA1c in T2DM patients treated with insulin[30].
Alternatively, the total glycated haemoglobin estimation, fructosamine, or glycated albumin measurement can be considered when there is an issue with HbA1c monitoring[22,27,28]. The total glycated haemoglobin estimation uses the boronate-affinity chromatography method to measure the HbA1c based on the separation of proteins resulting from structural differences. Although it is demonstrated to have the least analytical interference from the haemoglobin variants, it can be affected by the abnormal glycation of proteins. The fructosamine is obtained by measuring all the glycated proteins including albumin in plasma while the glycated albumin is measured and expressed as a percentage of total serum albumin. Both the fructosamine and glycated albumin are much shorter-term markers correlating to the glycaemic control over a 2–4-wk period, owing to their relatively rapid turnover rate. They are convenient and cost-effective to use. The reference range for both is assay, age, gender, and race dependent. While fructosamine reference intervals are now widely available, this is not the case for glycated albumin. The results for both the fructosamine and glycated albumin can be significantly affected in several conditions associated with altered protein metabolism and protein loss such as nephrotic syndrome, a hepatic disease with diminished protein synthesis, thyroid disease, and malnutrition states. In addition, glycated albumin can underestimate glycaemic control in overweight patients, particularly in those with BMI > 30 kg/m²[23,28,31]. The total HbA1c estimation or fructosamine estimation is currently recommended for use in the UK[22].
Continuous glucose monitoring (CGM) is an emerging method to monitor ambient glucose concentrations. It reveals the glycaemic variability which may have an association with an increased risk of micro- and macrovascular complications[32]. The CGM is being increasingly recommended in patients with T2DM who are on multiple daily injections and experience recurrent or severe hypoglycaemia, impaired hypoglycaemia awareness, conditions or disabilities impacting their ability to perform self-CBG monitoring or those who self-measure at least 8 times a day. This could be an option in the future but is not currently recommended for use in glycaemic control monitoring in the context of inaccurate HbA1c in the UK[22].
The obesity epidemic is affecting the T1DM population much greater than the general population. Its prevalence continues to be trending up in recent decades, with a rate of between 2.8% and 37.1% across the lifetime of T1DM patients. The associated incidence of metabolic syndrome increased from 4.9% among patients with normal weight to 35.3% among obese patients[33]. Several studies demonstrated that individuals with T1DM and obesity are at higher risk of micro- and macrovascular complications[34].
Double diabetes is a terminology used to describe patients with T1DM who are also showing clinical signs of T2DM such as obesity and insulin resistance. In addition to the state of insulin deficiency, the increased adiposity leads to the production of inflammatory cytokines and adipokines, resulting in a worsening of peripheral insulin resistance. Consequently, these patients require a higher amount of exogenous insulin to achieve satisfactory glycaemic control which is crucial in reducing the risk of long-term complications from diabetes. However, insulin is an anabolic hormone that slows basal metabolism, inhibits protein catabolism, and stimulates lipogenesis. The exogenous insulin also does not have first pass through the liver as in the case of endogenous insulin which suppresses hepatic gluconeogenesis in a non-diabetic individual. Hence, the use of intensive insulin therapy over time can cause increased fat accumulation and weight gain which further exacerbates the issue of obesity[33,34].
In addition to lifestyle modifications, several pharmacological approaches can be considered in the management of patients with double diabetes.
Metformin is known to decrease hepatic glucose production, increase insulin sensitivity, and decrease glucose absorption. The REMOVAL trial revealed a reduction in body weight, LDL cholesterol, and insulin dose requirement when Metformin was used in T1DM patients[35]. However, it did not result in a sustained effect in glycaemic control as measured with HbA1c or alter the atherosclerosis progression. Metformin is currently being used off-licensed in the UK as additional therapy in T1DM patients who have a BMI of > 25 kg/m² and in the context of improving glycaemic control while minimising the insulin dosage[36].
