Published online Apr 16, 2023. doi: 10.12998/wjcc.v11.i11.2423
Peer-review started: December 21, 2022
First decision: January 11, 2023
Revised: January 25, 2023
Accepted: March 17, 2023
Article in press: March 17, 2023
Published online: April 16, 2023
Processing time: 106 Days and 8.7 Hours
Hepatobiliary scintigraphy (HBS) is a useful diagnostic imaging technique that uses radiotracers to evaluate the function of the gallbladder (GB) and biliary system. In segmented GB, some HBS images reveal a discordant GB boundary as compared to anatomical images.
To evaluate the characteristics of HBS in segmented GB and determine the clinical relevance according to HBS characteristics.
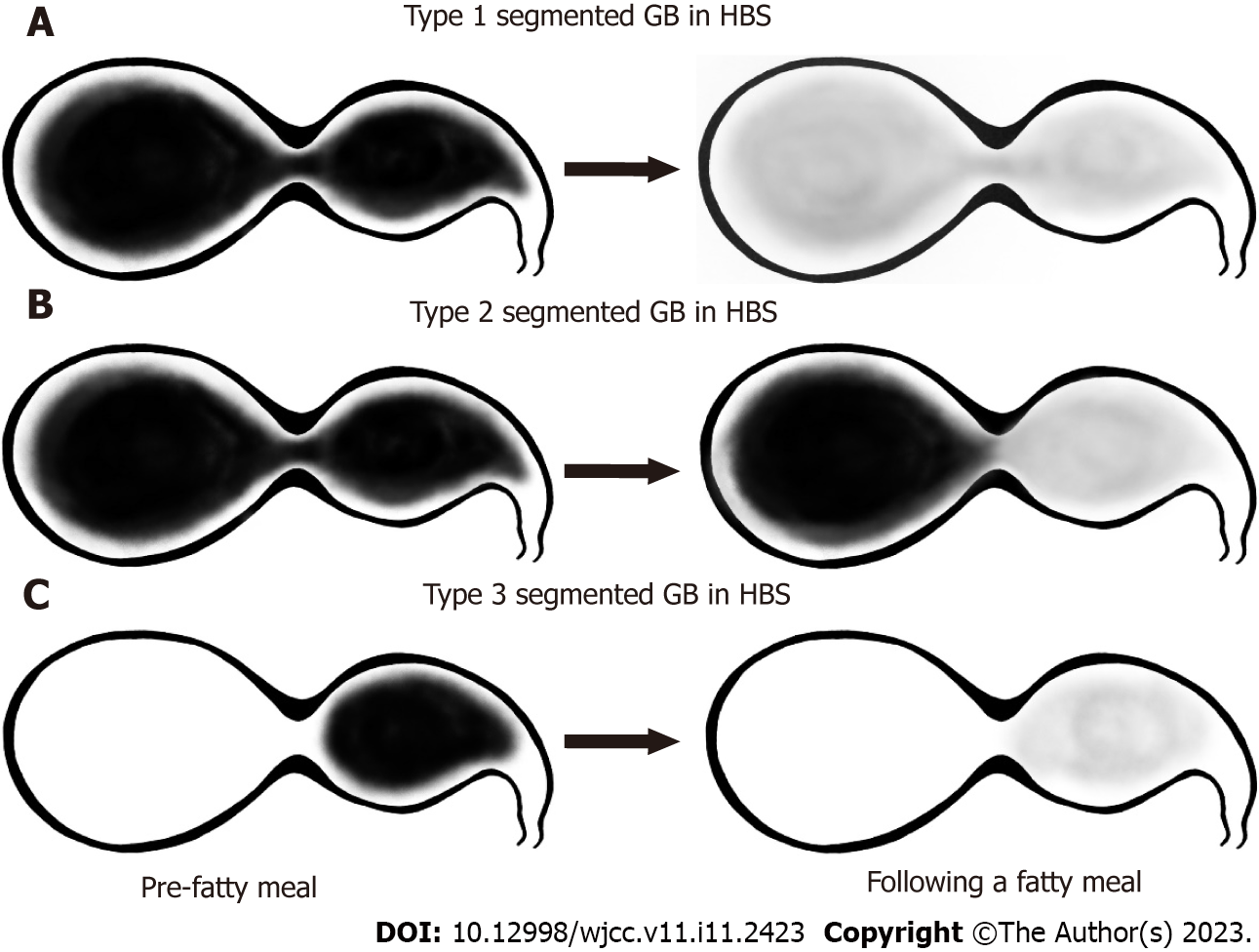
A total of 268 patients with chronic cholecystitis, gallstones, or biliary colic symptoms who underwent HBS between 2011 and 2020 were enrolled. Segmented GB was defined as segmental luminal narrowing of the GB body on computed tomography (CT) or magnetic resonance (MR) images, and HBS was examined 1 mo before or after CT or MR. Segmented GB was classified into 3 types based on the filling and emptying patterns of the proximal and distal segments according to the characteristics of HBS images, and GB ejection fraction (GBEF) was identified: Type 1 was defined as a normal filling and emptying pattern; Type 2 was defined as an emptying defect on the distal segment; and Type 3 was defined as a filling defect in the distal segment.
