Published online Apr 16, 2023. doi: 10.12998/wjcc.v11.i11.2559
Peer-review started: January 18, 2023
First decision: February 7, 2023
Revised: February 13, 2023
Accepted: March 23, 2023
Article in press: March 23, 2023
Published online: April 16, 2023
Processing time: 77 Days and 18 Hours
The incidence of colon cancer is increasing worldwide. Treatments for colon cancer include surgery and surgery combined with chemotherapy and radio
A 23-year-old woman was admitted to our hospital for further treatment with a history of acute abdominal pain, nausea, and vomiting. Her physical examination and computed tomography scan revealed an abdominal tumor. Transverse colectomy was successfully performed. Histopathological examination revealed that the tumor was a mucosecretory adenocarcinoma with signet ring cells. The patient inadvertently found a mass in the outer upper quadrant of the right breast after four cycles of XELOX chemotherapy [oxaliplatin 130 mg/m2, d1, intravenous (iv) drip for 2 h; capecitabine 1000 mg/m2, po, bid, d1–d14]. After discussion with the patient, we performed a lumpectomy and frozen biopsy. The latter revealed that the breast tumor was intestinal metastasis. Genetic testing showed wild-type RAS and BRAF. So we replaced the original chemotherapy with FOLFIRI [irinotecan 180 mg/m2, d1, iv drip for 3–90 min; leucovorin 400 mg/m2, d1, iv drip for 2 h; 5-fluorouracil (5-FU) 400 mg/m2, d1 and 5-FU 1200 mg/(m2 d) × 2 d, continuous iv drip for 46–48 h] + cetuximab (500 mg/m2, d1, iv drip for 2 h). Serum levels of tumor markers returned to normal after several treatment cycles, and there was no evidence of tumor recurrence or metastasis.
Breast metastasis from colon cancer is rare. Radical breast surgery should be avoided unless needed for palliation. Chemotherapy combined with targeted therapy should be the first choice.
Core Tip: Breast metastasis from colon cancer is rare. The prognosis is poor and conservative treatment is mainly adopted. We present a rare case of breast metastasis from colon cancer in a young patient who received chemotherapy (FOLFIRI) combined with targeted therapy (cetuximab) after undergoing transverse colectomy and lumpectomy. There was no recurrence or metastasis during the 16-mo follow-up after lumpectomy, and the treatment effect was good.
- Citation: Jiao X, Xing FZ, Zhai MM, Sun P. Successful treatment of breast metastasis from primary transverse colon cancer: A case report. World J Clin Cases 2023; 11(11): 2559-2566
- URL: https://www.wjgnet.com/2307-8960/full/v11/i11/2559.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i11.2559
Colon cancer is the third most common type of cancer worldwide and the fourth leading cause of cancer mortality[1]. Approximately 25% of patients with colon cancer have been diagnosed as having stage IV cancer, and the five-year survival rate for patients with stage IV tumors is less than 10%[2]. The metastatic pathways of colon cancer are similar to those of other malignancies, and include lymphatic, hematogenous, and distant metastases. Breast metastasis is rare, with an incidence of 0.5%–3%[3], and is especially rare from colon carcinoma. Since the first case reported in 1976[4], only no more than 30 cases have been reported[5,6]. Hsieh and Hsu[7] summarized 45 cases that were previously reported, and we found that about 60 studies have reported this rare case so far by searching the literature in the Reference Citation Analysis (https://www.referencecitationanalysis.com/) database, PubMed, and Embase. Prognosis is poor because it is usually indicative of disseminated disease. It is important to distinguish breast metastasis from primary breast carcinoma in order to plan the most suitable treatment[8]. Treatment experience is uncomfortable due to the rarity of the metastatic pattern. We report a new case of breast metastasis from colon carcinoma.
A 23-year-old woman was admitted to our hospital in February 2021 for further treatment with a history of acute abdominal pain, nausea, and vomiting.
The patient suddenly suffered from abdominal pain, nausea, and vomiting, without fever, chills, fatigue, melena, haematemesis, hematochezia, or other discomforts.
