Published online Apr 26, 2023. doi: 10.12998/wjcc.v11.i12.2780
Peer-review started: January 13, 2023
First decision: February 8, 2023
Revised: February 22, 2023
Accepted: March 30, 2023
Article in press: March 30, 2023
Published online: April 26, 2023
Processing time: 102 Days and 10.5 Hours
Pulmonary hypertension is a rare cardiopulmonary disease, with an insidious onset that usually worsens rapidly. Amyloid light chain (AL) amyloidosis is a rare systemic disease caused by extracellular deposition of pathologic, insoluble, and proteinaceous fibrils in organs and tissues; however, it is difficult to diagnose given its varied and nonspecific symptoms. To date, rare cases of amyloidosis with pulmonary hypertension have been reported. Of note, the optimal treat
We report a case of a 51-year-old woman who presented with progressively worsening dyspnea. Transthoracic echocardiography indicated severe pulmonary hypertension. Twenty-seven months after first admission, the patient returned with symptoms of progressive heart failure. A myocardial tissue sample stained with Congo red was positive, and the patient was ultimately diagnosed with AL amyloidosis with cardiac involvement.
Although pulmonary hypertension may be idiopathic, it is frequently associated with other conditions. In rare cases, pulmonary hypertension can be a com
Core Tip: Symptomatic pulmonary hypertension is only rarely described and, when present, is typically associated with progressive disease, such as elevated filling pressures secondary to cardiac amyloid. In this case, the patient initially presented with pulmonary hypertension, she was found, 2 years later, to have amyloid light chain (AL) amyloidosis with cardiac involvement. We highlight the diagnostic difficulties presented by pulmonary hypertension in a patient with AL amyloidosis, and illustrate the complicated progression of the disease, as well as the poor efficacy of current palliative medicine.
- Citation: Gao M, Zhang WH, Zhang ZG, Yang N, Tong Q, Chen LP. Cardiac amyloidosis presenting as pulmonary arterial hypertension: A case report. World J Clin Cases 2023; 11(12): 2780-2787
- URL: https://www.wjgnet.com/2307-8960/full/v11/i12/2780.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i12.2780
Pulmonary hypertension is a rare cardiopulmonary disease with an insidious onset that usually worsens rapidly, and, if left untreated, has a median survival of 2–3 years[1]. Although pulmonary hypertension may be idiopathic, it is frequently associated with other conditions. The most common clinical disorders that cause pulmonary hypertension include primary cardiac abnormalities, chronic pulmonary embolism, pulmonary parenchymal problems, obstructive sleep apnea, connective tissue disorders, cirrhosis with portal hypertension, and use of appetite suppressants[2]. Amyloid light chain (AL) amyloidosis is a rare systemic disease caused by extracellular deposition of pathologic, insoluble, and proteinaceous fibrils in organs and tissues[3]. Group I pulmonary hypertension is a rare complication of AL amyloidosis[4]. In addition to AL amyloidosis, transthyretin-related amyloidosis is considered a disease in the field of cardiology. Cardiac amyloidosis is confirmed by endomyocardial biopsy, with Congo red staining, nuclear scintigraphy and immunohistochemistry to determine the amyloid type[5]. To date, only a few, rare cases of amyloidosis with pulmonary hypertension have been reported. We present the following article in accordance with the CARE reporting checklist.
In this report, we highlight the diagnostic difficulties presented by pulmonary hypertension in a patient with AL amyloidosis, and illustrate the complicated progression of the disease, as well as the poor efficacy of current treatment strategies.
A 51-year-old woman was admitted to hospital due to dyspnea, occurring over a 1-mo period.
Physical examination revealed blood pressure of 101/55 mmHg, heart rate of 65 beats/min.
Plasma N-terminal brain natriuretic peptide (NT-proBNP) was elevated to 3780 pg/mL (normal range 0-125 pg/mL).