In our case, the patient was also trialled on the maximum dose of Liraglutide, a GLP-1RA usually used for treating patients with T2DM. The GLP-1RA has extensively demonstrated to have a good profile in promoting weight loss in both diabetic and non-diabetic populations[34,37,38]. However, there are relatively few studies that recruited patients with T1DM. The 2 Large trials: ADJUNCT ONE[37] and ADJUNCT TWO[38] which studied the efficacy and safety of the use of Liraglutide in the T1DM population showed benefits in HbA1c, insulin dose, and body weight reduction. Both these studies showed a statistically significant reduction in HbA1c with all doses of Liraglutide compared to placebo. However, the ADJUNCT 2 study observed no significant difference in the mean fasting plasma glucose. This could be explained by the mode of action of the GLP-1RA. In line with this, the studies demonstrated a dose-dependent reduction in total daily insulin dose, mainly contributed by a reduction in the bolus insulin requirement. Both studies also revealed a statistically significant weight loss in a dose-dependent manner, with a mean reduction in body weight of 5.1 kg with Liraglutide 1.8 mg being observed. The occurrence of adverse events associated with Liraglutide use is observed to be dose-dependent. Higher rates of hypoglycaemia and hyperglycaemia with ketosis were also reported[37,38].
The use of SGLT2 inhibitors in T1DM remained an area to be explored. A review of 8 randomised placebo-controlled trials which studied the use of SGLT2 inhibitors (Canagliflozin, Dapagliflozin, Empagliflozin, and Sotagliflozin) in T1DM patients have shown its effectiveness in lowering the HbA1c, averaging at 0.35%–0.54% after 24-26 weeks of treatment when added to the insulin therapy[39]. However, the benefit of sustained glycaemic efficacy beyond 1 year of therapy remained uncertain. All the studies also found a positive outcome in terms of weight loss and reduced requirement for a total daily dose of insulin. All the trials cut down on the insulin regime by 10%-30% at the initiation of treatment to mitigate the risk of SGLT2 inhibitors induced hypoglycaemia. Studies that used Dapagliflozin, Empagliflozin, and Sotagliflozin reported no increase in the risk of developing severe hypoglycaemia. However, a 5.8- fold increase in the risk of ketoacidosis was found in these studies and this occurred in a dose-dependent manner[39].
Overall, the experience of using other antidiabetic medications as add-on therapy to insulin in T1DM remained limited with all uses being unlicensed. The pros and cons of treatment need to be carefully weighed and discussed with patients. The individualised use of these medications especially in the management of double diabetes can achieve some good outcomes such as in the patient we showcased here. Largescale multicentre clinical trials are needed to provide us with more robust evidence of the benefits of such an approach and the risks associated.
Monogenic diabetes accounts for about 1%-3% of patients with young-onset diabetes[40]. These rare forms of diabetes caused by a single gene defect are mostly inherited in an autosomal dominant pattern, giving rise to 2 main clinical phenotypes: the maturity-onset diabetes of young (MODY) and neonatal diabetes. MODY, being the most common form of monogenic diabetes, is estimated to be present at 1 in 10000 adults and 1 in 23000 children according to studies that comprised mostly of the White European population[41]. 14 different gene mutations in MODY have been identified so far with the HNF1A, HNF4A, HNF1B, and GCK being the most common mutations[40,42]. Nearly all genes implicated in monogenic diabetes correspondingly encode a protein which involves in pancreatic beta cell development or function. For example, a mutation in HNF1A, HNF4A, and HNF1B leads to transcription factor defects disrupting the beta cell development and function while the GCK mutation accounts for impaired beta cell glucose sensing[43].
Detecting monogenic diabetes in clinical practice has remained challenging as it relies on a collection of clinical characteristics with no universally recognised criteria to prompt suspicion and investigations into the presence of the disease[43,44]. Investigations for monogenic diabetes should be considered in children who receive a diagnosis of diabetes within the first 6 mo of life, children, and young adults (< 25 years of age) who have hyperglycaemia without the typical characteristics of type 1 or type 2 diabetes. Other clinical characteristics that could be suggestive of monogenic diabetes include the presence of a personal or family history of transient neonatal hypoglycaemia or neonatal diabetes, absence of pancreatic antibodies, prolonged “honeymoon” phase with detectable C- peptide after more than 3-5 years and lack of ketoacidosis when insulin is omitted or is extremely sensitive to the sulphonylurea treatment[40,44]. It is recommended that a minimum of 3 antibodies, preferably GAD, IA2, and ZnT8 should be checked with the presence of any positive antibodies essentially excluding the diagnosis of MODY[41]. Due to the pattern of inheritance, a family history of diabetes in successive generations is a good pointer to prompt further investigations. The MODY calculator is a clinical prediction tool developed by the University of Exeter group which takes into account the current age, age at diagnosis, gender, ethnicity, BMI, HbA1c, treatment regimen, parental history of diabetes, and presence of certain medical conditions to calculate the post-test probability of MODY. The calculator is validated in individuals of < 35 years old and has a limitation of detecting MODY across different ethnic groups as it is developed based on the Caucasian group[41,42].