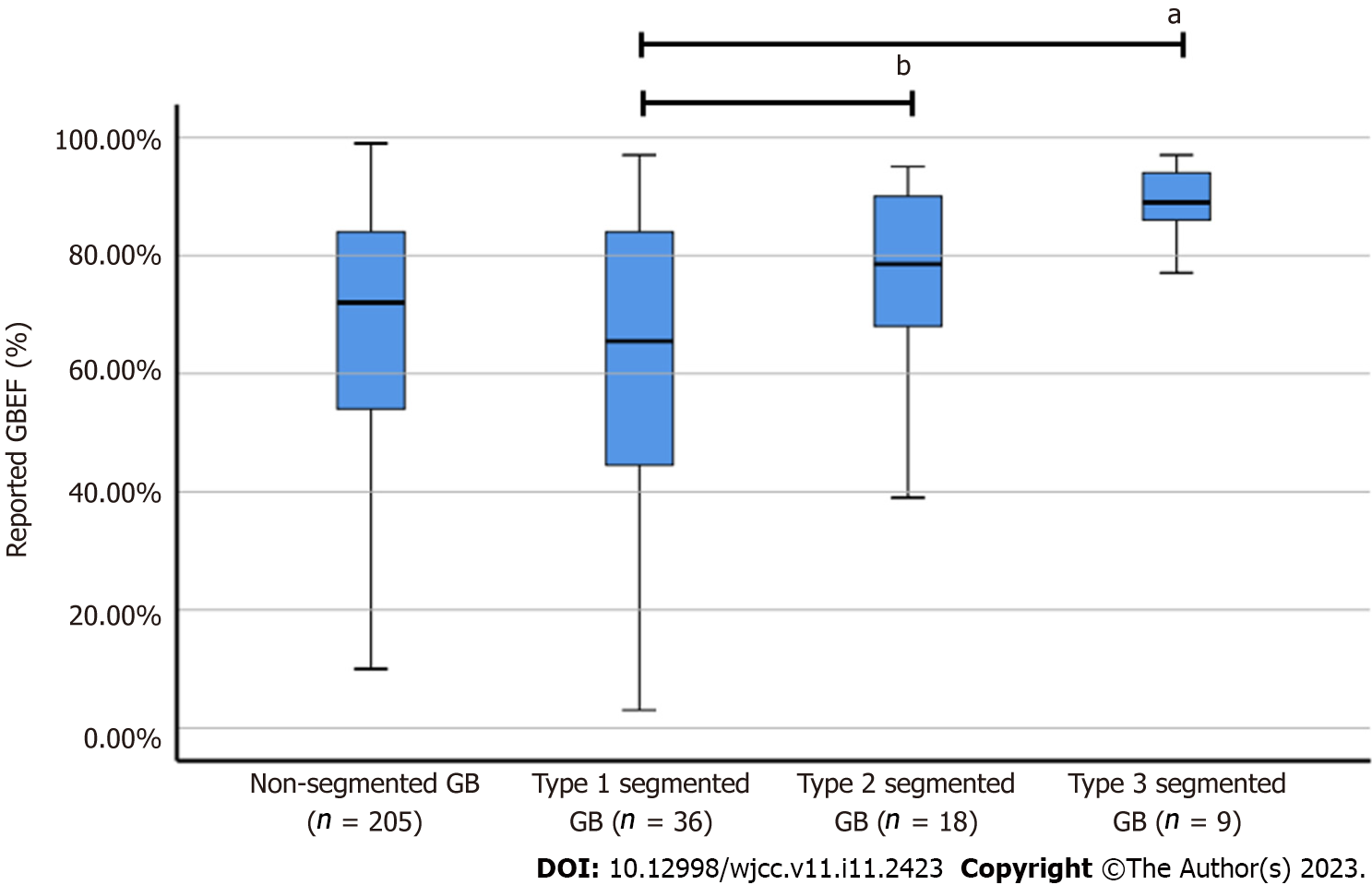
Segmented GB accounted for 63 cases (23.5%), including 36 patients (57.1%) with Type 1, 18 patients (28.6%) with Type 2, and 9 patients (14.3%) with Type 3 emptying pattern. Thus, approximately 43% of HBS images showed a discordant pattern as compared to anatomical imaging of segmented GB. Although there were no significant differences in clinical symptoms, rate of cholecystectomy, or pathological findings based on the type, most gallstones occurred in the distal segment. Reported GBEF was 62.50% ± 24.79% for Type 1, 75.89% ± 17.21% for Type 2, and 88.56% ± 7.20% for Type 3. Type 1 showed no difference in reported GBEF compared to the non-segmented GB group (62.50% ± 24.79% vs 67.40% ± 21.78%). In contrast, the reported GBEF was higher in Types 2 and 3 with defective emptying and filling when compared to Type 1 (80.11% ± 15.70% vs 62.57% ± 24.79%; P = 0.001).
In segmented GB, discordance in the filling patterns detected by HBS and anatomical imaging could lead to misinterpretation of GBEF. For this reason, clinicians should be cautious when interpreting HBS results in patients with segmented GB.
Core Tip: This retrospective study aimed to evaluate the clinical relevance of discrepancies between anatomical and hepatobiliary scintigraphy (HBS) images in patients with segmented gallbladder (GB). HBS images of segmented GB were classified according to filling and emptying patterns: Type 1 was a normal pattern; Type 2 was an emptying defect on the distal segment; and Type 3 was a filling defect in the distal segment. Types 2 and 3 had higher GB ejection fraction measurements than Type 1. Therefore, clinicians should be cautious when interpreting the results of HBS in patients with segmented GB.