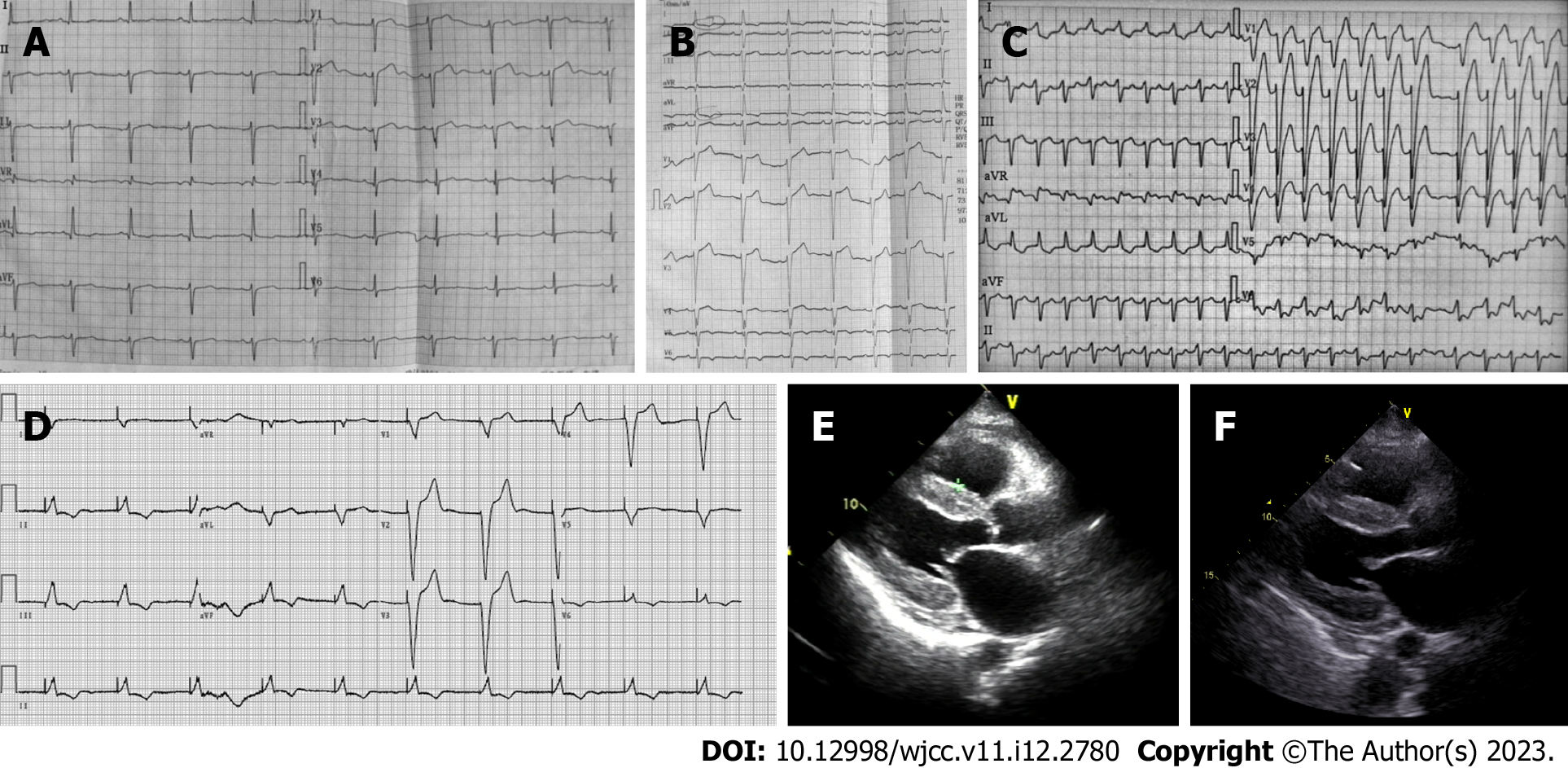
Electrocardiography (ECG) demonstrated a sinus rhythm with a heart rate of 65 bpm, a left anterior fascicular block, poor R-wave progression in leads V1-V3, and a non-specific T wave and ST-segment (Figure 1A). A 2-dimensional transthoracic echocardiogram revealed right ventricular enlargement, with a chamber diameter of 27 mm, a left ventricular cavity of normal diameter, an end diastolic chamber size of 42 mm and preserved systolic function, as well as an ejection fraction of 64%. The left atrium (42 mm) and right atrium (48 mm × 48 mm) were both enlarged. Transmitral Doppler flow was consistent with restrictive physiology. The left ventricle was revealed to have impaired diastolic dysfunction (mitral E wave velocity = 0.99 m/s, A wave (1.28 m/s, medial E/e’ > 15). Transthoracic echocardiography indicated severe pulmonary hypertension on the basis of the estimated right ventricular systolic pressures (the pulmonary arterial pressure was 51 mmHg). Pulmonary arterial hypertension is defined as pulmonary capillary wedge pressure < 15 mmHg and pulmonary vascular resistance > 3 Wood Units as assessed by right heart catheterization[6]. The criterion for clinically significant pulmonary hypertension when detected by Doppler echocardiography is not precisely defined. Commonly used definitions of pulmonary hypertension are a pulmonary artery systolic pressure > 35 mmHg or mean > 25 mmHg at rest or mean > 30 mmHg when exercising[7]. Color Doppler showed mild regurgitation at the mitral and pulmonary valves. Furthermore, moderate tricuspid valve regurgitation was observed (the tricuspid regurgitation area was 7.8 cm2).