Diagnosing monogenic diabetes via molecular genetic testing enables the practice of precision medicine in diabetes which allows future care and management to be tailored accordingly. This has a significant implication of allowing cost and treatment effectiveness with targeted therapies, as well as the cascade effect of identifying other family members who may have a similar diagnosis[42,44]. As portrayed in this case, achieving the diagnosis has allowed the patient’s glycaemic control to be optimised with sulfonylurea only. It has prevented the unnecessary use of exogenous insulin and its associated complications with prolonged use. Besides, achieving the right diagnosis enables her son to be investigated and managed appropriately at a younger age. Patients with HNF-1A mutation have a similar risk as patients with T2DM in all-cause mortality and cardiovascular disease. They are also at risk of developing microvascular complications such as retinopathy, nephropathy, and neuropathy[40]. Patients with HNF-1A MODY respond well to sulphonylurea, which acts on the potassium-sensitive ATP channels leading to increased insulin secretion. However, monotherapy with sulphonylurea may not be sufficient to control hyperglycaemia over time. Exogenous insulin or GLP-1RA can be considered at this point. GLP-1RA can act by stimulating insulin secretion and reducing postprandial glucose elevation. On the other hand, SGLT2-i is thought to cause a higher risk of dehydration, glycosuria, genital infections, and diabetic ketoacidosis in HNF-A1 MODY and therefore, not a choice of treatment currently[40,41].
A significant glycaemic deterioration in a diabetic patient with longstanding stable disease control should prompt screening for other causes of sudden hyperglycaemia. In addition to the common T1DM and T2DM, other causes of diabetes which could be considered depending on the clinical history and examination include: (1) Disorders of the pancreas such as pancreatitis, pancreatic neoplasm, or following pancreatectomy; (2) endocrinopathies such as Cushing’s syndrome or acromegaly; (3) drug-induced diabetes; (4) various infections; and (5) systemic diseases such as haemochromatosis[45].
Type 3C diabetes describes diabetes caused by the destruction of the pancreas. Its prevalence was approximately 1%-9% of all forms of diabetes. The majority of type 3C diabetes was due to chronic pancreatitis with only 8% of it being caused by pancreatic ductal adenocarcinoma[45]. The presence of diabetes was found to be significantly higher at 68% in patients with pancreatic ductal adenocarcinoma when compared to other types of cancers, such as lung, breast, colorectal, and prostate cancer[46]. New-onset of diabetes has been commonly reported preceding the diagnosis of pancreatic ductal adenocarcinoma. The incidence of impaired fasting glucose or glucose intolerance irrespective of the size and stage of the underlying pancreatic ductal adenocarcinoma was estimated to be about 80%[47]. Although the exact mechanism is yet to be fully understood, insulin resistance, the presence of adrenomedullin, beta cell loss, and dysfunction were among the proposed elements which are thought to be contributing to diabetes in patients with pancreatic ductal adenocarcinoma[47]. However, due to the high incidence of diabetes relative to the incidence of pancreatic cancer, routine screening for the presence of cancer in patients with new-onset diabetes is currently not deemed to be cost-effective[45].
There is no consensus on managing diabetes in this group of patients. The primary aim of antidiabetic treatment is to avoid acute metabolic complications due to uncontrolled hyperglycaemia rather than prevention of long-term complications considering the limited life expectancy associated with the diagnosis[47]. Symptomatic hyperglycaemia has a negative impact on the quality of life. Patients with diabetes who are on cancer treatment are at higher risk of infection. The glucocorticoid treatment which is commonly used in advanced cancer for symptom management and appetite stimulation can lead to worsening glycaemic control. It was also found that the concordance to antidiabetic treatment was lower in patients with cancer.