- Citation: Lee YC, Jung WS, Lee CH, Kim SH, Lee SO. Classification of hepatobiliary scintigraphy patterns in segmented gallbladder according to anatomical discordance. World J Clin Cases 2023; 11(11): 2423-2434
- URL: https://www.wjgnet.com/2307-8960/full/v11/i11/2423.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i11.2423
The gallbladder (GB) is a single pear-shaped chamber mainly located in a shallow depression below the right lobe of the liver. The GB can be anatomically divided into 4 parts: Fundus, body, infundibulum, and neck. The neck is connected to the common bile duct via the cystic duct. The liver secretes approximately 600 mL of bile per day (0.4 mL/min)[1], a major portion of which enters the GB during fasting with the rest bypassing the GB and entering the duodenum. In the basal state, bile moves within the GB from the long central axis toward the periphery in a lamellar fashion within approximately 30 min[2].
A segmented GB refers to a hyperplastic condition of uncertain etiology in which the organ is divided into two chambers by a fold or septum[3,4]. Nevertheless, it is unknown how GB segmentation affects hepatic bile entry and exit from the two segments. Studies have demonstrated a relationship between segmented GB and the development of cholestasis[4]. A few studies have proposed a relationship between cholestasis and GB cancer[4-6]. Thus, it is hypothesized that segmented GB influences bile flow and as such may be associated with GB-related diseases. Therefore, cholecystectomy can be considered for the treatment of segmented GB.
Hepatobiliary scintigraphy (HBS) is a diagnostic technique used in nuclear medicine to continuously capture the pattern of radiotracers ingested by hepatocytes and secreted together with bile acid. The radiotracer is administered intravenously, bound to albumin, transported to the liver, and drained into the duodenum through the GB and bile ducts. Normal hepatobiliary findings are characterized by the presence of hepatic parenchyma and rapid clearance of cardiac hematologic activity, followed sequentially by activities in the intrahepatic and extrahepatic biliary ductal system, GB, and upper small bowel within approximately 1 h[7]. Abdominal ultrasonography (US), computed tomography (CT), or magnetic resonance cholangiopancreatography (MRCP) can provide morphological information to aid in the diagnosis of hepatobiliary disease, whereas HBS can be used to delineate hepatic and GB function and the degree of cholestasis by tracking radiotracers in the bilirubin metabolic pathway into the bile duct[8].
HBS is used to assess the adequacy of GB contraction and calculate the GB ejection fraction (GBEF) to determine the need for surgery in patients with chronic cholecystitis and GB dyskinesia, and also in symptomatic patients without gallstones[8-11]. An absolute ejection fraction cutoff for surgery has not been definitively established, but has historically been suggested to be approximately 40%[10]. Because clinicians usually make the decision for surgery based on these results and patient symptoms, accurate measurement of GBEF is mandatory. However, it is generally accepted that scintigraphic findings are not always specific. Therefore, it is crucial to correlate HBS findings with clinical information and findings from other imaging modalities to arrive at optimal management strategy[7].
In segmented GB, changes in bile flow may affect HBS results. Some HBS images revealed discordant GB filling and emptying patterns when compared to anatomical images obtained using CT or MR techniques. Changes in these patterns may influence the interpretation of HBS findings and lead to miscalculation of GBEF. However, there are no studies on the clinical significance of HBS in segmented GB with a reasonable classification of the HBS pattern. Therefore, this study aimed to evaluate HBS image features in a segmented GB and determine the clinical impact of these features.
We performed a retrospective review of patients who underwent HBS at Jeonbuk National University Hospital from 2011 to 2020. Baseline demographic and clinical characteristics on biliary colic symptoms (right upper quadrant and epigastric pain or discomfort or postprandial discomfort) and atypical abdominal symptoms (dyspepsia or abdominal discomfort in an uncertain location) were obtained. In addition, the presence and location of any gallstones, sludge, or polyps was assessed. Laboratory testing [alkaline phosphatase, gamma (γ)-glutamyl transferase, aspartate aminotransferase, alanine aminotransferase, total bilirubin, direct bilirubin, carcinoembryonic antigen, and carbohydrate antigen 19-9] was performed near the date of HBS. Morphological characteristics of the GB were verified by CT or MRCP. The interval between CT or MRCP and HBS was no longer than 1 mo. GBEF and scanned images of the radiotracers were validated using HBS. This study was approved by the Institutional Review Board of Jeonbuk National University Hospital (IRB No. 2021-07-005).