When pulmonary hypertension was identified, the patient was further evaluated for an underlying etiology. A high resolution computed tomography angiogram of the chest did not show evidence of pulmonary embolism or signs of interstitial or other lung disease. The patient had negative findings upon spirometry, and denied a family history of pulmonary hypertension, sleep apnea, and premature death. No laboratory markers or clinical symptoms that suggested collagen vascular disease were detected in the patient. Furthermore, a Doppler ultrasound of the portal vein and liver scan did not show signs of portal hypertension. The patient also showed serologic test results that were negative for human immunodeficiency virus, and the patient refused right heart catheterization.
The patient was diagnosed with pulmonary hypertension based on echocardiographic finding. Pulmonary hypertension in heart failure with preserved ejection fraction represents the most complex situation. We cannot make a clear distinction between idiopathic pulmonary arterial hypertension (group 1 pulmonary hypertension) and pulmonary hypertension secondary to left heart disease (group 2 pulmonary hypertension) without right heart catheterization, Idiopathic pulmonary arterial hypertension was considered in our case who had an early indication of left ventricle diastolic dysfunction (E/e’ > 15 and enlarged left atria), but severe pulmonary hypertension.
The patient was treated with a phosphodiesterase 5 inhibitor (sildenafil, 20 mg, three times a day) and diuretics (furosemide, 20 mg, twice a day and spironolactone, 20 mg, three times a day), and showed subsequent improvement in signs and symptoms of right heart failure, in addition to a slight lowering of pulmonary hypertension.
However, the patient returned 11 mo after her first admission and presented with worsening dyspnea, paroxysmal nocturnal dyspnea, and complaints of an inability to lie flat on her back. Echocardiography showed newly-presenting left ventricular hypertrophy (the septal and left posterior wall thicknesses were 12 mm and 12 mm, respectively). NT-proBNP was elevated from 3780 pg/mL to 7910 pg/mL (normal range 0-125 pg/mL). ECG demonstrated a left anterior fascicular block, a new left bundle branch block, ST-segment depression in leads I and augmented unipolar limb lead (AVL) and T wave inversion in leads I, AVL, and V4-V6 (Figure 1B).
Ten months later (21 mo after first admission), the patient’s respiratory condition continued to deteriorate. The patient was admitted due to new appearance of orthopnea and worsening edema in the lower extremities. NT-proBNP was again elevated at 11600 pg/mL. Transthoracic echocardiography showed worsening systolic dysfunction, and echocardiography revealed progressive left ventricular hypertrophy (the septal and left posterior wall thicknesses were 14 mm and 13 mm, respectively). Furthermore, severe tricuspid regurgitation (13.2 cm2) and higher pulmonary artery pressure (58 mmHg) were also observed. ECG demonstrated atrial fibrillation, left bundle branch block with wider QRS wave group, ST segment elevation in leads V1-V3, and T wave inversion in leads I, AVL and V4-V6.
Six month later (27 mo after first admission), the patient returned again with symptoms of New York Heart Association class III heart failure. ECG showed atrial fibrillation with rapid ventricular response, left bundle branch block (Figure 1C), first degree atrioventricular block, Mobitz type I second-degree Atrioventricular block, and premature ventricular contraction on 24-h dynamic electrocardiography. Echocardiography revealed progressive atrial enlargement (the left atrial diameter was 51 mm, and the right atrial diameter was 52 mm × 62 mm). The patient also had worsening systolic dysfunction (ejection fraction 48%). The ventricle wall had a characteristic sparkling apperance, with a granular texture (Figure 1D). Immunohistochemistry of amyloid deposits was used to distinguish transthyretin from other proteins that may cause amyloidosis. Immunoglobulin light chain was associated with AL amyloidosis. Serum free light-chain analysis showed lambda light-chain was increased at 378 mg/L (normal range 8.3-27 mg/L), with an altered kappa/Lambda of 22/378. The lambda and kappa light-chain were not detected in the urine. Cardiac magnetic resonance imaging showed suspected delayed subendocardial gadolinium enhancement. The patient underwent endomyocardial biopsy at another hospital. Amyloid deposits were detected by Congo red staining. Myocardial tissue sample was positive, but periumbilical fat aspirates, as well as samples from the tongue, gums and bone marrow were all negative. The patient was ultimately diagnosed with stage III AL amyloidosis with cardiac involvement, per the Mayo 2012 staging system. In this patient, the most likely cause of pulmonary hypertension was deposition of amyloid in the pulmonary vasculature.
