Published online May 6, 2023. doi: 10.12998/wjcc.v11.i13.2855
Peer-review started: January 27, 2023
First decision: February 17, 2023
Revised: March 8, 2023
Accepted: April 4, 2023
Article in press: April 4, 2023
Published online: May 6, 2023
Processing time: 87 Days and 12.1 Hours
Fetal growth restriction (FGR), or intrauterine growth restriction (IUGR), is a complication of pregnancy where the fetus does not achieve its genetic growth potential. FGR is characterized by a pathological retardation of intrauterine growth velocity in the curve of intrauterine growth. However, the FGR definition is still debated, and there is a lack of a uniform definition in the literature. True IUGR, compared to constitutional smallness, is a pathological condition in which the placenta fails to deliver an adequate supply of oxygen and nutrients to the developing fetus. Infants with IUGR, compared to appropriately grown ges
Core Tip: Fetal growth restriction (FGR) is a common complication of pregnancy where the fetus does not achieve its genetic growth potential. It is well known that FGR appears to be a contributing factor for adult chronic diseases including cardiovascular disease, metabolic syndrome, diabetes, dyslipidemia, and hypertension. Several studies demonstrated how suboptimal fetal growth leads to long-lasting physiological alterations for the developing fetus as well as for the newborn and adult in the future. Preventive measures and treatments should be assessed and adopted to prevent chronic diseases in FGR patients.
- Citation: D'Agostin M, Di Sipio Morgia C, Vento G, Nobile S. Long-term implications of fetal growth restriction. World J Clin Cases 2023; 11(13): 2855-2863
- URL: https://www.wjgnet.com/2307-8960/full/v11/i13/2855.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i13.2855
Fetal growth restriction (FGR), or intrauterine growth restriction (IUGR), is a complication of pregnancy where the fetus does not achieve its genetic growth potential[1]. In FGR, intrauterine growth velocity is delayed, expressed by a characteristic kink in intrauterine curve of growth. Several definitions have been proposed and used in clinical practice. FGR has been defined as fetus with an estimated fetal weight or abdominal circumference of less than the 10th percentile for the specific gestational age[1]. There is variation among international society guidelines, with some including abdominal circumference thresholds < 10th or ≤ 5th percentile alone as a diagnostic criteria[2-4]. Based on a survey of expert opinion, FGR is defined by a birth weight < 3rd percentile or the combination of three criteria: (1) Birth weight < 10th percentile; (2) Head circumference < 10th percentile; (3) Birth length < 10th percentile (4) Antenatal FGR diagnosis; and (5) Prenatal risk factors associated with FGR[5]. Frequently, FGR results in the birth of a small for gestational age (SGA) infant. However, an infant can be SGA without having FGR, and some growth restricted infants can have a birth weight above the 10th percentile. In the literature, IUGR and SGA were often used as interchangeable terms even though often used improperly.
Several factors are involved in the development of FGR, such as genetic abnormalities, intrauterine infections, fetal structural anomalies, multiple gestations, and ischemic placental diseases[6]. According to the various definition of FGR, almost 9% pregnancies in wealthy countries and almost 30% in poor countries are prone developing FGR[7,8].
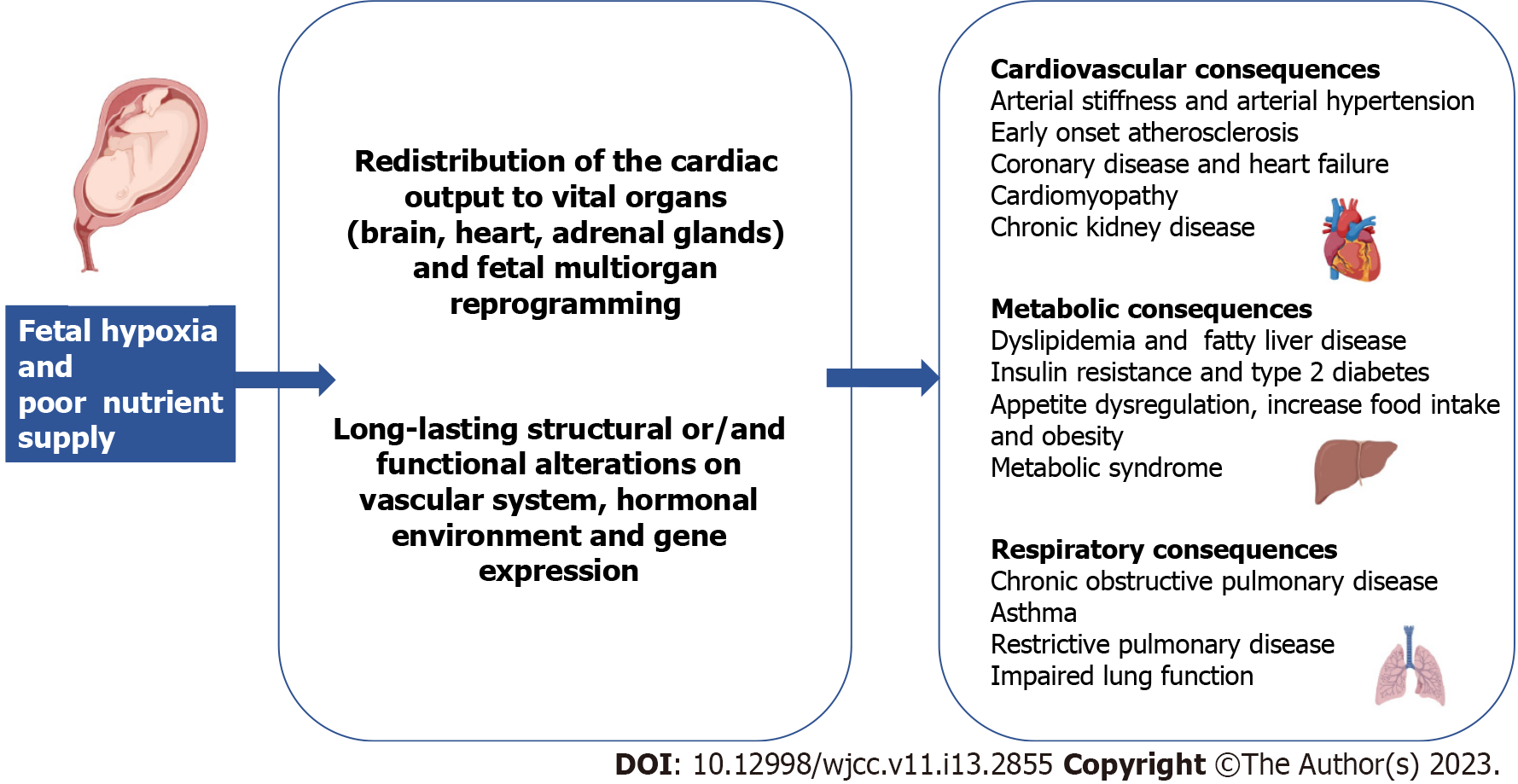