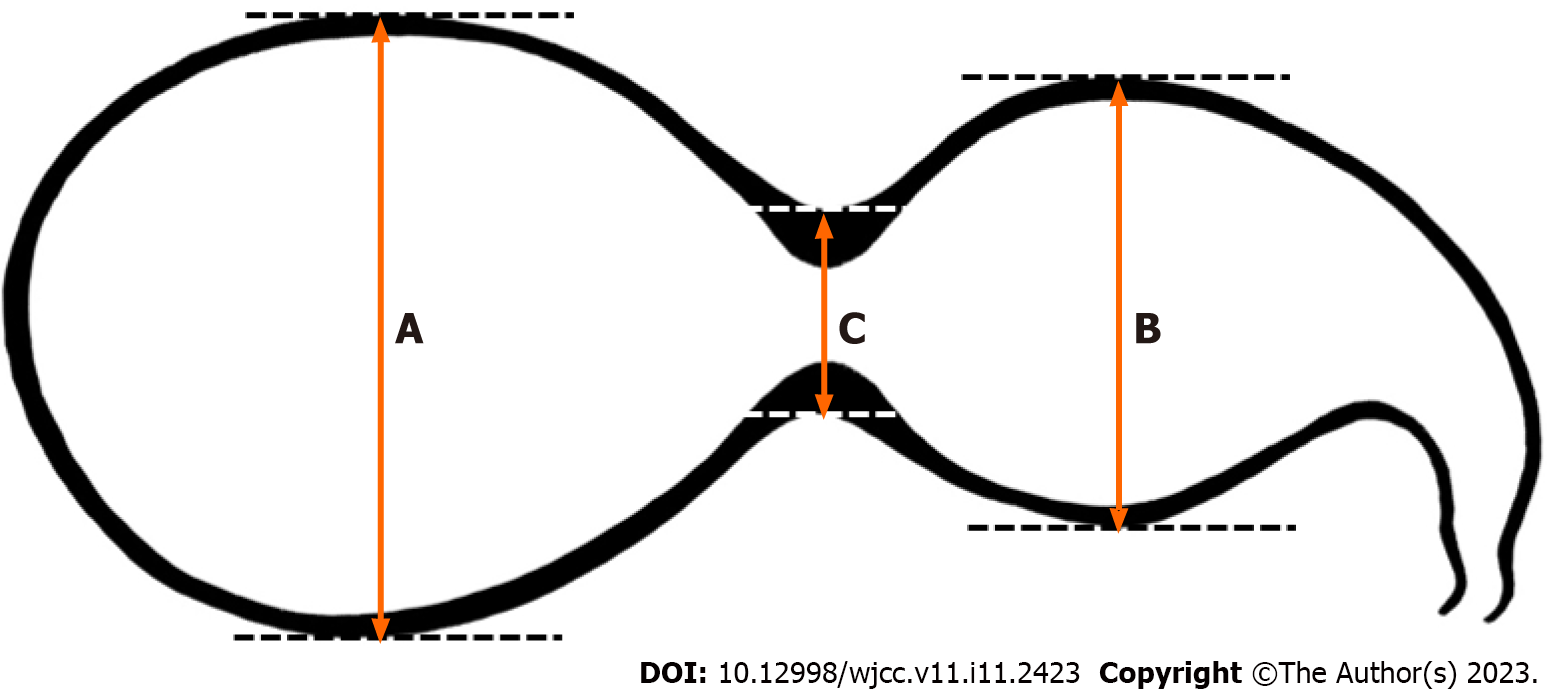
A segmented GB was defined as segmental luminal narrowing of the GB body observed on CT or MR imaging. Segmented GB contains a fold or septum that divides the GB lumen into two or more interconnected compartments: A neck proximal to the stricture and a fundus distal to the stricture; determination of the presence of a septum and compartments is based on a review of CT or MR images. A new approach was proposed to define segmented GB (Figure 1). The segmented GB was first defined as:
A + B > 3C
where A and B denote the long axes of the outer lumen of the distal and proximal portions, respectively. C is defined as the diameter of luminal narrowing based on the outer lumen of the GB body. This calculation was made after observing a segmented GB on CT or MR images and HBS images of an enrolled patient and measuring each GB area of that patient.
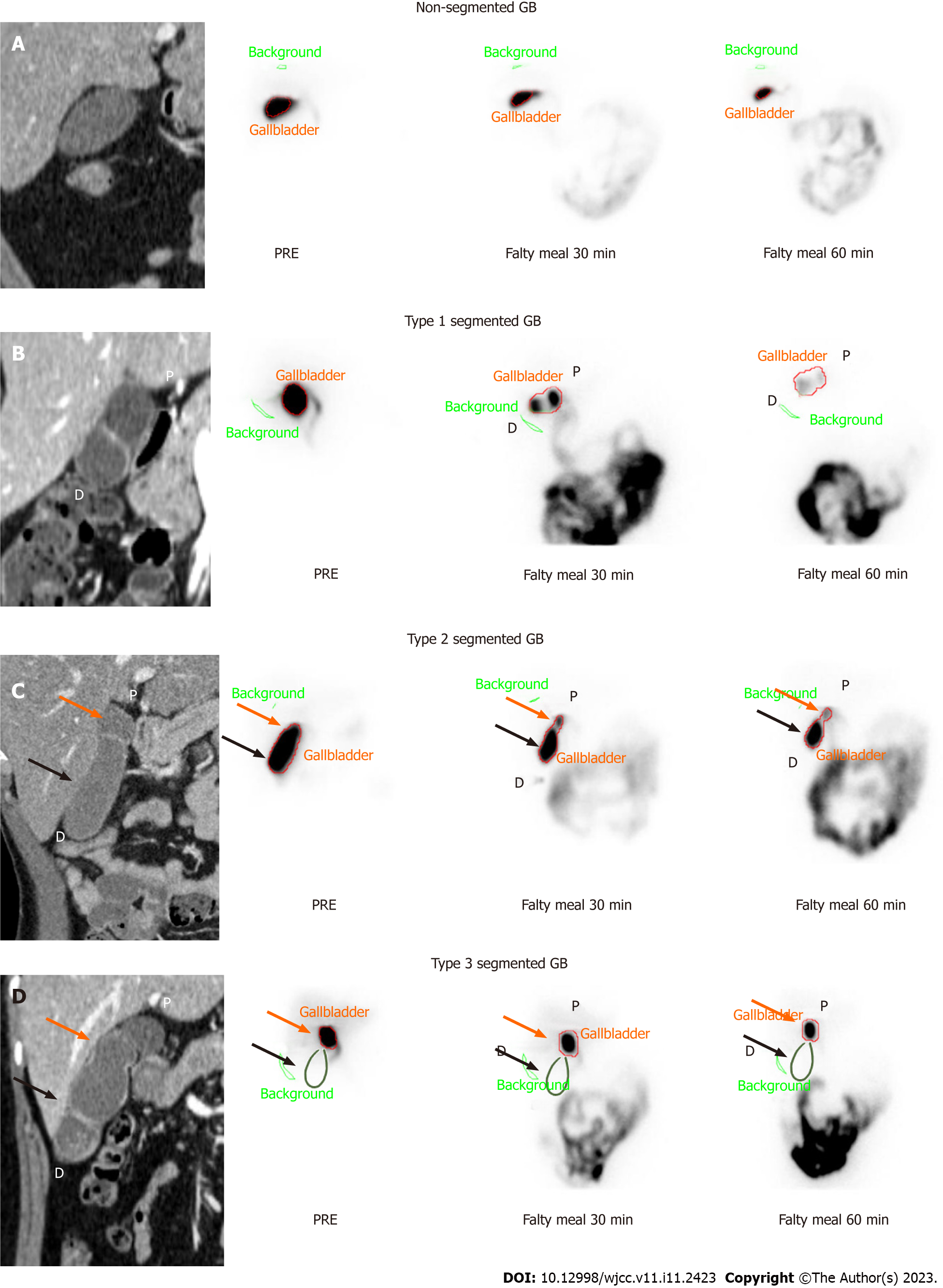
After a minimum of 6 h of fasting, each patient received 99mTc-mebrofenin intravenously while lying supine under a dual-head gamma camera fitted with a low-energy parallel-hole collimator, with a detector centered over the abdomen covering the region between the heart and pelvis in the field of view. GB phase images were obtained 60 min after 99mTc-mebrofenin injection. Following a fatty meal, GB phase images were obtained at 30 and 60 min. GBEF was measured using immediate pre- and post-fatty meal images, and the regions of interest were drawn around the GB (considering the patient’s movement) and adjacent liver (background). The GBEF was calculated as the ratio of the difference between the maximum and minimum signals to the maximum signal and corrected for the background signal[7].