The patient’s therapeutic plan was to receive three cycles of therapy consisting of cyclophosphamide/bortezomib/dexamethasone (CyBorD). Bortezomib (1.3 mg/sqm/day) and cyclophosphamide (300 mg/day) were administered on the 1st, 5th, 15th, and 22nd day of each 35-d course, and dexamethasone (20 mg/day) was administered on days 1, 2, 8, 9, 15, 16, 21, and 22. Unfortunately, 1 d after the first cycle of therapy, the patient experienced an episode of cardiopulmonary arrest due to cardiac arrest, with a quick return of spontaneous circulation after a brief cardiopulmonary resuscitation. The next day, the patient experienced cardiac arrest 4 times, returning to spontaneous circulation after cardiopulmonary resuscitation in each case. A pacemaker was implanted to address the recurrent cardiac arrests and the pacing electrocardiogram is shown in Figure 1E. Echocardiography revealed atrial enlargements and worsening systolic dysfunction (ejection fraction 48%) (Figure 1F).
It was unclear whether there was a causal relationship between CyBorD and cardiac arrest. However, the patient refused CyBorD chemotherapy for cardiac amyloidosis. The patient died 5 years after the first admission and 3 years after the diagnosis of cardiac amyloidosis.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient.
Cardiac amyloidosis is a rare disease, and patients frequently experience significant delays between the onset of non-specific symptoms and a confirmed amyloidosis diagnosis. Symptomatic pulmonary hypertension is only rarely described and, when present, is typically associated with progressive disease, such as elevated filling pressures secondary to cardiac amyloidosis (Table 1)[4,8]. Although our patient initially presented with pulmonary hypertension, she was found, 2 years later, to have AL amyloidosis with cardiac involvement. This report further emphasizes the insidious nature of amyloidosis that makes it difficult to diagnose. Multiple underlying factors and a long term follow-up must be considered for patients with pulmonary hypertension in order to reduce the time to diagnosis.
| Case | Ref. | Age (year)/sex | Biopsy site | Type of amyloid | MPAP (mmHg) | MM | Treatment | Time to death (mo) |
| 1 | Chapman et al[19], 1999 | 91/F | Lung, heart | AL | NI | (-) | NI | NI |
| 2 | Cirulis et al[4], 2016 | 53/F | Lung, heart | IgGκ AL | 46 | (+) | Bortezomib-thalidomide-dexamethasone, sildenafil | Survival |
| 3 | D'Aloia et al[20], 2008 | 75/M | Fat, heart | AL | NI | (-) | NI | NI |
| 4 | Dingli et al[10], 2001 | 61/F | BM, fat | IgGκ AL | 58 | (+) | Diuretics, melphalan, prednisone, cyclophosphamide, vincristine doxorubicin, dexamethasone | 1 |
| 5 | Dingli et al[10], 2001 | 64/F | BM | IgGλ AL | NI | (+) | Diuretics, vincristine, BCNU, Melphalan, cyclophosphamide, prednisone | 29 |
| 6 | Dingli et al[10], 2001 | 82/M | BM | IgGλ AL | NI | (-) | Diuretics, digoxin, calcium channel blocker | 32 |
| 7 | Dingli et al[10], 2001 | 54/F | Lung | IgGκ AL | 48 | (-) | Diuretics, calcium channel blocker, aspirin | 2 |
| 8 | Dingli et al[10], 2001 | 48/F | Liver | Amyloid A AL | 62 | (-) | Calcium channel blocker, colchicine | 2 |
| 9 | Eder et al[8], 2007 | 73/F | BM | AL | 90 | (+) | Prednisone, chlorambucil, dexamethasone, cyclophosphamide | 8 |
| 10 | Hashimoto et al[21], 2015 | 85/F | BM | IgGκ AL | 60 | (+) | Melphalan-prednisolone, Lenalidomide- dexamethasone, bortezomib, cyclophosphamide, vincristine, and dacarbazine | 29 |
| 11 | Kruczak et al[22], 2013 | 67/F | Lung, colon mucosa | Transthyretin AL | 95 | (-) | Methylprednisolone, melphalan and cyclophosphamide | 1 |