Most cases of pancreatic ductal adenocarcinoma are associated with a poor prognosis due to late diagnosis and limited treatment options. Patients with pancreatic malignancy with concurrent diabetes are observed to have poorer overall survival[48]. In addition to physical illness, the patient and families also undergo psychological distress. Palliative care involvement is therefore vital in supporting the management of the symptoms and preserving the quality of life as best as possible[49]. Diabetes care during the end-of-life requires a holistic approach and acknowledgement that the focus and principles of treatment have now changed to avoid hypoglycaemia, diabetic ketoacidosis, hyperosmolar hyperglycaemic state, or symptomatic hyperglycaemia. Discussions with patients and families to provide reassurance, respect, support, and preserve one’s ability to self-manage their diabetes are pivotal[50]. During the last days of life, a CBG range of 6-15 mmol/L is generally well accepted to accommodate the minimal number of glucose testings as possible.
The diabetes epidemic continues to grow. Over the recent decades, there have been many advances in diabetes research leading to more sophisticated diabetes care algorithms in the modern world. As the complexity of the disease unfolded such as with the discovery of some rarer causes of diabetes via genomic testing and recognising diabetes as part of a bigger picture of the metabolic disease, having a better understanding of the underlying pathophysiology contributing to various forms of diabetes enables the practice of precision medicine in diabetes. Acknowledging diabetes as a multifaceted disease and the development of various newer hypoglycaemic agents are important milestones in the paradigm of enhanced diabetes care. This should always be reflected in our clinical practice to individualise diabetes care with the best evidence-based approach.
Although we presented 5 unique case scenarios to highlight the importance of individualized diabetes care in day-to-day clinical practice with this paper, we cannot make any firm recommendations for the management of every patient that physicians come across in their diabetes clinics based on this paper. We need to adhere to appropriate clinical guidelines from various professional bodies for the usual care of diabetes patients. However, when the situation demands as in the case snippets discussed above, we need to “think out of the box” to change the usual algorithms to ensure that our patients get the optimal benefit from scientific medicine based on the latest evidence available to us.
| 1. | IDF Diabetes Atlas 2021. IDF Atlas 10th Edition, International Diabetes Federation. IDF Diabetes Atlas 2021 | IDF Diabetes Atlas. Accessed on 20th September 2022. |
| 2. | Dineen-Griffin S, Garcia-Cardenas V, Williams K, Benrimoj SI. Helping patients help themselves: A systematic review of self-management support strategies in primary health care practice. PLoS One. 2019;14:e0220116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 117] [Cited by in RCA: 341] [Article Influence: 48.7] [Reference Citation Analysis (0)] |
| 3. | Coulter A, Entwistle VA, Eccles A, Ryan S, Shepperd S, Perera R. Personalised care planning for adults with chronic or long-term health conditions. Cochrane Database Syst Rev. 2015;2015:CD010523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 319] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 4. | Ray JG, O'Brien TE, Chan WS. Preconception care and the risk of congenital anomalies in the offspring of women with diabetes mellitus: a meta-analysis. QJM. 2001;94:435-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 312] [Cited by in RCA: 268] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 5. | World Health Organisation. Obesity. Assessed on 22nd September 2022. Available from: https://www.who.int/health-topics/obesity#tab=tab_1. |
| 6. | Pappachan JM, Viswanath AK. Medical Management of Diabesity: Do We Have Realistic Targets? Curr Diab Rep. 2017;17:4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 44] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 7. | Pappachan JM, Fernandez CJ, Chacko EC. Diabesity and antidiabetic drugs. Mol Aspects Med. 2019;66:3-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 72] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 8. | Jakhar K, Vaishnavi S, Kaur P, Singh P, Munshi A. Pharmacogenomics of GLP-1 receptor agonists: Focus on pharmacological profile. Eur J Pharmacol. 2022;936:175356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 9. | Sarma S, Palcu P. Weight loss between glucagon-like peptide-1 receptor agonists and bariatric surgery in adults with obesity: A systematic review and meta-analysis. Obesity (Silver Spring). 2022;30:2111-2121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 72] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 10. | Buse JB, Henry RR, Han J, Kim DD, Fineman MS, Baron AD; Exenatide-113 Clinical Study Group. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in sulfonylurea-treated patients with type 2 diabetes. Diabetes Care. 2004;27:2628-2635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 955] [Cited by in RCA: 926] [Article Influence: 42.1] [Reference Citation Analysis (0)] |