The patient had no previous medical history.
There was no personal or family history of acute or chronic disease. There was no family history of tumors.
Physical examination revealed abdominal tension, pressing pain, and rebound pain. There was a large abdominal mass with hard texture, unclear boundary, and uncertain size, and tenderness of the left abdomen.
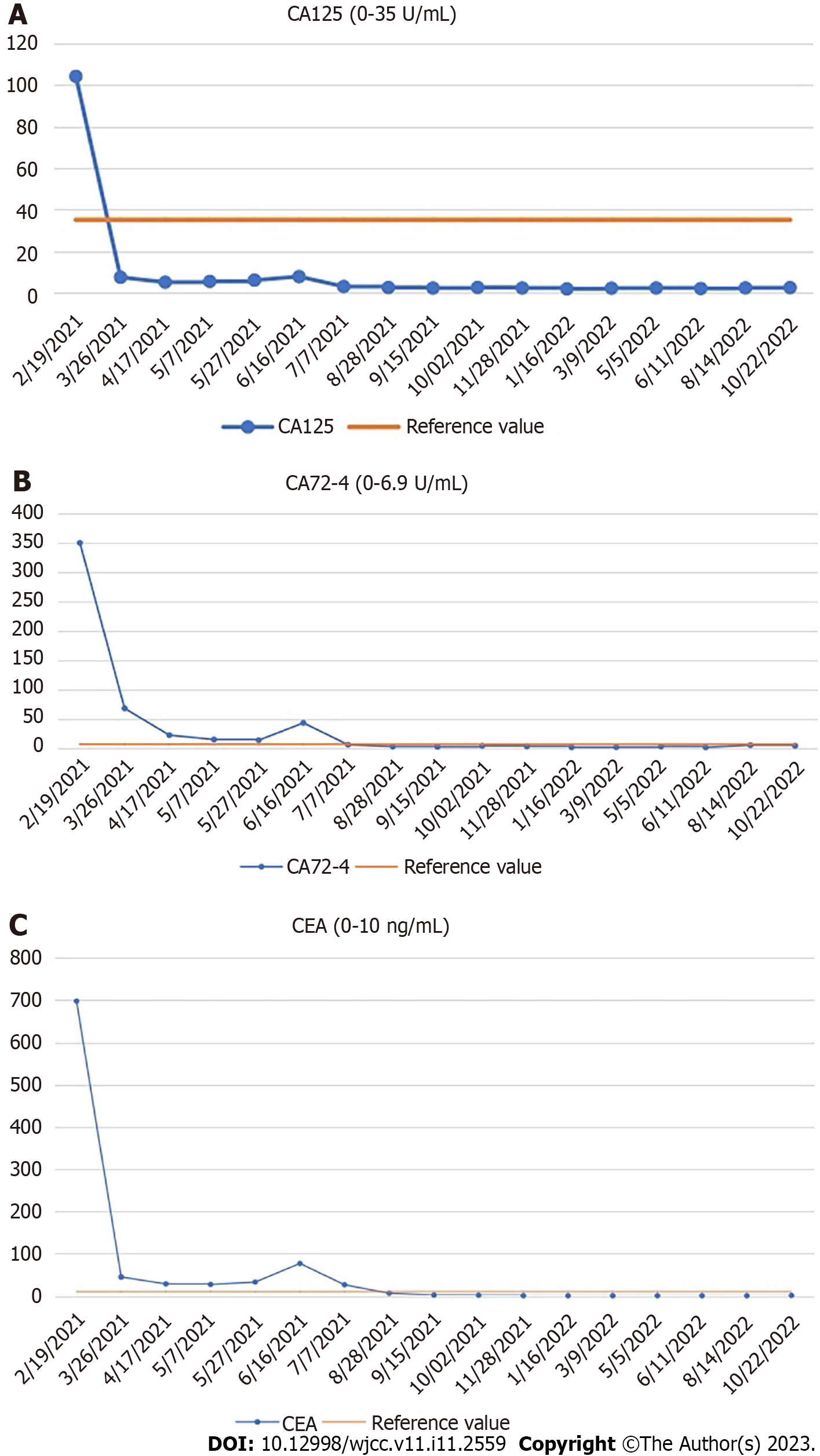
Laboratory examination showed a significant increase in serum levels of tumor markers: Carcinoembryonic antigen (CEA), 697.90 ng/mL (normal range: 0–10 ng/mL); carbohydrate antigen (CA)125, 104.20 U/mL (normal range: 0–35 U/mL); and CA72-4, > 300.0 U/ mL (normal range: 0–6.9 U/mL). The level of CA19-9 (3.49 U/mL) was normal (normal range: 0–27 U/mL).