FGR is well known to contribute to adult chronic diseases including cardiovascular disease (CVD), metabolic syndrome, diabetes and chronic kidney disease[9]. Several studies have demonstrated how suboptimal fetal growth leads to long-lasting physiological alterations for the developing fetus as well as for the newborn and adult in the future. The primary cause of fetus growth restriction is a decreased oxygen and nutrient supply, which causes chronic hypoxia. As a response, the fetus redistributes the cardiac output to the brain, the heart, and the adrenals in order to preserve function in these vital organs[10] (Figure 1). Potentially, all the organs may be affected by growth restriction.
This review focuses on the long-term impacts of FGR on the cardiovascular, metabolic, and respiratory systems and discusses pathophysiologic mechanisms and preventive measures for FGR.
Several studies have reported the association between FGR and subsequent development of disease, like obesity, metabolic syndrome, CVD and cancer. A possible explanation has been proposed by developmental origin of health and disease concept[9]. According to this hypothesis, fetuses developing in an adverse intrauterine environment adapt through changing their endocrine-metabolic status to save energy and redirect nutrients to essential organs. The reprogramming at hepatic level predisposes to future dyslipidemia, vascular modifications induce endothelial damage and future hypertension, and insulin resistance contributes to the development of metabolic syndrome (dyslipidemia, fatty liver, arterial hypertension, and type 2 diabetes mellitus)[11].
The proposed pathophysiologic factors underlying these changes include epigenetic modifications of the expression of genes[12]. These modifications could induce appetite dysregulation and increase food intake and adipogenesis, resulting in future obesity and cardiovascular risk. The increased risk of metabolic syndrome and CVD may also be found during childhood, particularly in cases of rapid weight gain during infancy[13,14]. In a recent study from Singapore, a rapid weight gain from 0 to 2 years, with or without prior fetal growth deceleration, was associated with unfavorable cardiometabolic markers at 3 years of life[15]. Similarly, Norris et al[16] reported that the adverse consequences of rapid infant weight gain in the first 2 years of life may occur regardless of FGR occurrence.
Other important determinants of adult anthropometric and inflammatory alterations are fetal growth trajectories, as reported in a relatively small Australian cohort[17]. A relationship between small fetal head and abdominal circumference and higher adult blood pressure was described, independent of confounding variables, such as adult adiposity[17]. In a later report, a significant association between fetal growth patterns and markers of adiposity [body mass index (BMI), waist circumference] and inflammation [C-reactive protein (CRP)] was found in 27-year-old subjects. Good growth in early gestation had a protective effect on adiposity in later life, whereas reduced early growth was associated to adiposity. For example, a very-low-to-rising femoral length trajectory was associated with higher adult BMI, as confirmed by other studies in different populations[18].
Average or above-average abdominal growth from early-mid pregnancy with later deceleration was associated with lower adult BMI and abdominal circumference. Decreased waist circumference during gestation was related to higher CRP level in adulthood, while increased abdominal and head circumference was associated with lower CRP, even after adjustments for several factors, including postnatal lifestyle factors and maternal and pregnancy covariates. These effects were more pronounced in females than in males. However, it should be noted that obesity is a complex phenomenon involving multiple genetic and environmental factors, and the reported observations do not clarify the pathophysiology of adult obesity in former FGR individuals.