| 11. | Holman RR, Bethel MA, Mentz RJ, Thompson VP, Lokhnygina Y, Buse JB, Chan JC, Choi J, Gustavson SM, Iqbal N, Maggioni AP, Marso SP, Öhman P, Pagidipati NJ, Poulter N, Ramachandran A, Zinman B, Hernandez AF; EXSCEL Study Group. Effects of Once-Weekly Exenatide on Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med. 2017;377:1228-1239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1599] [Cited by in RCA: 1606] [Article Influence: 178.4] [Reference Citation Analysis (0)] |
| 12. | Garber A, Henry R, Ratner R, Garcia-Hernandez PA, Rodriguez-Pattzi H, Olvera-Alvarez I, Hale PM, Zdravkovic M, Bode B; LEAD-3 (Mono) Study Group. Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD-3 Mono): a randomised, 52-week, phase III, double-blind, parallel-treatment trial. Lancet. 2009;373:473-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 752] [Cited by in RCA: 807] [Article Influence: 47.5] [Reference Citation Analysis (0)] |
| 13. | Pratley RE, Aroda VR, Lingvay I, Lüdemann J, Andreassen C, Navarria A, Viljoen A; SUSTAIN 7 investigators. Semaglutide versus dulaglutide once weekly in patients with type 2 diabetes (SUSTAIN 7): a randomised, open-label, phase 3b trial. Lancet Diabetes Endocrinol. 2018;6:275-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 632] [Cited by in RCA: 577] [Article Influence: 72.1] [Reference Citation Analysis (0)] |
| 14. | Garvey WT, Birkenfeld AL, Dicker D, Mingrone G, Pedersen SD, Satylganova A, Skovgaard D, Sugimoto D, Jensen C, Mosenzon O. Efficacy and Safety of Liraglutide 3.0 mg in Individuals With Overweight or Obesity and Type 2 Diabetes Treated With Basal Insulin: The SCALE Insulin Randomized Controlled Trial. Diabetes Care. 2020;43:1085-1093. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 124] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 15. | Rubino DM, Greenway FL, Khalid U, O'Neil PM, Rosenstock J, Sørrig R, Wadden TA, Wizert A, Garvey WT; STEP 8 Investigators. Effect of Weekly Subcutaneous Semaglutide vs Daily Liraglutide on Body Weight in Adults With Overweight or Obesity Without Diabetes: The STEP 8 Randomized Clinical Trial. JAMA. 2022;327:138-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 457] [Cited by in RCA: 636] [Article Influence: 159.0] [Reference Citation Analysis (0)] |
| 16. | Giugliano D, Scappaticcio L, Longo M, Caruso P, Maiorino MI, Bellastella G, Ceriello A, Chiodini P, Esposito K. GLP-1 receptor agonists and cardiorenal outcomes in type 2 diabetes: an updated meta-analysis of eight CVOTs. Cardiovasc Diabetol. 2021;20:189. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 178] [Article Influence: 35.6] [Reference Citation Analysis (0)] |
| 17. | Blüher M, Ceriello A, Davies M, Rodbard H, Sattar N, Schnell O, Tonchevska E, Giorgino F. Managing weight and glycaemic targets in people with type 2 diabetes-How far have we come? Endocrinol Diabetes Metab. 2022;5:e00330. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 18. | Lazzaroni E, Ben Nasr M, Loretelli C, Pastore I, Plebani L, Lunati ME, Vallone L, Bolla AM, Rossi A, Montefusco L, Ippolito E, Berra C, D'Addio F, Zuccotti GV, Fiorina P. Anti-diabetic drugs and weight loss in patients with type 2 diabetes. Pharmacol Res. 2021;171:105782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 121] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 19. | Milder TY, Stocker SL, Abdel Shaheed C, McGrath-Cadell L, Samocha-Bonet D, Greenfield JR, Day RO. Combination Therapy with an SGLT2 Inhibitor as Initial Treatment for Type 2 Diabetes: A Systematic Review and Meta-Analysis. J Clin Med. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 20. | Castellana M, Cignarelli A, Brescia F, Perrini S, Natalicchio A, Laviola L, Giorgino F. Efficacy and safety of GLP-1 receptor agonists as add-on to SGLT2 inhibitors in type 2 diabetes mellitus: A meta-analysis. Sci Rep. 2019;9:19351. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 61] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 21. | Deol H, Lekkakou L, Viswanath AK, Pappachan JM. Combination therapy with GLP-1 analogues and SGLT-2 inhibitors in the management of diabesity: the real world experience. Endocrine. 2017;55:173-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 22. | National Institute for Health and Clinical Care Excellence. Type 2 diabetes in adults: management [NICE guideline no. 28]; Assessed on 24th September 2022. Available from: https://www.nice.org.uk/guidance/ng28. |