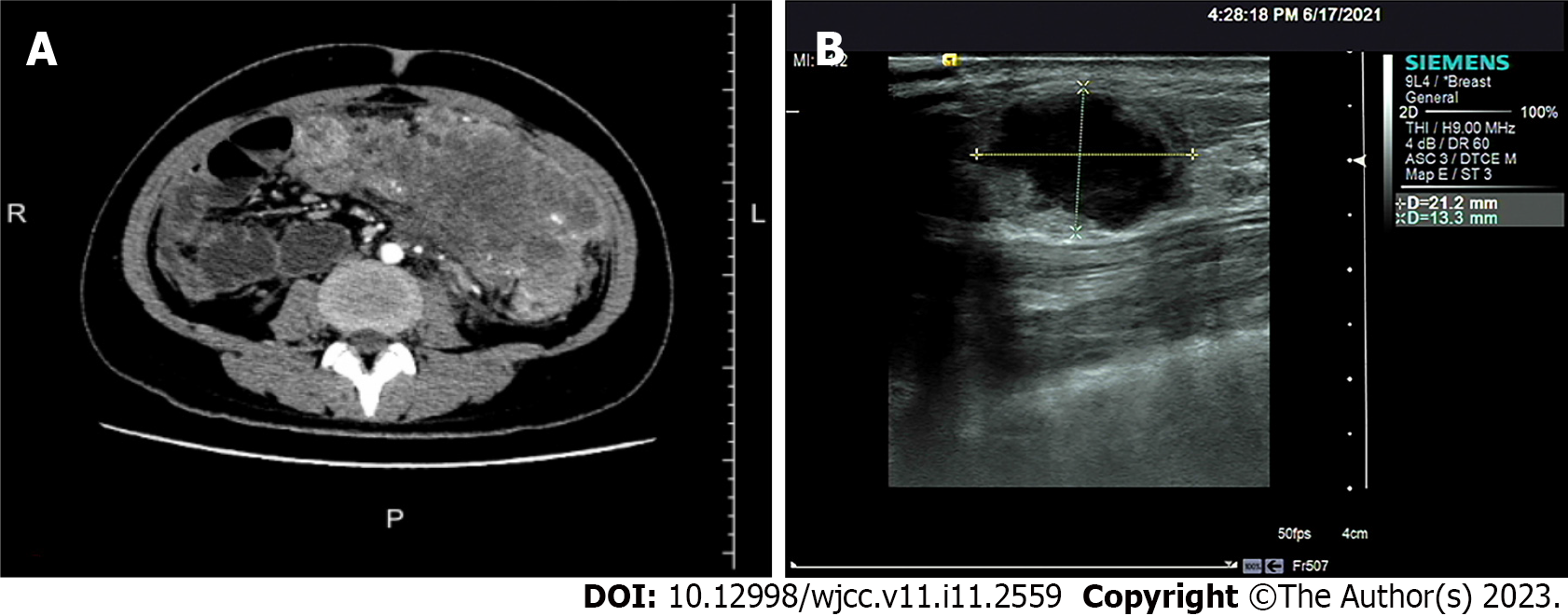
Computed tomography (CT) and abdominal enhanced CT showed that the abdominal tumor measured 7.5 cm × 10.9 cm, with uneven density, and unclear boundary between the tumor and surrounding tissues (Figure 1A). There were swollen lymph nodes in the mesentery. Abdominal enhanced CT was highly suspicious for gastrointestinal stromal tumors, and abdominal and retroperitoneal lymph nodes were swollen. Preoperative chest CT showed no abnormalities.