Potential preventive measures and treatments for the onset of metabolic complications include breastfeeding, adequate nutrition and physical exercise starting from early childhood, growth hormone, and metformin[10]. However, the studies have included small numbers of patients and need to be replicated in larger cohorts. Moreover, in the follow-up of FGR children, pediatricians should perform routine blood pressure monitoring, advice on healthy diet, and encourage physical activity.
FGR compared to normal growth is associated with a significantly higher incidence of CVD later in life[19]. FGR is also associated with metabolic syndrome and the effect of IUGR on cardiovascular system may be mediated by diabetes, dyslipidemia, or hypertension. An increased risk of high systolic blood pressure, arterial stiffness, and reduced renal functional reserve have been described in young adults born after FGR[20]. Hypertension, coronary disease, cardiomyopathy, and heart failure have been found extensively in adulthood and older age[21]. However, growing evidence suggests that FGR is the direct cause of cardiovascular alterations independently from pre-existing metabolic disease[22], which can increase the level of mortality and morbidity among IUGR patients.
Several studies have examined the relationship between FGR and the development of CVD later in life[9,19]. Leon et al[23] were the first to conduct a large epidemiological study of about 15000 births in Sweden and reported a statistically significant relationship between low birth weight and mortality from CVD in male individuals aged > 65 years. Moreover, another cohort study showed an inverse correlation between birth weight and systolic pressure in 50-year-old patients in the United Kingdom[24].
Cardiovascular impairment may already exist in growth restricted children preclinically in childhood, before the clinical development of CVD in adulthood. Long-term exposure to hypoxemia may be associated with permanent alterations in the structure and function of the cardiovascular system. To date, available evidence suggests that chronic hypoxemia in utero induces physiological modifications of autonomic nervous system function, oxidative stress, impaired secretion of hormones, and functional and structural modifications of the blood vessels[25]. A more spherical shape is typically evident in the heart of a restricted fetus, which can evolve into hypertrophy in the most severe cases[26]. Furthermore, prenatal echocardiography shows reduced longitudinal myocardial motion, abnormal transmitral E/A ratios (a marker of left ventricular function and late diastolic filling), prolonged isovolumic relaxation time, and decreased diastolic annular peak velocities. These modifications are functional to ensure an efficient stroke volume output and tolerance to pressure overload[27].
Interestingly, biomarkers of cardiac dysfunction and damage, such as B-type natriuretic peptide and troponin[27,28], have been found to be increased in the cord blood of FGR fetuses, potentially explaining the cardiac impairment caused by a suboptimal intrauterine environment. The altered prenatal echocardiographic findings were also confirmed in the 1st days after birth[29]. In fact, decreased absolute “E” and “A2 wave velocities”, higher “E/A” ratio, a prolonged isovolumic relaxation time, and reduced contractility and cardiac output have been described in these neonates, leading to increased blood pressure and both diastolic and systolic dysfunction[30].
The same findings were also identified by other studies including infants from FGR pregnancies aged 3-4 months[31]. Interestingly, a prospective study of 150 infants conducted by Crispi et al[32] compared cardiovascular morphology of 3-year-old to 6-year-old FGR infants with a control group. The authors showed that FGR children were more likely to present globular-shaped hearts, increased cardiac output, and left ventricular thickening. Similar findings were found by Rodríguez-López et al[33] in children aged 8-12 years. Altered vascular elastin and collagen content, extracellular matrix remodeling, and endothelial dysfunction are some of the prenatal circulatory modifications found in growth restricted offspring[25]. Multiple molecular mechanisms are involved in the pathogenesis of endothelial dysfunction in FGR patients such as the disruption in placental-mTORC and transforming growth factor beta signaling cascades, and changes in expression of endothelial nitric oxide synthase, as clearly explained in a recent review by Amruta et al[34]. Vascular changes may persist after birth and cause early onset preclinical atherosclerosis in children. For example, carotid artery thickness was found by Martin et al[35] in 3-year-old to 6-year-old children, and this evidence was confirmed by autopsy studies[36].
It is important to underline that other factors may influence the development of CVD in FGR patients. For example, pre-eclampsia, obesity, maternal diabetes, and prematurity are independent risk factors of hypertension during childhood.
To summarize, even if epidemiologic studies showed an association between FGR and late complications, the underlying mechanisms may be numerous. Some of these have recently been described and may coexist. Finally, there is an urgent need for studies for the evaluation of preventive measures in the FGR population.
Lung development occurs through several stages, namely embryonic, pseudoglandular, canalicular, saccular, and alveolar[37]. In many growth restricted infants, placental insufficiency occurs in late pregnancy in parallel with distal lung development (acinar and alveolar structures), suggesting that FGR may especially impact distal lung development[38]. Clinical observations in newborns show that SGA infants have a more severe early respiratory course and increased risk of developing bronchopulmonary dysplasia[39,40].
The long-term effects of FGR may be due to adaptations to poor oxygen exposure and nutrient supply that might result in structural or functional alterations[41]. FGR impacts lung function through molecular and cellular events, involving parenchyma, airway, and vasculature[38]. In fact, evidence showed that perinatal undernutrition changed the hormonal environment, which has an important impact on lung development and function, conditioning a higher risk for lung pathology in adulthood. Particularly, a deficit of retinol, cholecalciferol, leptin, ghrelin, and GLP-1 could be present in undernutrition in pregnancy and play a role in lung development, suggesting a correction of these deficiencies with diet supplementation during gestation[42,43].
