Published online Apr 16, 2023. doi: 10.12998/wjcc.v11.i11.2452
Peer-review started: August 13, 2022
First decision: November 11, 2022
Revised: February 22, 2023
Accepted: March 17, 2023
Article in press: March 17, 2023
Published online: April 16, 2023
Processing time: 236 Days and 5.2 Hours
IFIH1 is a protein-coding gene. Disorders associated with IFIH1 include Aicardi-Goutières syndrome (AGS) type 7 and Singleton-Merten syndrome type 1. Related pathways include RIG-I/MDA5-mediated induction of the interferon (IFN)-α/β pathway and the innate immune system. AGS type 7 is an autosomal dominant inflammatory disorder characterized by severe neurological impairment. In infancy, most patients present with psychomotor retardation, axial hypotonia, spasticity, and brain imaging changes Laboratory assessments showed increased IFN-α activity with upregulation of IFN signaling and IFN-stimulated gene expression. Some patients develop normally in the early stage, and then have episodic neurological deficits.
The 5-year-old girl presented with postpartum height and weight growth retardation, language retardation, brain atrophy, convulsions, and growth hormone deficiency. DNA samples were obtained from peripheral blood from the child and her parents for whole-exome sequencing and test of genome-wide copy number variation. Heterozygous mutations in the IFIH1 gene were found. Physical examination at admission found that language development was delayed, the reaction to name calling was average, there was no communication with people, but there was eye contact, no social smile, and no autonomous language. However, the child had rich gesture language and body language, could understand instructions, had bad temper. When she wants to achieve something, she starts crying or shouting. Cardiopulmonary examination showed no obvious abnormality, and abdominal examination was normal. Bilateral muscle strength and muscle tone were symmetrical and slightly decreased. Physiological reflexes exist, but pathological reflexes were not elicited.
We reported the clinical characteristics of a Chinese child with a clinical diagnosis of AGS type 7, which expanded the mutational spectrum of the IFIH1 gene.
Core Tip: We report a 5-year-old girl with Aicardi-Goutières syndrome type 7. The clinical characteristics included postnatal height and weight retardation, delayed language development, brain atrophy, convulsions, and growth hormone deficiency. Whole exome test showed c.1093A>G (p.Lys365Glu) and heterozygous mutation in IFIH1 gene, and the mutation source was her father. This report provides a molecular basis for etiological diagnosis and treatment of the child, as well as for genetic counseling for the pedigree.
- Citation: Lin SZ, Yang JJ, Xie TL, Li JY, Ma JQ, Wu S, Wang N, Wang YJ. Aicardi-Goutières syndrome type 7 in a Chinese child: A case report. World J Clin Cases 2023; 11(11): 2452-2456
- URL: https://www.wjgnet.com/2307-8960/full/v11/i11/2452.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i11.2452
Aicardi-Goutières syndrome (AGS) is a rare, genetically determined early-onset progressive encephalopathy[1-4]. Individuals affected with AGS typically suffer from progressive microcephaly associated with severe neurological symptoms, such as hypotonia, dystonia, seizures, spastic quadriplegia[5,6], and severe developmental delay. On brain imaging, AGS is characterized by basal ganglia calcification, white matter abnormalities, and cerebral atrophy cerebrospinal fluid analyses show chronic lymphocytosis and elevated levels of the interferon (IFN)-α[7] and neopterin. AGS-affected individuals are often misdiagnosed as having intrauterine infections, such as Pseudo-TORCH syndrome[8] (Pseudo-TORCH syndrome is a rare, chronic disorder that is characterised by dimorphic features such as microcephaly, intracranial calcification, seizures, mental retardation, hepatosplenomegaly and coagulation disorders), because of the similarities of these disorders, particularly the intracranial calcifications. AGS type 7 (AGS7) is an autosomal dominant inflammatory disorder characterized by severe neurological impairment. Episodic neurological deficits occur after the onset. Therefore, diseases related to mutations at the genetic locus must be diagnosed in the early phase so as to be treated in timely.