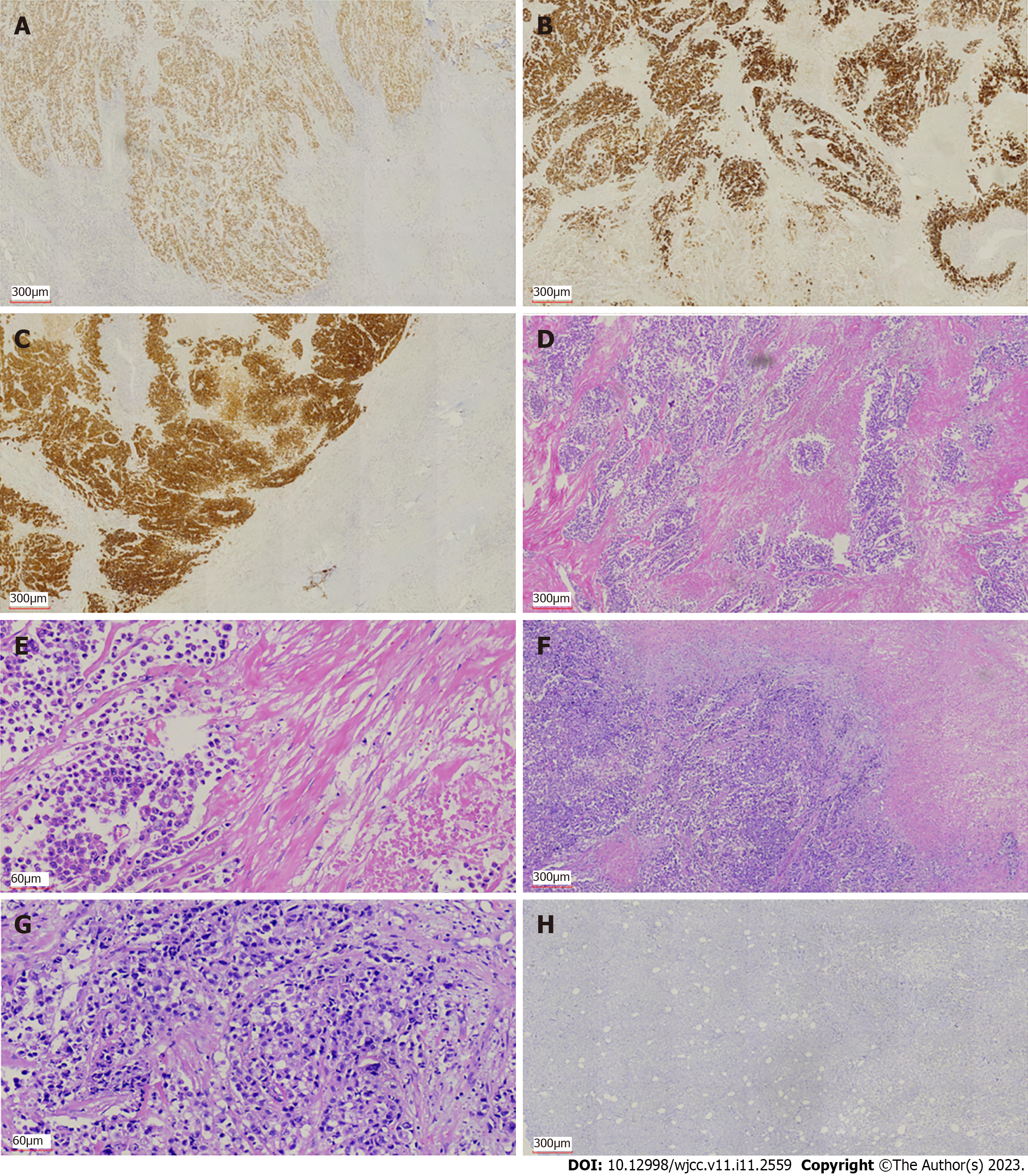
We performed exploratory laparotomy. A tumor with hard texture was located in the middle of the transverse colon, causing peritonitis because of perforation. The diameter of the tumor was approximately 10 cm. Transverse colectomy was successfully performed. The lymph nodes around the transverse colon were cleared, but the retroperitoneal lymph nodes could not be removed. The favorable recovery period for the patient was approximately 2 wk. Immunohistochemistry was negative for CD20 and CD117, but positive for CK20, caudal-related homeobox transcription factor 2 (CDX-2) (Figure 2A), mucin 2 (Figure 2B), villin (Figure 2C), delay of germination 1, leukocyte common antigen, and CD3. Histopathological examination revealed that the tumor was a mucosecretory adenocarcinoma with signet ring cells (Figure 2D and E). Lymph node metastasis was evident (of the 24 detected lymph nodes, 23 were metastatic lymph nodes), but no distant metastasis was detected. The pathological stage was IIIC.