Epidemiological studies showed that changes in lung development impacted both lung function and respiratory disease in early life, as well as in adulthood, particularly reduced forced expiratory volume in 1 s (FEV1) and chronic obstructive lung disease[38,44].
Much of our understanding of the relationship between FGR and lung development comes from animal studies. Maritz et al[45] showed that structural alterations induced by growth restriction during fetal lung development were still evident in adult sheep and were similar both qualitatively and quantitatively to those observed at 8 weeks, suggesting that restricted growth may induce permanent alterations in the morphology of the offspring's lungs as well as faster lung aging[46]. Adult FGR animals have fewer alveoli (larger than in controls), thickening of the interalveolar septa and basement membrane due to the accumulation of extracellular matrix[46], and inhibition of surfactant maturation[47]. FGR rats experienced significant pulmonary arterial hypertension and pulmonary vascular remodeling secondary to epigenetic mechanisms and pulmonary artery endothelial cell dysfunction[48]. Another study in sheep demonstrated that chronic placental insufficiency and subsequent FGR during late gestation resulted in alveolar simplification after birth, without concomitant alteration in lung weight and reduced septation[49]. This observation was in contrast to a previous study from the same group[50] in which lungs were inspected for a short time after the onset of placental insufficiency and FGR, supporting the concept that prolonged exposure to chronic hypoxia negatively influences lung growth, whereas exposure for short time did not.
Other studies have evaluated the relationship of FGR and functional respiratory values. A recent study showed a lower FEV1 Z-score in subjects aged 8-15 years who were born preterm and with a diagnosis of FGR, suggesting a worse conducting airway function. In this study, confounding factors, potentially contributing themselves to the lung function impairment, were prematurity and bronchodysplasia[51]. The study of Nikolajev et al[52] showed that FGR has its most pronounced effect on airway dynamics. Particularly, no differences were found in FEV1 or peak expiratory flow between FGR children and controls, but mid-expiratory flow measurements were significantly lower, suggesting that FGR has a more pronounced effect on airway development than on lung volumes. FGR has an impact on lung function not fully understood, as current evidence is mainly based on studies in children born SGA or low birth weight but not necessarily with FGR.
In a recent systematic review, different lung function trajectories were described, and low birth weight was associated with subnormal lung function trajectories[53]. Karmaus et al[54] described a relationship between ‘low’ FEV1 trajectories in both genders and ‘low’ FEV1/forced vital capacity (FVC) trajectories in females between ages 10 years and 26 years, whereas other authors reported ‘low’ FEV1, FVC, and FEV1/FVC trajectories in FGR individuals aged 15 to 22[55]. However, other studies found only modest associations for low birth weight[56]. Stein et al[57] studied the potential association between fetal growth and adult lung function in South India. They found an association between low birth weight/small head circumference at birth and reduced FEV1, independent from age and current stature; FVC was similarly associated with low birth weight. Canoy et al[58] followed a large population from fetal period until adulthood showing that adult FEV1 and FVC increased linearly with birth weight, and that the reduction in lung function was more pronounced in adults with lower birth weight.
Several studies showed that low birth weight is an important determinant for later development of chronic obstructive pulmonary disease[59,60]. On the other hand, a meta-analysis reported a significant association between birth weight and adult FVC, indicative of restrictive pattern, and weaker evidence for airflow obstruction[61].
A crucial point to investigate is the relationship between FGR and the subsequent risk of asthma. Källén et al[62] found that FGR is associated with an increased risk of asthma, even if a stronger predisposing factor is prematurity. A study evaluated the association between fetal growth and childhood asthma, showing that it is independent of gestational age, familial context, and genetic factors[63]. In fact, a cohort study of twins described the association between lower birth weight and increased risk of asthma, suggesting that this association is not influenced by shared environmental or genetic factors as twins are theoretically exposed to the same factors[64].
Further studies are required to evaluate the impact of FGR, based on a consensus-defined definition, and long-term pulmonary outcomes. In fact, the confusion between IUGR, SGA, and low birth weight confound the interpretation of the literature, and there is the risk of over/underestimating the relationship between the two entities. Several animal studies demonstrated the impact of FGR on both short-term and long-term structure and function of the lung. The association between FGR and impaired functional respiratory values is controversial, and it is still not clear whether the impairment, if any, is mainly due to a restrictive or obstructive pattern.