She was 3 years old at the time of her first visit and presented on 16 September 2020 due to language delay. She is 5 years old now.
Since age 6 mo, she had had obvious slow growth in height and weight. Language development was delayed. Her response to name calling was normal but there was no communication with people. Her eyes could not be met and there was no social smile. There was no autonomous language, However, the child had rich gesture language and body language, she could understand instructions, had a short temper, and when she wants to achieve something, she starts crying or shouting. She was thin, with a weight of 9 kg and height of 83 cm.
There was no obvious abnormality at birth, weight 3 kg, height 50 cm. She raised her head at 3 mo, crawled at 7 mo, and walked at 15 mo. She had a history of febrile convulsions twice, each lasting about 2 min, which resolved spontaneously, and a 1-year history of ulcerative colitis.
Both parents were healthy.
Body temperature was 36.2 °C, heart rate 96 beats/min, breathing 24 beats/min, blood pressure 100/60 mmHg, height 83.0 cm and weight 9 kg. There was no special sick face, and cardiopulmonary and abdominal examinations showed no obvious abnormalities. Bilateral muscle strength and muscle tone were symmetrical and slightly decreased. Physiological reflexes were present but pathological reflexes were not elicited.
Routine blood and urine examinations, myocardial enzymes, lactic acid, liver function, renal function, electrolytes, blood glucose, thyroid function, and hematuria showed no obvious abnormalities. The peak growth hormone challenge test was 5.77 ng/mL.
Video electroencephalography showed no abnormalities. Magnetic resonance imaging (MRI) of the brain showed mild atrophy.
AGS7 caused by a missense mutation in the IFIH1 gene.
Symptomatic nutritional support and rehabilitation were undertaken.