| 12 | Lehtonen and Kettunen[23], 2007 | 48/M | BM, fat | AL | 46 | (+) | Sildenafil, melphalan, prednison | NI |
| 13 | Lutz et al[24], 1995 | 61/F | Heart, lung | β2M AL | 75 | (-) | Antihypertensive medication | 12 |
| 14 | Shiue and McNally[25], 1988 | 65/F | BM, lung, rectal | AL | 39 | (+) | Melphalan, prednisolone, diuretics | 1 |
| 15 | Sullivan and Schwarz[26], 1994 | 72/F | BM, lung | AL | 56 | (+) | NI | 6 |
| 16 | Krishnan et al[27], 2015 | 69/F | BM, heart, renal | AL | 41 | (+) | Sildenafil, bumetadine, warfarin, metoprolol succinate, ambrisentan | NI |
| 17 | Present case, 2019 | 51/F | BM, heart, fat | IgGλ AL | 51 | (-) | Sildenafil, cyclophosphamide, bortezomib, dexamethasone | 60 |
Doppler echocardiography is a commonly used method for diagnosing pulmonary hypertension[9]. Echocardiography provides an estimate of the systolic pressure in the pulmonary artery. However, a definitive diagnosis of pulmonary hypertension requires a direct pulmonary arterial pressure measurement via right heart catheterization. Generally pressures obtained via echocardiography are similar to those obtained by catheterization, if they are in the lower pressure ranges. Based on criteria from the National Institutes of Health, a mean pulmonary artery pressure ≥ 25 mmHg at rest (> 30 mmHg with exercise) is the standard for the diagnosis of pulmonary hypertension[9].
Due to etiologic uncertainty and several possible contributing factors, further diagnostic evaluation was pursued in our patient. However, the underlying disease causing pulmonary hypertension was unclear at the time of the initial presentation. Two years later, our patient was finally diagnosed with amyloidosis following progressively worsening symptoms and echocardiographic evidence indicating characteristic sparkling and granular texture of the ventricle wall.
In patients with AL amyloidosis, the most common etiologies of pulmonary hypertension are left-side restrictive cardiomyopathy from amyloid deposition or diffuse lung disease[8,10]. Pulmonary amyloidosis rarely causes symptoms despite the fact that it is commonly found in bronchoscopic lung biopsy[11]. The main patterns of pulmonary involvement are tracheobronchial or parenchymal, the latter being further classified radiographically, either as solitary/multiple nodular parenchymal, or as a diffuse alveolar septal pattern[12]. A tracheobronchial pattern is a common form of respiratory amyloidosis, in which amyloid is found in the subepithelial interstitial tissue and often surrounds tracheobronchial ducts and acini. Nodular parenchymal amyloidosis is rare and amyloid is often present only in the alveolar interstitium at nodule peripheries. A diffuse parenchymal pattern is the least common form of respiratory amyloidosis, in which amyloid is present in the media of small blood vessels and in the parenchymal interstitium. In all reported cases, vascular obstructions due to amyloid deposits are considered the cause of increased pulmonary vascular resistance[13]. Amyloid deposition in blood vessel walls can result in endothelial dysfunction. The abnormal endothelial cells express lower levels of nitric oxide synthase and cyclooxygenase, as well as increased levels of endothelia, and promote the onset of vasoconstriction and smooth muscle proliferation. It is possible that similar mechanisms operate in the pulmonary condition, lead to vasoconstriction and pulmonary hypertension, even in the absence of severe intra-vascular amyloid deposits. An increase in pulmonary vascular resistance requires a higher pulmonary arterial pressure to maintain the same right ventricular output, which eventually leads to pulmonary arterial hypertension.