The patient received chemotherapy according to the National Comprehensive Cancer Network guidelines for colorectal cancer. We retested the serum tumor markers after surgery in March 2021, which revealed significant changes: CEA, 44.65 ng/mL (normal range: 0–10 ng/mL); CA125, 7.33 U/mL (normal range: 0–35 U/mL); and CA72-4, 68.0 U/mL (normal range: 0–6.9 U/mL). The level of CA19-9 was normal (< 0.6 U/mL; normal range: 0–27 U/mL). The effect of tumor resection was remarkable. The patient inadvertently found a mass in the outer upper quadrant of the right breast after receiving four cycles of chemotherapy (XELOX: Oxaliplatin 130 mg/m2, d1, iv drip for 2 h; capecitabine 1000 mg/m2, po, bid, d1–d14). Physical examination revealed that the breast mass was 1.5 cm × 2 cm in size, with hard texture, and clear boundary between the mass and surrounding tissues. Further examination was performed. Breast ultrasound showed a tumor of 2.1 cm × 1.5 cm × 1.3 cm, with unclear boundary and echo heterogenicity. Color Doppler flow imaging showed blood flow signal (Figure 1B). Axillary lymph nodes were normal. It was not clear whether the breast tumor was a primary cancer or metastasis from colon cancer. There were slight elevations in serum tumor markers: CEA, 76.7 ng/mL (normal range: 0–10 ng/mL); CA125, 7.66 U/mL (normal range: 0–35 U/mL); and CA72-4, 43.20 U/mL (normal range: 0–6.9 U/mL). The level of CA19-9 (8.83 U/mL; normal range: 0–27 U/mL) was still normal. After discussion with the patient, we decided to perform a lumpectomy and frozen biopsy in June 2021. Frozen biopsy revealed that the breast tumor was intestinal metastasis. Routine hematoxylin and eosin staining of the breast tumor revealed breast metastasis from mucosecretory adenocarcinoma possibly of colonic origin (Figure 2F and G). The tumor was negative for estrogen receptor, progesterone receptor, GATA binding protein-3 (GATA3) (Figure 2H), gross cystic disease fluid protein, human epidermalgrowth factor receptor-2, and CK7, but positive for CDX-2, villin, and special AT-rich binding protein (SATB). GATA3 is an important indicator of primary breast cancer; however, CDX-2, villin, and SATB are important indicators of enterogenous breast cancer. The disease was considered to be disseminated.
Based on the pathological findings and clinical results, the final diagnosis was breast metastasis from transverse colon carcinoma.