Baseline images used for classification were those obtained 60 min after 99mTc-mebrofenin injection and the GB phase obtained 30 min and 60 min after fatty meal intake. HBS images were compared with CT or MR images and classified into 3 groups according to the filling and emptying patterns according to the actual anatomical morphology of the GB. Type 1 was defined as a normal filling and emptying pattern, Type 2 was defined as an emptying defect on the distal segment, and Type 3 was defined as a filling defect in the distal segment (Figure 2). Figure 3 depicts the anatomical boundaries of segmented GB as shown in typical CT images, the scanned images of HBS in normal GB, and each segmented GB subtype classified according to the filling and emptying pattern.
Results were reported as numbers (percentages) or as mean ± standard deviation. Continuous variables were compared using the Student’s t-test, and categorical data were compared using Pearson’s chi-squared test and Fisher’s exact test. Statistical significance was set at P < 0.05. Data were analyzed using SPSS software (version 25.0; IBM Corp., Armonk, NY, United States).
A total of 268 adult patients 18 years or older underwent HBS for chronic cholecystitis, gallstones, or biliary colic. The patients’ baseline characteristics are listed in Table 1. The mean age was 51.65 years ± 13.10 years and men constituted 43.2% of the study population. Approximately 20% of patients complained of biliary colic pain. Among these, 63 (23.51%) belonged to the segmented GB group. There were no differences in laboratory findings between the non-segmented and segmented GB groups. Based on radiological findings, adenomyomatosis and chronic cholecystitis were diagnosed more frequently in the segmented GB group (18/63, 28.6% and 45/63, 71.4%, respectively) than in the non-segmented GB group (12/205, 5.9% and 107/205, 52.2%, respectively) (P < 0.001 and P = 0.009, respectively). Reported GBEF was 68.01% ± 22.05%, and there was no difference between the two groups (61.70% ± 21.78% vs 70.05% ± 22.97%; P = 0.405). Cholecystectomy was performed more frequently in the segmented GB group (45/63, 71.4%) than in the non-segmented GB group (73/205, 35.6%; P < 0.001).