| 23. | Liu J, Li L, Li S, Jia P, Deng K, Chen W, Sun X. Effects of SGLT2 inhibitors on UTIs and genital infections in type 2 diabetes mellitus: a systematic review and meta-analysis. Sci Rep. 2017;7:2824. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 170] [Cited by in RCA: 182] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 24. | Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE; EMPA-REG OUTCOME Investigators. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N Engl J Med. 2015;373:2117-2128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7124] [Cited by in RCA: 8762] [Article Influence: 796.5] [Reference Citation Analysis (2)] |
| 25. | Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Silverman MG, Zelniker TA, Kuder JF, Murphy SA, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH, Ruff CT, Gause-Nilsson IAM, Fredriksson M, Johansson PA, Langkilde AM, Sabatine MS; DECLARE–TIMI 58 Investigators. Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med. 2019;380:347-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4652] [Cited by in RCA: 4543] [Article Influence: 649.0] [Reference Citation Analysis (0)] |
| 26. | Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, Shaw W, Law G, Desai M, Matthews DR; CANVAS Program Collaborative Group. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N Engl J Med. 2017;377:644-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4498] [Cited by in RCA: 5678] [Article Influence: 630.9] [Reference Citation Analysis (0)] |
| 27. | Sodi R, McKay K, Dampetla S, Pappachan JM. Monitoring glycaemic control in patients with diabetes mellitus. BMJ. 2018;363:k4723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 28. | Bergman M, Abdul-Ghani M, DeFronzo RA, Manco M, Sesti G, Fiorentino TV, Ceriello A, Rhee M, Phillips LS, Chung S, Cravalho C, Jagannathan R, Monnier L, Colette C, Owens D, Bianchi C, Del Prato S, Monteiro MP, Neves JS, Medina JL, Macedo MP, Ribeiro RT, Filipe Raposo J, Dorcely B, Ibrahim N, Buysschaert M. Review of methods for detecting glycemic disorders. Diabetes Res Clin Pract. 2020;165:108233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 137] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 29. | Radin MS. Pitfalls in hemoglobin A1c measurement: when results may be misleading. J Gen Intern Med. 2014;29:388-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 242] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 30. | Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, Peters AL, Tsapas A, Wender R, Matthews DR; American Diabetes Association (ADA); European Association for the Study of Diabetes (EASD). Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2012;35:1364-1379. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2563] [Cited by in RCA: 2626] [Article Influence: 187.6] [Reference Citation Analysis (4)] |
| 31. | Danese E, Montagnana M, Nouvenne A, Lippi G. Advantages and pitfalls of fructosamine and glycated albumin in the diagnosis and treatment of diabetes. J Diabetes Sci Technol. 2015;9:169-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 145] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 32. | Umpierrez GE, P Kovatchev B. Glycemic Variability: How to Measure and Its Clinical Implication for Type 2 Diabetes. Am J Med Sci. 2018;356:518-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 96] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 33. | Vilarrasa N, San Jose P, Rubio MÁ, Lecube A. Obesity in Patients with Type 1 Diabetes: Links, Risks and Management Challenges. Diabetes Metab Syndr Obes. 2021;14:2807-2827. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 60] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 34. | Mottalib A, Kasetty M, Mar JY, Elseaidy T, Ashrafzadeh S, Hamdy O. Weight Management in Patients with Type 1 Diabetes and Obesity. Curr Diab Rep. 2017;17:92. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 87] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 35. | Petrie JR, Chaturvedi N, Ford I, Brouwers MCGJ, Greenlaw N, Tillin T, Hramiak I, Hughes AD, Jenkins AJ, Klein BEK, Klein R, Ooi TC, Rossing P, Stehouwer CDA, Sattar N, Colhoun HM; REMOVAL Study Group. Cardiovascular and metabolic effects of metformin in patients with type 1 diabetes (REMOVAL): a double-blind, randomised, placebo-controlled trial. Lancet Diabetes Endocrinol. 2017;5:597-609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 260] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 36. | National Institute for Health and Care Excellence. Type 1 diabetes in adults: diagnosis and management [NICE guideline no. 17]; Assessed on 26th September 2022. Available from: https://www.nice.org.uk/guidance/ng17. |