Genetic testing showed that RAS and BRAF were wild type. According to the treatment guidelines for distant metastasis of colon cancer, we replaced the original chemotherapy with FOLFIRI + cetuximab [irinotecan 180 mg/m2, d1, iv drip for 30–90 min; leucovorin 400 mg/m2, d1, iv drip for 2 h; 5-fluorouracil (5-FU) 400 mg/m2, d1 and 5-FU 1200 mg/(m2 d) × 2 d, continuous iv drip for 46–48 h; cetuximab 500 mg/m2, d1, iv drip for 2 h]. The serum levels of tumor markers were significantly reduced in July 2021 after one treatment cycle: CEA, 26.20 ng/mL (normal range: 0–10 ng/mL); CA125, 2.84U/mL (normal range: 0–35 U/mL); and CA72-4, 5.96 U/mL (normal range: 0–6.9 U/mL). The level of CA19-9 (7.76 U/mL; normal range: 0–27 U/mL) was still normal. The patient received 10 cycles of FOLFIRI + cetuximab. The serum levels of tumor markers (CEA, CA12-5, CA72-4, and CA19-9) were normal, and there was no evidence of tumor recurrence or metastasis. The patient has been reviewed regularly without further treatment.
The patient did not receive further treatment after 10 cycles of FOLFIRI + cetuximab. During the whole treatment process, the patient underwent chest and abdominal CT examinations every 3 mo, and there was no evidence of distant metastasis. The serum levels of tumor markers (CEA, CA12-5, CA72-4, and CA19-9) were normal (Figure 3). The patient is in no evidence of disease state.
Breast metastasis from colon cancer without liver metastasis, lung metastasis, or peritoneal dissemination is extremely rare. The prognosis for metastatic breast cancer remains poor overall, due to disseminated disease. The breast lump can be mistaken for primary breast cancer[9]. Histopathological examination revealed that the tumor in the present case was a mucosecretory adenocarcinoma with signet ring cells, which is a type of colon cancer with poor prognosis. In addition, there was a large number of lymph node metastases. Metastatic breast cancer appeared about 4 mo after surgery.
In the present case, rapid growth of the breast mass indicated high malignancy of the colon tumor. Signet ring cells were found in the colon and breast tumors. Typically, colorectal carcinoma is positive for CK20, whereas this is exceptional in primary breast carcinoma. Metastatic breast cancer is usually negative for estrogen and progesterone receptors. Aspiration and excision biopsies are feasible for diagnosis of breast metastases. Previously, some authors have advised against extensive resection of metastatic breast tumor[5]. Thus, surgical treatment of metastatic breast tumor should be both diagnostic and palliative. However, when the tumor is large or painful, mastectomy is sometimes required.
Breast metastasis from colon cancer should be considered as systemic metastasis, although the metastatic pathway is not clear. The most common forms of metastasis of colon cancer are lymph node and hematogenous metastases[10]. The lymphatic network of the breast is rich, with a large distribution of lymph flowing to the axillary lymph nodes. The lymphatic drainage of the breast can flow to the parasternal lymphatic vessels through the intercostal lymphatic vessels, or it can flow back to the abdominal organs along the rectus abdominis sheath[11]. Therefore, we consider that there may be two possible pathways for metastasis to the breast from colon carcinoma. The first possibility is that colon cancer cells invade the blood circulation, and tumor cells are planted in the breast and grow into a mass similar to primary breast cancer. Another possibility is that colon cancer cells invade the lymphatic vessels of the abdominal wall and communicate with the lymphatic vessels of the breast along the intercostal lymphatic vessels or along the rectus abdominis sheath. In the present case, we prefer the second explanation. Previous reports have hardly mentioned the route of breast metastasis of colon cancer.