| Variables | Non-segmented GB, n = 205 | Segmented GB, n = 63 | P value | |
| Age in year | 51.8 ± 13.6 | 51.1 ± 11.3 | 0.664 | |
| Male | 87 (42.4%) | 29 (46.0%) | 0.72 | |
| Atypical symptoms | 52 (25.4%) | 9 (14.3%) | 0.085 | |
| Biliary colic pain | 50 (24.4%) | 10 (15.9%) | 0.171 | |
| Laboratory findings | ||||
| ALP in IU/L | 67.6 ± 24.6 | 68.0 ± 20.5 | 0.911 | |
| GGT in IU/L | 37.1 ± 54.9 | 33.1 ± 28.4 | 0.58 | |
| AST in IU/L | 24.2 ± 8.7 | 26.3 ± 14.7 | 0.163 | |
| ALT in IU/L | 23.5 ± 12.7 | 26.3 ± 15.4 | 0.148 | |
| Total bilirubin in mg/dL | 1.05 ± 2.60 | 0.86 ± 0.41 | 0.583 | |
| Direct bilirubin in mg/dL | 0.31 ± 0.87 | 0.25 ± 0.12 | 0.59 | |
| CEA in ng/mL | 1.58 ± 1.31 | 1.35 ± 0.83 | 0.31 | |
| Radiologic findings | ||||
| GB stone | 110 (53.7%) | 37 (58.7%) | 0.563 | |
| GB sludge | 7 (3.4%) | 2 (3.2%) | 0.999 | |
| GB polyp | 14 (6.8%) | 8 (12.7%) | 0.186 | |
| Adenomyomatosis | 12 (5.9%) | 18 (28.6%) | < 0.001 | |
| Chronic cholecystitis | 107 (52.2%) | 45 (71.4%) | 0.009 | |
| Reported GBEF, % | 67.40 ± 21.78 | 70.05 ± 22.97 | 0.405 | |
| Cholecystectomy | 73 (35.6%) | 45 (71.4%) | < 0.001 | |
| Pathology1 | ||||
| Adenomyomatosis | 3 (4.1%) | 5 (11.1%) | 0.257 | |
| Chronic cholecystitis | 68 (93.2%) | 41 (91.1%) | 0.73 | |
| Cholesterol polyp | 16 (21.9%) | 7 (15.6%) | 0.478 | |
According to the HBS patterns defined for segmented GB, 36 patients (57.1%) with Type 1, 18 patients (28.6%) with Type 2, and 9 patients (14.3%) with Type 3 were identified. A comparison of variables according to the type of segmented GB is presented in Table 2. Demographic, radiological, and pathological findings did not differ according to the type of segmented GB. In addition, the rates of atypical symptoms and biliary colic pain did not differ between the groups. GB stones occurred more frequently in the distal segment in both groups; however, no significant intergroup difference was found. Type 1, with normal emptying and filling patterns, showed no difference in the reported GBEF compared to the non-segmented GB group. Interestingly, the reported GBEF was higher in Types 2 and 3 with defective emptying and filling than in Type 1 (80.11% ± 15.70% vs 62.57% ± 24.79%; P = 0.001).
| Variables | Type 1, n = 36 | Types 2 & 3, n = 27 | P value | ||
| Age in year | 50.2 ± 9.6 | 52.3 ± 13.2 | 0.483 | ||
| Male | 14 (38.9%) | 15 (55.6%) | 0.212 | ||
| Atypical symptoms | 4 (11.1%) | 5 (18.5%) | 0.48 | ||
| Biliary colic pain | 7 (19.4%) | 3 (11.1%) | 0.494 | ||
| Radiological findings | |||||
| GB stone | 22 (61.1%) | 15 (55.6%) | 0.797 | ||
| Location of GB stone | 0.147 | ||||
| Proximal | 5 (22.7%) | 0 (0.0%) | 0.067 | ||
| Distal | 15 (68.2%) | 13 (86.7%) | 0.262 | ||
| Both | 2 (9.1%) | 2 (13.3%) | 0.999 | ||
| GB sludge | 1 (2.8%) | 1 (3.7%) | 0.999 | ||
| GB polyp | 5 (13.9%) | 3 (11.1%) | 0.999 | ||
| Adenomyomatosis | 10 (27.8%) | 8 (29.6%) | 0.999 | ||
| Chronic cholecystitis | 25 (69.4%) | 20 (74.1%) | 0.782 | ||
| Reported GBEF, % | 62.50 ± 24.79 | 80.11 ± 15.70 | < 0.001 | ||
| Cholecystectomy | 26 (72.2%) | 19 (70.4%) | 0.999 | ||
| Pathology1 | |||||
| Adenomyomatosis | 3 (11.5%) | 2 (10.5%) | 0.999 | ||
| Chronic cholecystitis | 24 (92.3%) | 17 (89.5%) | 0.999 | ||
| Cholesterol polyp | 4 (15.4%) | 3 (15.8%) | 0.999 | ||
The reported GBEF was 62.50% ± 24.79% for Type 1, 75.89% ± 17.21% for Type 2, and 88.56% ± 7.20% for Type 3 (Figure 4). Notably, a significant difference was observed between the reported GBEF of Types 1 and 3 (P = 0.005). The mean GBEF trended toward being higher in Type 2 than in Type 1, but the difference was not significant (P = 0.082).
The segmentation of the GB into two chambers by a fold or septum is a hyperplastic condition of uncertain etiology[3,4]. GB segmentation in adults is often attributed to adenomyomatosis or congenital septa[12,13]. The term segmented GB is used in a broader fashion to include segmental adenomyosis of the GB. This term is not commonly used; however, GB segmentation without adenomyomatosis is often observed in imaging studies of patients of any age. Krishnamurthy et al[14] reported that microscopic examination of a segmented GB revealed chronic inflammatory changes, fibrosis, and wall thickening[14]. The strictures caused by the annular thickening of the GB wall may be narrow[2]. The anatomical stricture of a segmented GB leads to difficult bile movement and causes cholestasis, which may lead to chronic inflammatory changes, cholelithiasis, and tumorigenesis[4,5,13]. Therefore, clinical symptoms and the patient’s physiological status should be considered; however, surgical treatment is recommended in the management of segmented GB.