Treating pulmonary hypertension in patients with amyloidosis can be a challenging. Despite a paucity of data, diuretics and vasodilators, with calcium channel blockers, are often appropriate therapies used to treat patients with pulmonary hypertension[14]. However, patients with amyloidosis often have orthostatic hypotension and cannot tolerate the high doses required for successful treatment. The pulmonary hypertension-specific drugs that have emerged over the past 2 decades have largely focused on targeting the underlying complex etiology via the endothelial, prostacyclin, and nitric oxide pathways. Phosphodiesterase type 5 inhibitors, such as Sildenafil, are a class of drugs used to prolong the physiological effects of Nitric oxide–cyclic guanosine monophosphate (NO/cGMP) signaling by inhibiting cGMP degradation[15]. Sildenafil is approved for treatment of pulmonary hypertension as a class I indication in World Health Organization-Functional Class (WHO-FC) II and III, and as a class IIa indication in WHO-FC IV patients[16]. However, pulmonary hypertension-specific drugs are not recommended treatments for patients with pulmonary hypertension related to left heart disease[17]. Since 2013, several randomized controlled trials have been completed in patients with pulmonary hypertension related to left heart disease, and no effect was observed on the primary end-point of mean pulmonary artery pressure. The principle of the treatment applied is always related to the underlying disease. Our patient was first treated with sildenafil; however, her symptoms continued to gradually progress which is in accordance with those in previous studies. However, it was difficult to determine whether the clinical deterioration was related to the use of sildenafil in our patient. Such an event, underscores the importance of a treatment strategy that addresses the underlying etiology in order to effectively improve pulmonary hypertension. Therapeutic interventions for AL amyloidosis are controversial. Definitive management involves stopping production of the paraprotein responsible for amyloid formation. The combination of CyBorD, as a first-line treatment, has shown signs of early promise, with high rates of hematologic responses in many patients[18]. Unfortunately, our patient suffered cardiac arrests during CyBorD chemotherapy. Therefore, the patient declined further CyBorD treatment and we did not observed any changes in pulmonary hypertension following treatment of the underlying process driving amyloid deposition.
Although pulmonary hypertension may be idiopathic, it is frequently associated with other conditions. Pulmonary hypertension is a rare complication of AL amyloidosis, which is associated with a poor prognosis and significant mortality. Further studies are required to develop targeted therapies to effectively improve outcomes among patients with pulmonary hypertension, and in those with other comorbidities due to AL amyloidosis.
| 1. | Kiely DG, Lawrie A, Humbert M. Screening strategies for pulmonary arterial hypertension. Eur Heart J Suppl. 2019;21 Suppl:K9-K20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 56] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 2. | Barnett CF, Alvarez P, Park MH. Pulmonary Arterial Hypertension: Diagnosis and Treatment. Cardiol Clin. 2016;34:375-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 3. | Wechalekar AD, Gillmore JD, Hawkins PN. Systemic amyloidosis. Lancet. 2016;387:2641-2654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 557] [Cited by in RCA: 704] [Article Influence: 70.4] [Reference Citation Analysis (0)] |
| 4. | Cirulis MM, Emerson LL, Bull DA, Hatton N, Nativi-Nicolai J, Hildebrandt GC, Ryan JJ. Pulmonary arterial hypertension in primary amyloidosis. Pulm Circ. 2016;6:244-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 5. | Koike H, Okumura T, Murohara T, Katsuno M. Multidisciplinary Approaches for Transthyretin Amyloidosis. Cardiol Ther. 2021;10:289-311. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 6. | Simonneau G, Montani D, Celermajer DS, Denton CP, Gatzoulis MA, Krowka M, Williams PG, Souza R. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J. 2019;53. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1668] [Cited by in RCA: 2746] [Article Influence: 392.3] [Reference Citation Analysis (0)] |