Because of early diagnosis and new therapeutic methods, the overall incidence and mortality of colorectal cancer have declined significantly[12,13]. However, metastasis remains a significant challenge in prolonging survival. In particular, we have no experience in the treatment of breast metastasis from colon cancer, and there is no clear statement in the relevant guidelines. For the treatment of metastatic colorectal cancer, KRAS, NRAS, RAS, and BRAF should be tested first by genetic testing, which is the basis for treatment with cetuximab or bevacizumab. We selected cetuximab according to the results of genetic testing (wild-type KRAS, NRAS, RAS, and BRAF). After we replaced FOLFIRI + cetuximab with XELOX, the serum levels of CEA, CA125, and CA72-4 decreased dramatically. According to the treatment guidelines for distant metastasis of colon cancer, there is no mistake in giving chemotherapy and targeted treatment. Diagnosis of breast metastasis from colon cancer has been reported, but there are few reports on treatment and metastasis route[14,15]. The prognosis of such patients is poor. Barthelmes reported a case of breast metastasis from colon cancer who died 4 mo later from advanced intra-abdominal carcinomatosis[5]. In our case, the patient survived for 16 mo after receiving lumpectomy, which was due to the application of chemotherapeutic and targeted drugs, especially cetuximab. Cetuximab is used for the treatment of metastatic colorectal cancer with overexpression of epidermal growth factor (EGF) receptor and resistance to irinotecan-based chemotherapy. Cetuximab is an immunoglobulin G1 monoclonal antibody against EGF receptor. After specific binding, the two antibodies block the intracellular signal transduction pathway by inhibiting the binding of ligands to EGF receptor, resulting in inhibition of proliferation of cancer cells, inducing apoptosis of cancer cells, and reducing the production of matrix metalloproteinase and vascular endothelial growth factor[16]. Wang et al[6] performed treatment with XELOX chemotherapy regimen in combination with bevacizumab in a patient with breast metastasis from colon cancer for 8 cycles, increasing successful treatment experience for patients with this rare metastasis. This is similar to our research. In addition, most of the other reports only reported the diagnosis of this rare metastasis. The average survival time is 14.9 mo according to the literature[6]. Only one case had a more than 5-year overall survival with colon cancer metastasis to the breast[6]. The patient had a 16-mo disease-free survival in our case, which has exceeded the average level, indicating that our treatment is effective.
Our patient received chemotherapy and targeted therapy after surgery. At the same time, the patient was regularly reviewed, including chest and abdominal CT, and gastrointestinal tumor markers. The dramatic decrease in tumor markers (CEA, CA125, and CA72-4) was due to the removal of colon tumor after transverse colectomy. When breast metastasis occurs, tumor markers begin to increase. However, in our case, the levels of tumor markers (CEA, CA125, and CA72-4) decreased and remained within the normal range after treatment with FOLFIRI + cetuximab. CEA is usually highly expressed in digestive system tumors, especially in some patients with advanced gastric cancer and colorectal cancer[17]. As a specific tumor marker, CA72-4 is used to monitor the recurrence and prognosis of gastric cancer. CA125 is a sensitive marker for ovarian cancer[18], and is also elevated in gastrointestinal tumors. CA125 seems to be a better tumor marker than CEA for predicting progressive disease in colorectal cancer in both men and women[19]. The changes of CEA, CA125, and CA72-4 reflected disease progression and effect of treatment in our patient. These three tumor markers play an important role in evaluating disease status, tumor recurrence, tumor metastasis, and prognosis of gastrointestinal cancer patients. They also indicate postoperative follow-up of patients with gastrointestinal tumors. We will continue to evaluate the condition of the patient by monitoring the levels of tumor markers and CT changes, which are beneficial for treatment planning.
Breast metastasis from colon cancer is a manifestation of advanced cancer. There are a variety of treatment methods, but they are complex and difficult. In addition to chemotherapy and targeted therapy, immunotherapy may also become a new choice, which needs further clinical research. For this patient, we will continue to observe the effect of treatment by follow-up and decide whether to give further treatment according to the review results.
Breast metastasis from colon cancer is rare. Typically, it has a poor prognosis because it is associated with disseminated metastatic disease. Radical breast surgery should be avoided unless needed for palliation. The surgical treatment of a metastatic breast tumor should be both diagnostic and palliative. Chemotherapy combined with targeted therapy should be the first choice. The mechanism of metastasis and better treatment need to be studied.