Gallstones are one of the most common biliary tract diseases, and its prevalence is estimated at 5.9%-21.9% in the West and 3.1%-10.7% in Asia[15]. Diagnosis of gallstones is often incidental, and most patients remain asymptomatic throughout their lives. During a follow-up period of 10–15 years, symptoms appear in approximately 15%–25% of patients, and the risk of developing biliary pain due to complications is reported to be approximately 2%–3% annually[16-18]. Patients with symptomatic gallstones are at high risk of gallstone-related complications, and cholecystectomy is recommended in such cases[19]. In addition, cholecystectomy is recommended if there are risk factors for GB cancer [e.g., anomalous pancreatic ductal drainage, GB adenoma, porcelain GB, or large gallstones (especially those larger than 3 cm)][20-22]. However, if a patient with gallstones has ambiguous symptoms, it is often difficult to distinguish gallstone-related symptoms; therefore, blood tests, ultrasound, HBS, and the patient's personal circumstances are considered to determine the need for surgery.
HBS is a radionuclide diagnostic imaging modality that is used to evaluate hepatocellular function and the biliary system by tracing the synthesis and flow of bile from the liver and its passage through the biliary system into the small intestine[7]. The technique assesses the function of hepatocytes, the patency and integrity of the biliary ducts, GB contractility, and sphincter of Oddi function[23]. HBS has frequently been used to investigate the physiological parameters of the GB during emptying and filling[24]. A reduced GBEF is observed in calculous and acalculous biliary diseases, such as chronic acalculous cholecystitis, biliary dyskinesia, and sphincter of Oddi dysfunction. It may also be associated with various nonbiliary diseases and conditions as well as with the use of various medications (e.g., morphine, atropine, calcium channel blockers, octreotide, etc)[7,25]. To this end, a meta-analysis concluded that the utility of a low GBEF in deciding whether to perform cholecystectomy in individuals with GB dyskinesia is still unclear[10]. However, the reduced GBEF in HBS has been advocated as a diagnostic parameter for the clinical evaluation of individuals presenting with suspected biliary pain or gallstones and altered GB morphology (such as segmented GB) in deciding whether to proceed with cholecystectomy.
The GBEF as evaluated in this study did not differ between patients with segmented or non-segmented GB. In a previous study, the GBEF measured in segmented GB was lower than that in non-segmented GB[14]. In this study, we observed the appearance of partial filling and emptying events in each lumen in segmented GB using HBS imaging. The anatomical features of annular strictures in segmented GB may alter the emptying of radiotracers without completely replenishing them. Defective HBS images in segmented GB have been reported[26]; however, they have never been classified according to filling and emptying patterns on scanned images. HBS images were divided into three categories. Type 1 was defined as a normal filling and emptying pattern. Type 2 was defined as emptying defects in the distal portion, and Type 3 was characterized by filling defects in the distal portion.
Furthermore, GBEF varied across segmented GB types depending on the HBS imaging pattern. We have described GBEF as "reported GBEF" because GBEF may be incorrectly measured depending on the type. We measured GBEF in Type 1 as 62.50%, with higher measured GBEF in Types 2 and 3 with defective filling or emptying (P = 0.001). Filling or emptying defects in HBS images of a segmented GB are attributable to the undesirable effects of bile flow. However, the higher GBEF of Types 2 and 3 compared with that of Type 1 renders the results unreliable and inconsistent with those of a previous study of 7 patients with segmented GB analyzed via HBS[14]. In this study, the total GBEF was determined by measuring the ejection fraction (EF) of the proximal and distal segments of the GB using HBS. Distal EF was lower in the segmented GB group than the proximal EF. The total EF in the segmented GB group was also lower than that in the non-segmented GB group. However, in the non-segmented GB group (n = 10), distal EF was higher than proximal EF. Decreased emptying (low EF) of the distal segment indicates that the septum acts as a one-way valve, allowing normal bile entry into the distal segment, but does not allow an easy exit. However, the total GBEF of segmented GB measured in our hospital was often good, suggesting normal GB contractility. This phenomenon indicates that the measurement of total GBEF via HBS in segmented GB does not reflect the actual contractility of the organ. Total GBEF in Types 2 and 3 was primarily measured based on the contractility of the proximal part rather than the overall contractility as the GB boundary was not adequately demonstrated in the HBS images.
Changes in bile acid filling and emptying and the effect of the segmented GB on GBEF can be determined from the location of gallstones. GB stones were located mainly in the distal part and were related to the contraction and imbalance of the bile flow between the two segments. Nishimura et al[4] investigated the association between segmental adenomyomatosis of the GB and gallstones[4]. In addition, the contribution of gallstones to adenomyomatosis was investigated by examining their components. According to this study, the incidence of gallstones was higher in segmental adenomyomatosis than in fundal and diffuse adenomyomatosis, and distal compartment stones were detected in 80.6% (58/72). In our study, 75.7% (28/37) of stones were found in distal segments, 10.8% (4/37) in both segments, and only 13.5% (5/37) in the proximal segment of segmented GB. Although the size or composition of gallstones was not investigated in our study, the narrowed passageway was considered to obstruct the flow of bile and facilitate the production of gallstones at the distal part of the segmented GB.