Several preventive measures have been identified and considered to promote long-term health in former FGR individuals (Table 1). A useful antenatal measure is an improved identification of subjects with increased risk of complications (i.e. earlier/more frequent ecographic growth assessment). Other strategies could include the promotion of dietary modifications during gestation to facilitate normalization of body weight, micronutrient levels, glycemia and blood pressure, lifestyle measures (i.e. avoidance of alcohol and smoke, enhancement of maternal education, reduction of stress and exposure to pollution), and control of chronic diseases. Some of these are currently being evaluated by clinical studies[65-68].
| Prenatal interventions | Postnatal interventions |
| Early detection of fetal growth restriction | Breastfeeding |
| Dietary modifications/supplementations during pregnancy | Adequate nutrition in childhood |
| Normalization of body weight, glycemia and blood pressure control during pregnancy | Growth follow-ups and blood pressure monitoring |
| Lifestyle measures (i.e. avoidance of alcohol and tobacco, maximization of maternal education, reduced stress) | Lifestyle measures (i.e. avoidance of alcohol and tobacco, reduced stress, avoid overweight) |
| Management of maternal chronic diseases | Pharmacological interventions: Growth hormone, metformin |
Postnatal early-life interventions include: Breastfeeding promotion, provision of adequate nutrition and growth, follow-up of high risk patients, and appropriate resource distribution[41]. Maternal and offspring microbiota modifications (i.e. dietary supplementation with docosahexaenoic acid and arachidonic acid to improve neurodevelopmental outcomes)[65], pre-probiotics are a potential interventions needing further studies. Lactoferrin and stem cell administration are under investigation.
In this review, we reported the most important complications of FGR with their proposed pathophysiology, according to the most recent literature. FGR is not only a complication of pregnancy but a condition with relevant short- and long-term unfavorable outcomes for children and adults. Potential benefits from the research in this area could include reduced stillbirths and neonatal deaths and improved outcomes in pregnancies affected by FGR. Moreover, the prevention, detection, and treatment of FGR might have important positive reflections on public health worldwide, and it is expected that these themes will be on the next research agenda. Indeed, in recent years a number of government agencies extensively funded research studies in this area (i.e. European Commission’s 7th Framework Programme, United States National Institutes of Health, among others).
Moreover, the interplay between FGR and other environmental exposures (i.e. microbiome, smoking, pollution, malnutrition, etc.) will be another interesting area of research likely to be covered by future studies.
| 1. | Society for Maternal-Fetal Medicine (SMFM), Martins JG, Biggio JR, Abuhamad A. Society for Maternal-Fetal Medicine Consult Series #52: Diagnosis and management of fetal growth restriction: (Replaces Clinical Guideline Number 3, April 2012). Am J Obstet Gynecol. 2020;223:B2-B17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 353] [Article Influence: 58.8] [Reference Citation Analysis (0)] |
| 2. | Lees CC, Stampalija T, Baschat A, da Silva Costa F, Ferrazzi E, Figueras F, Hecher K, Kingdom J, Poon LC, Salomon LJ, Unterscheider J. ISUOG Practice Guidelines: diagnosis and management of small-for-gestational-age fetus and fetal growth restriction. Ultrasound Obstet Gynecol. 2020;56:298-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 547] [Article Influence: 91.2] [Reference Citation Analysis (0)] |
| 3. | Molina LCG, Odibo L, Zientara S, Običan SG, Rodriguez A, Stout M, Odibo AO. Validation of Delphi procedure consensus criteria for defining fetal growth restriction. Ultrasound Obstet Gynecol. 2020;56:61-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 53] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 4. | Resnik R. Intrauterine growth restriction. Obstet Gynecol. 2002;99:490-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 257] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 5. | Beune IM, Bloomfield FH, Ganzevoort W, Embleton ND, Rozance PJ, van Wassenaer-Leemhuis AG, Wynia K, Gordijn SJ. Consensus Based Definition of Growth Restriction in the Newborn. J Pediatr. 2018;196:71-76.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 132] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 6. | Nardozza LM, Caetano AC, Zamarian AC, Mazzola JB, Silva CP, Marçal VM, Lobo TF, Peixoto AB, Araujo Júnior E. Fetal growth restriction: current knowledge. Arch Gynecol Obstet. 2017;295:1061-1077. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 403] [Article Influence: 44.8] [Reference Citation Analysis (0)] |
| 7. | Lee AC, Kozuki N, Cousens S, Stevens GA, Blencowe H, Silveira MF, Sania A, Rosen HE, Schmiegelow C, Adair LS, Baqui AH, Barros FC, Bhutta ZA, Caulfield LE, Christian P, Clarke SE, Fawzi W, Gonzalez R, Humphrey J, Huybregts L, Kariuki S, Kolsteren P, Lusingu J, Manandhar D, Mongkolchati A, Mullany LC, Ndyomugyenyi R, Nien JK, Roberfroid D, Saville N, Terlouw DJ, Tielsch JM, Victora CG, Velaphi SC, Watson-Jones D, Willey BA, Ezzati M, Lawn JE, Black RE, Katz J; CHERG Small-for-Gestational-Age-Preterm Birth Working Group. Estimates of burden and consequences of infants born small for gestational age in low and middle income countries with INTERGROWTH-21(st) standard: analysis of CHERG datasets. BMJ. 2017;358:j3677. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 255] [Cited by in RCA: 264] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 8. | Miller SL, Huppi PS, Mallard C. The consequences of fetal growth restriction on brain structure and neurodevelopmental outcome. J Physiol. 2016;594:807-823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 342] [Cited by in RCA: 450] [Article Influence: 45.0] [Reference Citation Analysis (0)] |