| 1. | Taieb J, Karoui M, Basile D. How I treat stage II colon cancer patients. ESMO Open. 2021;6:100184. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 2. | Wang Z, Song J, Azami NLB, Sun M. Identification of a Novel Immune Landscape Signature for Predicting Prognosis and Response of Colon Cancer to Immunotherapy. Front Immunol. 2022;13:802665. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 3. | Tsung SH. Metastasis of Colon Cancer to the Breast. Case Rep Oncol. 2017;10:77-80. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 4. | McIntosh IH, Hooper AA, Millis RR, Greening WP. Metastatic carcinoma within the breast. Clin Oncol. 1976;2:393-401. [PubMed] |
| 5. | Arnaout K, Hawa N, Agha S, Kadoura L, Aloulou M, Ayoub K. A case report of multiple bilateral breast metastases after colorectal cancer. Int J Surg Case Rep. 2021;81:105759. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Wang X, Zhang H, Lu Y. Breast metastasis of signet ring cell carcinoma from the colon: a case report. World J Surg Oncol. 2022;20:376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 7. | Hsieh TC, Hsu CW. Breast metastasis from colorectal cancer treated by multimodal therapy: Case report and literature review. Medicine (Baltimore). 2019;98:e18016. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 8. | Fernández de Bobadilla L, García Villanueva A, Collado M, de Juan A, Rojo R, Pérez J, Lisa E, Aguilera A, Mena A, González-Palacios F. Breast metastasis of primary colon cancer. Rev Esp Enferm Dig. 2004;96:415-7; 418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 9. | Taccogna S, Gozzi E, Rossi L, Caruso D, Conte D, Trenta P, Leoni V, Tomao S, Raimondi L, Angelini F. Colorectal cancer metastatic to the breast: A case report. World J Gastrointest Oncol. 2020;12:1073-1079. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 10. | Gallagher DJ, Kemeny N. Metastatic colorectal cancer: from improved survival to potential cure. Oncology. 2010;78:237-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 179] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 11. | Lane K, Worsley D, McKenzie D. Exercise and the lymphatic system: implications for breast-cancer survivors. Sports Med. 2005;35:461-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 60] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 12. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9172] [Cited by in RCA: 9981] [Article Influence: 907.4] [Reference Citation Analysis (15)] |
| 13. | Zhao L, Xu J, Liang F, Li A, Zhang Y, Sun J. Effect of Chronic Psychological Stress on Liver Metastasis of Colon Cancer in Mice. PLoS One. 2015;10:e0139978. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 14. | Mihai R, Christie-Brown J, Bristol J. Breast metastases from colorectal carcinoma. Breast. 2004;13:155-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 40] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 15. | Wakeham NR, Satchithananda K, Svensson WE, Barrett NK, Comitis S, Zaman N, Ralleigh G, Sinnett D, Shousha S, Lim AK. Colorectal breast metastases presenting with atypical imaging features. Br J Radiol. 2008;81:e149-e153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 16. | Kopetz S, Guthrie KA, Morris VK, Lenz HJ, Magliocco AM, Maru D, Yan Y, Lanman R, Manyam G, Hong DS, Sorokin A, Atreya CE, Diaz LA, Allegra C, Raghav KP, Wang SE, Lieu CH, McDonough SL, Philip PA, Hochster HS. Randomized Trial of Irinotecan and Cetuximab With or Without Vemurafenib in BRAF-Mutant Metastatic Colorectal Cancer (SWOG S1406). J Clin Oncol. 2021;39:285-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 183] [Article Influence: 36.6] [Reference Citation Analysis (0)] |
| 17. | Zheng TH, Zhao JL, Guleng B. Advances in Molecular Biomarkers for Gastric Cancer. Crit Rev Eukaryot Gene Expr. 2015;25:299-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (1)] |
| 18. | Bast RC Jr, Feeney M, Lazarus H, Nadler LM, Colvin RB, Knapp RC. Reactivity of a monoclonal antibody with human ovarian carcinoma. J Clin Invest. 1981;68:1331-1337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1132] [Cited by in RCA: 1136] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 19. | Huang CJ, Jiang JK, Chang SC, Lin JK, Yang SH. Serum CA125 concentration as a predictor of peritoneal dissemination of colorectal cancer in men and women. Medicine (Baltimore). 2016;95:e5177. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D, D, D
Grade E (Poor): 0
P-Reviewer: Dambrauskas Z, Lithuania; Faraji N, Iran; Pattarajierapan S, Thailand S-Editor: Zhao S L-Editor: Wang TQ P-Editor: Zhao S