| 37. | Mathieu C, Zinman B, Hemmingsson JU, Woo V, Colman P, Christiansen E, Linder M, Bode B; ADJUNCT ONE Investigators. Efficacy and Safety of Liraglutide Added to Insulin Treatment in Type 1 Diabetes: The ADJUNCT ONE Treat-To-Target Randomized Trial. Diabetes Care. 2016;39:1702-1710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 236] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 38. | Ahrén B, Hirsch IB, Pieber TR, Mathieu C, Gómez-Peralta F, Hansen TK, Philotheou A, Birch S, Christiansen E, Jensen TJ, Buse JB; ADJUNCT TWO Investigators. Efficacy and Safety of Liraglutide Added to Capped Insulin Treatment in Subjects With Type 1 Diabetes: The ADJUNCT TWO Randomized Trial. Diabetes Care. 2016;39:1693-1701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 184] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 39. | Taylor SI, Blau JE, Rother KI, Beitelshees AL. SGLT2 inhibitors as adjunctive therapy for type 1 diabetes: balancing benefits and risks. Lancet Diabetes Endocrinol. 2019;7:949-958. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 90] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 40. | Naylor RN, Philipson LH. Diagnosis and Clinical Management of Monogenic Diabetes. 2020 Nov 5. In: Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000–. [PubMed] |
| 41. | Broome DT, Pantalone KM, Kashyap SR, Philipson LH. Approach to the Patient with MODY-Monogenic Diabetes. J Clin Endocrinol Metab. 2021;106:237-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 87] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 42. | Tosur M, Philipson LH. Precision diabetes: Lessons learned from maturity-onset diabetes of the young (MODY). J Diabetes Investig. 2022;13:1465-1471. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 43] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 43. | Zhang H, Colclough K, Gloyn AL, Pollin TI. Monogenic diabetes: a gateway to precision medicine in diabetes. J Clin Invest. 2021;131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 124] [Article Influence: 24.8] [Reference Citation Analysis (1)] |
| 44. | Colclough K, Patel K. How do I diagnose Maturity Onset Diabetes of the Young in my patients? Clin Endocrinol (Oxf). 2022;97:436-447. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 45. | Feingold KR. Atypical Forms of Diabetes. 2022 Feb 24. In: Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000–. [PubMed] |
| 46. | Aggarwal G, Kamada P, Chari ST. Prevalence of diabetes mellitus in pancreatic cancer compared to common cancers. Pancreas. 2013;42:198-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 123] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 47. | Hart PA, Bellin MD, Andersen DK, Bradley D, Cruz-Monserrate Z, Forsmark CE, Goodarzi MO, Habtezion A, Korc M, Kudva YC, Pandol SJ, Yadav D, Chari ST; Consortium for the Study of Chronic Pancreatitis, Diabetes, and Pancreatic Cancer(CPDPC). Type 3c (pancreatogenic) diabetes mellitus secondary to chronic pancreatitis and pancreatic cancer. Lancet Gastroenterol Hepatol. 2016;1:226-237. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 448] [Cited by in RCA: 364] [Article Influence: 36.4] [Reference Citation Analysis (0)] |
| 48. | Joharatnam-Hogan N, Carter TJ, Reynolds N, Ho JH, Adam S, Board R. Diabetes Mellitus in People with Cancer. 2021 Dec 12. In: Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000–. [PubMed] |
| 49. | Chung V, Sun V, Ruel N, Smith TJ, Ferrell BR. Improving Palliative Care and Quality of Life in Pancreatic Cancer Patients. J Palliat Med. 2022;25:720-727. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 57] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 50. | Diabetes UK. For Healthcare Professionals: End of Life Guidance for Diabetes Care. November 2021. Assessed on 29th November 2022https://diabetes-resources-production.s3.eu-west-1.amazonaws.com/resources-s3/public/2021-11/EoL_TREND_FINAL2_0.pdf. |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country/Territory of origin: United Kingdom
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Arumugam VA, India; Cigrovski Berkovic M, Croatia S-Editor: Liu JH L-Editor: A P-Editor: Yuan YY