| 9. | Barker DJ. The fetal and infant origins of adult disease. BMJ. 1990;301:1111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2075] [Cited by in RCA: 1646] [Article Influence: 45.7] [Reference Citation Analysis (0)] |
| 10. | Rock CR, White TA, Piscopo BR, Sutherland AE, Miller SL, Camm EJ, Allison BJ. Cardiovascular and Cerebrovascular Implications of Growth Restriction: Mechanisms and Potential Treatments. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 11. | Hales CN, Barker DJ. The thrifty phenotype hypothesis. Br Med Bull. 2001;60:5-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1807] [Cited by in RCA: 1721] [Article Influence: 68.8] [Reference Citation Analysis (0)] |
| 12. | Gluckman PD, Hanson MA, Buklijas T, Low FM, Beedle AS. Epigenetic mechanisms that underpin metabolic and cardiovascular diseases. Nat Rev Endocrinol. 2009;5:401-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 464] [Cited by in RCA: 455] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 13. | Eriksson J, Forsén T, Tuomilehto J, Osmond C, Barker D. Fetal and childhood growth and hypertension in adult life. Hypertension. 2000;36:790-794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 331] [Cited by in RCA: 299] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 14. | Stettler N, Stallings VA, Troxel AB, Zhao J, Schinnar R, Nelson SE, Ziegler EE, Strom BL. Weight gain in the first week of life and overweight in adulthood: a cohort study of European American subjects fed infant formula. Circulation. 2005;111:1897-1903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 270] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 15. | Ong YY, Sadananthan SA, Aris IM, Tint MT, Yuan WL, Huang JY, Chan YH, Ng S, Loy SL, Velan SS, Fortier MV, Godfrey KM, Shek L, Tan KH, Gluckman PD, Yap F, Choo JTL, Ling LH, Tan K, Chen L, Karnani N, Chong YS, Eriksson JG, Wlodek ME, Chan SY, Lee YS, Michael N. Mismatch between poor fetal growth and rapid postnatal weight gain in the first 2 years of life is associated with higher blood pressure and insulin resistance without increased adiposity in childhood: the GUSTO cohort study. Int J Epidemiol. 2020;49:1591-1603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 16. | Norris T, Crozier SR, Cameron N, Godfrey KM, Inskip H, Johnson W. Fetal growth does not modify the relationship of infant weight gain with childhood adiposity and blood pressure in the Southampton women's survey. Ann Hum Biol. 2020;47:150-158. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 17. | Yadav A, Beilin LJ, Huang RC, Vlaskovsky P, Newnham JP, White SW, Mori TA. The relationship between intrauterine foetal growth trajectories and blood pressure in young adults. J Hypertens. 2022;40:478-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 18. | Rytter D, Bech BH, Frydenberg M, Henriksen TB, Olsen SF. Fetal growth and cardio-metabolic risk factors in the 20-year-old offspring. Acta Obstet Gynecol Scand. 2014;93:1150-1159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 20. | Chan PY, Morris JM, Leslie GI, Kelly PJ, Gallery ED. The long-term effects of prematurity and intrauterine growth restriction on cardiovascular, renal, and metabolic function. Int J Pediatr. 2010;2010:280402. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 72] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 21. | Demicheva E, Crispi F. Long-term follow-up of intrauterine growth restriction: cardiovascular disorders. Fetal Diagn Ther. 2014;36:143-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 73] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 22. | Menendez-Castro C, Rascher W, Hartner A. Intrauterine growth restriction - impact on cardiovascular diseases later in life. Mol Cell Pediatr. 2018;5:4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 23. | Leon DA, Lithell HO, Vâgerö D, Koupilová I, Mohsen R, Berglund L, Lithell UB, McKeigue PM. Reduced fetal growth rate and increased risk of death from ischaemic heart disease: cohort study of 15 000 Swedish men and women born 1915-29. BMJ. 1998;317:241-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 537] [Cited by in RCA: 536] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 24. | Martyn CN, Barker DJ, Jespersen S, Greenwald S, Osmond C, Berry C. Growth in utero, adult blood pressure, and arterial compliance. Br Heart J. 1995;73:116-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 228] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 25. | Visentin S, Londero AP, Calanducci M, Grisan E, Bongiorno MC, Marin L, Cosmi E. Fetal Abdominal Aorta: Doppler and Structural Evaluation of Endothelial Function in Intrauterine Growth Restriction and Controls. Ultraschall Med. 2019;40:55-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 26. | Crispi F, Miranda J, Gratacós E. Long-term cardiovascular consequences of fetal growth restriction: biology, clinical implications, and opportunities for prevention of adult disease. Am J Obstet Gynecol. 2018;218:S869-S879. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 257] [Article Influence: 32.1] [Reference Citation Analysis (0)] |