The child was followed up for 1 year and 9 mo. Her weight increased to 12 kg and height to 90 cm. Language ability improved, and simple conversations could be conducted.
Our patient presented with growth retardation, language retardation, brain atrophy and convulsions as the main clinical manifestations. Among the various examination indicators of our patient, we particularly noticed that the peak value of growth hormone was decreased, and MRI of the brain showed brain atrophy, Combined with the patient's clinical symptoms and physical and chemical examination results, we considered the possibility of hereditary metabolic disease or genetic disease. Subsequent genetic metabolic screening for hematuria showed no obvious abnormality.
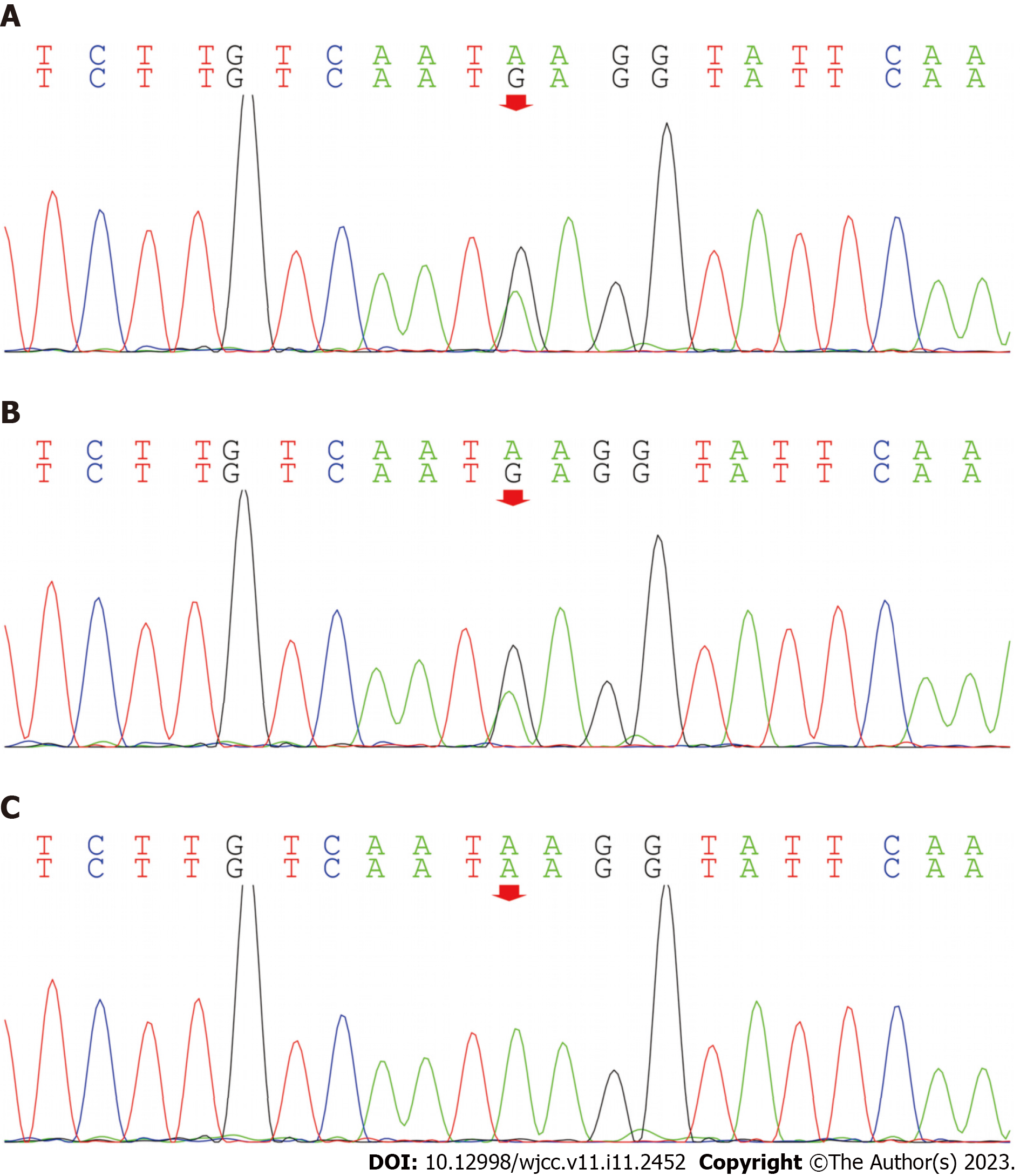
Whole exome analysis showed that the IFIH1 gene had a c.1093A>G (p.Lys365Glu) heterozygous mutation, and the source of the mutation was her father (Figure 1A-C). IFIH1 gene can cause two diseases, AGS7, and Singleton-Merten syndrome type 1. According to their clinical manifestations, children are more likely to have AGS7[1]. AGS (MIM 615846) is a rare genetic disorder characterized by aberrant type 1 IFN production and systemic, chronic inflammation. IFIH1 gene[2-4] may lead to changes in MDA5[7] and type 1 IFN (Mutations in the IFIH1 gene lead to overproduction of type 1 interferons, resulting in the AGS phenotype). AGS 7 may lead to an increase in type 1 IFN, and there are clinical manifestations caused by elevated type 1 IFN. Our patient had similar manifestations, but due to the patient’s clinical conditions, we did not examine the IFN concentration.
It has been reported that the IFIH1 gene is inherited in an autosomal dominant manner, with high heterogeneity and incomplete penetrance. Our patient had c.1093A>G (p.Lys365Glu) heterozygous mutation, and the pathogenicity of this variant has been reported previously. The clinical manifestations of the child were consistent with the clinical features of AGS7.