| 7. | Barst RJ, McGoon M, Torbicki A, Sitbon O, Krowka MJ, Olschewski H, Gaine S. Diagnosis and differential assessment of pulmonary arterial hypertension. J Am Coll Cardiol. 2004;43:40S-47S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 632] [Cited by in RCA: 593] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 8. | Eder L, Zisman D, Wolf R, Bitterman H. Pulmonary hypertension and amyloidosis--an uncommon association: a case report and review of the literature. J Gen Intern Med. 2007;22:416-419. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 9. | Dodson MW, Brown LM, Elliott CG. Pulmonary Arterial Hypertension. Heart Fail Clin. 2018;14:255-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 54] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 10. | Dingli D, Utz JP, Gertz MA. Pulmonary hypertension in patients with amyloidosis. Chest. 2001;120:1735-1738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 33] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 11. | Utz JP, Swensen SJ, Gertz MA. Pulmonary amyloidosis. The Mayo Clinic experience from 1980 to 1993. Ann Intern Med. 1996;124:407-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 217] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 12. | Scala R, Maccari U, Madioni C, Venezia D, La Magra LC. Amyloidosis involving the respiratory system: 5-year's experience of a multi-disciplinary group's activity. Ann Thorac Med. 2015;10:212-216. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Milani P, Basset M, Russo F, Foli A, Palladini G, Merlini G. The lung in amyloidosis. Eur Respir Rev. 2017;26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 125] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 14. | Hansen L, Burks M, Kingman M, Stewart T. Volume Management in Pulmonary Arterial Hypertension Patients: An Expert Pulmonary Hypertension Clinician Perspective. Pulm Ther. 2018;4:13-27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 15. | Ataya A, Cope J, Alnuaimat H. A Review of Targeted Pulmonary Arterial Hypertension-Specific Pharmacotherapy. J Clin Med. 2016;5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 16. | Galiè N, Corris PA, Frost A, Girgis RE, Granton J, Jing ZC, Klepetko W, McGoon MD, McLaughlin VV, Preston IR, Rubin LJ, Sandoval J, Seeger W, Keogh A. Updated treatment algorithm of pulmonary arterial hypertension. J Am Coll Cardiol. 2013;62:D60-D72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 491] [Cited by in RCA: 470] [Article Influence: 39.2] [Reference Citation Analysis (0)] |
| 17. | Berthelot E, Bailly MT, Hatimi SE, Robard I, Rezgui H, Bouchachi A, Montani D, Sitbon O, Chemla D, Assayag P. Pulmonary hypertension due to left heart disease. Arch Cardiovasc Dis. 2017;110:420-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 18. | Popkova T, Hajek R, Jelinek T. Monoclonal antibodies in the treatment of AL amyloidosis: co-targetting the plasma cell clone and amyloid deposits. Br J Haematol. 2020;189:228-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 19. | Chapman AD, Brown PA, Kerr KM. Right heart failure as the dominant clinical picture in a case of primary amyloidosis affecting the pulmonary vasculature. Scott Med J. 1999;44:116-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 20. | D'Aloia A, Vizzardi E, Chiari E, Faggiano P, Squeri A, Ugo F, Dei Cas L. Cardiac arrest in a patient with a mobile right atrial thrombus in transit and amyloidosis. Eur J Echocardiogr. 2008;9:141-142. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 21. | Hashimoto H, Kurata A, Mizuno H, Nashiro T, Hangaishi A, Kuroda M, Usuki K, Horiuchi H. Pulmonary arterial hypertension due to pulmonary vascular amyloid deposition in a patient with multiple myeloma. Int J Clin Exp Pathol. 2015;8:15391-15395. [PubMed] |
| 22. | Kruczak K, Duplaga M, Sanak M, Papla B, Soja J, Niżankowska-Mogilnicka E, Sładek K. Transthyretin amyloidosis with pulmonary involvement in a patient with monoclonal gammapathy. Pneumonol Alergol Pol. 2013;81:537-541. [PubMed] |
| 23. | Lehtonen J, Kettunen P. Pulmonary hypertension as a dominant clinical picture in a case of amyloidosis and smoldering multiple myeloma. Int J Cardiol. 2007;115:e29-e30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 24. | Lutz AE, Schneider U, Ehlerding G, Frenzel H, Koch KM, Kühn K. Right ventricular cardiac failure and pulmonary hypertension in a long-term dialysis patient--unusual presentation of visceral beta 2-microglobulin amyloidosis. Nephrol Dial Transplant. 1995;10:555-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 25. | Shiue ST, McNally DP. Pulmonary hypertension from prominent vascular involvement in diffuse amyloidosis. Arch Intern Med. 1988;148:687-689. [PubMed] |
| 26. | Sullivan EJ, Schwarz MI. Pulmonary hypertension resulting from primary pulmonary amyloidosis. Semin Respir Crit Care Med. 1994;15:238-242. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 27. | Krishnan U, Mark TM, Niesvizky R, Sobol I. Pulmonary hypertension complicating multiple myeloma. Pulm Circ. 2015;5:590-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gao Y, China; Koike H, Japan S-Editor: Zhao S L-Editor: A P-Editor: Zhao S