| 27. | Mäkikallio K, Vuolteenaho O, Jouppila P, Räsänen J. Ultrasonographic and biochemical markers of human fetal cardiac dysfunction in placental insufficiency. Circulation. 2002;105:2058-2063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 101] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 28. | Girsen A, Ala-Kopsala M, Mäkikallio K, Vuolteenaho O, Räsänen J. Cardiovascular hemodynamics and umbilical artery N-terminal peptide of proB-type natriuretic peptide in human fetuses with growth restriction. Ultrasound Obstet Gynecol. 2007;29:296-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 67] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 29. | Larsen LU, Petersen OB, Sloth E, Uldbjerg N. Color Doppler myocardial imaging demonstrates reduced diastolic tissue velocity in growth retarded fetuses with flow redistribution. Eur J Obstet Gynecol Reprod Biol. 2011;155:140-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 30. | Sehgal A, Doctor T, Menahem S. Cardiac function and arterial indices in infants born small for gestational age: analysis by speckle tracking. Acta Paediatr. 2014;103:e49-e54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 31. | Änghagen O, Engvall J, Gottvall T, Nelson N, Nylander E, Bang P. Developmental Differences in Left Ventricular Strain in IUGR vs. Control Children the First Three Months of Life. Pediatr Cardiol. 2022;43:1286-1297. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 32. | Crispi F, Hernandez-Andrade E, Pelsers MM, Plasencia W, Benavides-Serralde JA, Eixarch E, Le Noble F, Ahmed A, Glatz JF, Nicolaides KH, Gratacos E. Cardiac dysfunction and cell damage across clinical stages of severity in growth-restricted fetuses. Am J Obstet Gynecol. 2008;199:254.e1-254.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 219] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 33. | Rodríguez-López M, Cruz-Lemini M, Valenzuela-Alcaraz B, Garcia-Otero L, Sitges M, Bijnens B, Gratacós E, Crispi F. Descriptive analysis of different phenotypes of cardiac remodeling in fetal growth restriction. Ultrasound Obstet Gynecol. 2017;50:207-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 78] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 34. | Amruta N, Kandikattu HK, Intapad S. Cardiovascular Dysfunction in Intrauterine Growth Restriction. Curr Hypertens Rep. 2022;24:693-708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 35. | Martin H, Hu J, Gennser G, Norman M. Impaired endothelial function and increased carotid stiffness in 9-year-old children with low birthweight. Circulation. 2000;102:2739-2744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 190] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 36. | Napoli C, Glass CK, Witztum JL, Deutsch R, D'Armiento FP, Palinski W. Influence of maternal hypercholesterolaemia during pregnancy on progression of early atherosclerotic lesions in childhood: Fate of Early Lesions in Children (FELIC) study. Lancet. 1999;354:1234-1241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 473] [Cited by in RCA: 477] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 37. | Herriges M, Morrisey EE. Lung development: orchestrating the generation and regeneration of a complex organ. Development. 2014;141:502-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 374] [Cited by in RCA: 437] [Article Influence: 36.4] [Reference Citation Analysis (0)] |
| 38. | Pike K, Jane Pillow J, Lucas JS. Long term respiratory consequences of intrauterine growth restriction. Semin Fetal Neonatal Med. 2012;17:92-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 82] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 39. | Nobile S, Marchionni P, Gidiucci C, Correani A, Palazzi ML, Spagnoli C, Rondina C; Marche Neonatal Network, Carnielli VP. Oxygen saturation/FIO2 ratio at 36 wk' PMA in 1005 preterm infants: Effect of gestational age and early respiratory disease patterns. Pediatr Pulmonol. 2019;54:637-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 40. | Nobile S, Marchionni P, Carnielli VP. Neonatal outcome of small for gestational age preterm infants. Eur J Pediatr. 2017;176:1083-1088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 41. | Pike KC, Hanson MA, Godfrey KM. Developmental mismatch: consequences for later cardiorespiratory health. BJOG. 2008;115:149-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 42. | Briana DD, Malamitsi-Puchner A. Small for gestational age birth weight: impact on lung structure and function. Paediatr Respir Rev. 2013;14:256-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 43. | Fandiño J, Toba L, González-Matías LC, Diz-Chaves Y, Mallo F. Perinatal Undernutrition, Metabolic Hormones, and Lung Development. Nutrients. 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 44. | Arigliani M, Spinelli AM, Liguoro I, Cogo P. Nutrition and Lung Growth. Nutrients. 2018;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 71] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 45. | Maritz GS, Cock ML, Louey S, Joyce BJ, Albuquerque CA, Harding R. Effects of fetal growth restriction on lung development before and after birth: a morphometric analysis. Pediatr Pulmonol. 2001;32:201-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 86] [Article Influence: 3.4] [Reference Citation Analysis (1)] |
| 46. | Maritz GS, Cock ML, Louey S, Suzuki K, Harding R. Fetal growth restriction has long-term effects on postnatal lung structure in sheep. Pediatr Res. 2004;55:287-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 84] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 47. | Orgeig S, Crittenden TA, Marchant C, McMillen IC, Morrison JL. Intrauterine growth restriction delays surfactant protein maturation in the sheep fetus. Am J Physiol Lung Cell Mol Physiol. 2010;298:L575-L583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 81] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 48. | Xu XF, Lv Y, Gu WZ, Tang LL, Wei JK, Zhang LY, Du LZ. Epigenetics of hypoxic pulmonary arterial hypertension following intrauterine growth retardation rat: epigenetics in PAH following IUGR. Respir Res. 2013;14:20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 49. | Polglase GR, Barbuto J, Allison BJ, Yawno T, Sutherland AE, Malhotra A, Schulze KE, Wallace EM, Jenkin G, Ricardo SD, Miller SL. Effects of antenatal melatonin therapy on lung structure in growth-restricted newborn lambs. J Appl Physiol (1985). 