In summary, we reported the clinical characteristics of a Chinese child with a clinical diagnosis of AGS7, which expanded the mutational spectrum of the IFIH1 gene.
| 1. | Oda H, Nakagawa K, Abe J, Awaya T, Funabiki M, Hijikata A, Nishikomori R, Funatsuka M, Ohshima Y, Sugawara Y, Yasumi T, Kato H, Shirai T, Ohara O, Fujita T, Heike T. Aicardi-Goutières syndrome is caused by IFIH1 mutations. Am J Hum Genet. 2014;95:121-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 177] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 2. | Adang LA, Frank DB, Gilani A, Takanohashi A, Ulrick N, Collins A, Cross Z, Galambos C, Helman G, Kanaan U, Keller S, Simon D, Sherbini O, Hanna BD, Vanderver AL. Aicardi goutières syndrome is associated with pulmonary hypertension. Mol Genet Metab. 2018;125:351-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 45] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 3. | Amari S, Tsukamoto K, Ishiguro A, Yanagi K, Kaname T, Ito Y. An extremely severe case of Aicardi-Goutières syndrome 7 with a novel variant in IFIH1. Eur J Med Genet. 2020;63:103646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 4. | Rice GI, Del Toro Duany Y, Jenkinson EM, Forte GM, Anderson BH, Ariaudo G, Bader-Meunier B, Baildam EM, Battini R, Beresford MW, Casarano M, Chouchane M, Cimaz R, Collins AE, Cordeiro NJ, Dale RC, Davidson JE, De Waele L, Desguerre I, Faivre L, Fazzi E, Isidor B, Lagae L, Latchman AR, Lebon P, Li C, Livingston JH, Lourenço CM, Mancardi MM, Masurel-Paulet A, McInnes IB, Menezes MP, Mignot C, O'Sullivan J, Orcesi S, Picco PP, Riva E, Robinson RA, Rodriguez D, Salvatici E, Scott C, Szybowska M, Tolmie JL, Vanderver A, Vanhulle C, Vieira JP, Webb K, Whitney RN, Williams SG, Wolfe LA, Zuberi SM, Hur S, Crow YJ. Gain-of-function mutations in IFIH1 cause a spectrum of human disease phenotypes associated with upregulated type I interferon signaling. Nat Genet. 2014;46:503-509. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 401] [Cited by in RCA: 497] [Article Influence: 41.4] [Reference Citation Analysis (0)] |
| 5. | Liu N, Chen J, Xu C, Shi T, Li J. Hereditary spastic paraplegia associated with a rare IFIH1 mutation: a case report and literature review. Hereditas. 2019;156:28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 6. | Crow YJ, Zaki MS, Abdel-Hamid MS, Abdel-Salam G, Boespflug-Tanguy O, Cordeiro NJ, Gleeson JG, Gowrinathan NR, Laugel V, Renaldo F, Rodriguez D, Livingston JH, Rice GI. Mutations in ADAR1, IFIH1, and RNASEH2B presenting as spastic paraplegia. Neuropediatrics. 2014;45:386-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 67] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 7. | Lamborn IT, Jing H, Zhang Y, Drutman SB, Abbott JK, Munir S, Bade S, Murdock HM, Santos CP, Brock LG, Masutani E, Fordjour EY, McElwee JJ, Hughes JD, Nichols DP, Belkadi A, Oler AJ, Happel CS, Matthews HF, Abel L, Collins PL, Subbarao K, Gelfand EW, Ciancanelli MJ, Casanova JL, Su HC. Recurrent rhinovirus infections in a child with inherited MDA5 deficiency. J Exp Med. 2017;214:1949-1972. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 114] [Cited by in RCA: 120] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 8. | Meuwissen ME, Schot R, Buta S, Oudesluijs G, Tinschert S, Speer SD, Li Z, van Unen L, Heijsman D, Goldmann T, Lequin MH, Kros JM, Stam W, Hermann M, Willemsen R, Brouwer RW, Van IJcken WF, Martin-Fernandez M, de Coo I, Dudink J, de Vries FA, Bertoli Avella A, Prinz M, Crow YJ, Verheijen FW, Pellegrini S, Bogunovic D, Mancini GM. Human USP18 deficiency underlies type 1 interferonopathy leading to severe pseudo-TORCH syndrome. J Exp Med. 2016;213:1163-1174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 177] [Cited by in RCA: 230] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Genetics and heredity
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Mirsalehi M, Iran S-Editor: Gong ZM L-Editor: A P-Editor: Gong ZM