2017;123:1195-1203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 50. | Sutherland AE, Crossley KJ, Allison BJ, Jenkin G, Wallace EM, Miller SL. The effects of intrauterine growth restriction and antenatal glucocorticoids on ovine fetal lung development. Pediatr Res. 2012;71:689-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 51. | Arigliani M, Stocco C, Valentini E, De Pieri C, Castriotta L, Ferrari ME, Canciani C, Driul L, Orsaria M, Cattarossi L, Cogo P. Lung function between 8 and 15 years of age in very preterm infants with fetal growth restriction. Pediatr Res. 2021;90:657-663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 52. | Nikolajev K, Heinonen K, Hakulinen A, Länsimies E. Effects of intrauterine growth retardation and prematurity on spirometric flow values and lung volumes at school age in twin pairs. Pediatr Pulmonol. 1998;25:367-370. [PubMed] [DOI] [Full Text] |
| 53. | Okyere DO, Bui DS, Washko GR, Lodge CJ, Lowe AJ, Cassim R, Perret JL, Abramson MJ, Walters EH, Waidyatillake NT, Dharmage SC. Predictors of lung function trajectories in population-based studies: A systematic review. Respirology. 2021;26:938-959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 44] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 54. | Karmaus W, Mukherjee N, Janjanam VD, Chen S, Zhang H, Roberts G, Kurukulaaratchy RJ, Arshad H. Distinctive lung function trajectories from age 10 to 26 years in men and women and associated early life risk factors - a birth cohort study. Respir Res. 2019;20:98. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 48] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 55. | Weber P, Menezes AMB, Gonçalves H, Perez-Padilla R, Jarvis D, de Oliveira PD, Wehrmeister FC. Characterisation of pulmonary function trajectories: results from a Brazilian cohort. ERJ Open Res. 2020;6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 56. | Belgrave DCM, Granell R, Turner SW, Curtin JA, Buchan IE, Le Souëf PN, Simpson A, Henderson AJ, Custovic A. Lung function trajectories from pre-school age to adulthood and their associations with early life factors: a retrospective analysis of three population-based birth cohort studies. Lancet Respir Med. 2018;6:526-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 236] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 57. | Stein CE, Kumaran K, Fall CH, Shaheen SO, Osmond C, Barker DJ. Relation of fetal growth to adult lung function in south India. Thorax. 1997;52:895-899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 114] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 58. | Canoy D, Pekkanen J, Elliott P, Pouta A, Laitinen J, Hartikainen AL, Zitting P, Patel S, Little MP, Järvelin MR. Early growth and adult respiratory function in men and women followed from the fetal period to adulthood. Thorax. 2007;62:396-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 104] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 59. | Savran O, Ulrik CS. Early life insults as determinants of chronic obstructive pulmonary disease in adult life. Int J Chron Obstruct Pulmon Dis. 2018;13:683-693. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 68] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 60. | Duan P, Wang Y, Lin R, Zeng Y, Chen C, Yang L, Yue M, Zhong S, Zhang Q. Impact of early life exposures on COPD in adulthood: A systematic review and meta-analysis. Respirology. 2021;26:1131-1151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 61. | Saad NJ, Patel J, Burney P, Minelli C. Birth Weight and Lung Function in Adulthood: A Systematic Review and Meta-analysis. Ann Am Thorac Soc. 2017;14:994-1004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 56] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 62. | Källén B, Finnström O, Nygren KG, Otterblad Olausson P. Association between preterm birth and intrauterine growth retardation and child asthma. Eur Respir J. 2013;41:671-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 62] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 63. | Ortqvist AK, Lundholm C, Carlström E, Lichtenstein P, Cnattingius S, Almqvist C. Familial factors do not confound the association between birth weight and childhood asthma. Pediatrics. 2009;124:e737-e743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 67] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 64. | Villamor E, Iliadou A, Cnattingius S. Is the association between low birth weight and asthma independent of genetic and shared environmental factors? Am J Epidemiol. 2009;169:1337-1343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 45] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 65. | Heath RJ, Klevebro S, Wood TR. Maternal and Neonatal Polyunsaturated Fatty Acid Intake and Risk of Neurodevelopmental Impairment in Premature Infants. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 66. | Petersen AB, Ogunrinu T, Wallace S, Yun J, Belliard JC, Singh PN. Implementation and Outcomes of a Maternal Smoking Cessation Program for a Multi-ethnic Cohort in California, USA, 2012-2019. J Community Health. 2022;47:257-265. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 67. | van Hoorn F, de Wit L, van Rossem L, Jambroes M, Groenendaal F, Kwee A, Lamain-de Ruiter M, Franx A, van Rijn BB, Koster MPH, Bekker MN. A prospective population-based multicentre study on the impact of maternal body mass index on adverse pregnancy outcomes: Focus on normal weight. PLoS One. 2021;16:e0257722. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 68. | McCarthy EK, Murray DM, Kiely ME. Iron deficiency during the first 1000 days of life: are we doing enough to protect the developing brain? Proc Nutr Soc. 2022;81:108-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kumar S, India; Tolunay HE, Turkey S-Editor: Liu XF L-Editor: A P-Editor: Zhao S