The total GBEF (80.11% ± 15.70%) of the groups with defective filling or emptying (Types 2 and 3) suggested an overestimation of the proximal portion compared with the normal filling and emptying group (62.57% ± 24.79%). The difference between the groups underscores the need to interpret the HBS results in segmented GB in combination with GB morphology assessed by other imaging modalities (US, CT, or MRCP).
This study had some limitations. Our study was a relatively small, single-center cohort study. In addition, EFs of the proximal and distal GB were not investigated, which precluded the determination of the contribution of each GB part to total EF. The filling and emptying radiotracer volume of bile flow could not be confirmed; therefore, no quantitative results could be reported. However, quantitative studies require the use of single-photon emission CT. Lastly, non-specific fatty meals were used to stimulate GB contraction, as cholecystokinin (CCK) was unavailable for use at our institute. Although CCK would have offered a more reliable GB stimulus, a previous study did not show any significant difference between CCK and fatty meals in measuring GBEF[27].
Despite these limitations, our findings provide a better understanding of the results of HBS in segmented GB. Clinically, segmented GB is associated with a higher incidence of GB-related diseases than are other morphological features. Therefore, cholecystectomy is often indicated for the surgical treatment of segmented GB. In addition to the assessment of patient symptoms, reduced contractility in segmented GB measured by HBS is critical in determining the need for cholecystectomy. However, GBEF measurement in segmented GB may be misinterpreted depending on the type of HBS images. In particular, the GBEF in Type 2 and Type 3 HBS scans can be overestimated, which can complicate the development of an ideal treatment plan.
In segmented GB, discordance between HBS and anatomical imaging (CT or MR techniques) could lead to inaccurate estimation of GBEF. Therefore, clinicians should be cautious when interpreting GB contractility as measured by HBS in patients with segmented GB.
Hepatobiliary scintigraphy (HBS) is a useful diagnostic imaging technique that uses radioactive tracers to evaluate the function of the gallbladder (GB) and biliary tract. In segmented GB, some HBS images show inconsistent GB boundaries as compared to anatomical imaging, limiting the evaluation of GB contractility through HBS.
Cholecystectomy is sometimes necessary in patients with gallstones or chronic cholecystitis. In addition, in the case of anatomically segmented GB, cholecystectomy is recommended due to the higher risk of future GB disease. Because there are patients who are reluctant to undergo cholecystectomy, cholecystectomy is recommended again if cholecystectomy decreases after evaluating the GB contractability using HBS. In previous studies, the GB ejection fraction (GBEF) was reduced in the case of segmental GB, but GBEF was often normal when measured in our study.
We evaluated the characteristics of HBS in segmented GB and investigated the effect of segmented GB on the measurement of GBEF using HBS.
From a larger group of patients with chronic cholecystitis, gallstones, or biliary colic, those who underwent HBS were identified. Patients with segmented GB features with segmental lumen stricture were identified using computed tomography (CT) or magnetic resonance (MR) imaging. Patients were asked whether CT or MR was performed either 1 mo before or after HBS. Segmented GB was classified into 3 types based on the filling and emptying patterns of the proximal and distal segments according to the characteristics of HBS images, and GBEF was measured. Type 1 was defined as a normal filling and emptying pattern, Type 2 was defined as an emptying defect on the distal segment, and Type 3 was defined as a filling defect in the distal segment.
Segmented GB accounted for 63 cases (23.5%), including 36 patients (57.1%) with Type 1, 18 patients (28.6%) with Type 2, and 9 patients (14.3%) with Type 3. Thus, approximately 43% of segmented GB cases demonstrated discordance between HBS and anatomical imaging. Although there were no significant differences in clinical symptoms, rate of cholecystectomy, or pathological findings based on the type, most gallstones occurred in the distal segment. Reported GBEF was 62.50% ± 24.79% for Type 1, 75.89% ± 17.21% for Type 2, and 88.56% ± 7.20% for Type 3. Type 1 showed no difference in reported GBEF as compared to the non-segmented GB group (62.50% ± 24.79% vs 67.40 ± 21.78%). In contrast, the reported GBEF was higher in Types 2 and 3 with defective emptying and filling than in Type 1 (80.11% ± 15.70% vs 62.57% ± 24.79%; P = 0.001).
In segmented GB, discordance between HBS and anatomical imaging (CT or MR) could lead to inaccurate estimation of GBEF. Therefore, clinicians should use caution when interpreting GB contractility through HBS in patients with segmented GB.
Since the presence or absence of gallstones may affect HBS, additional studies may be conducted to examine the difference in GBEF according to the presence or absence of gallstones in patients with segmented GB. Further studies using single-photon emission CT to evaluate discrepancies between HBS and anatomical imaging are expected to help determine the need for cholecystectomy.
| 1. | Sherlock S, Dooley J. Diseases of the Liver and Biliary System. 11th ed. New York: John Wiley & Sons, 2008. [DOI] [Full Text] |
| 2. | Krishnamurthy GT, Krishnamurthy S. Hepatic bile entry into and transit pattern within the gallbladder lumen: a new quantitative cholescintigraphic technique for measurement of its concentration function. J Nucl Med. 2002;43:901-908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 3. | Golse N, Lewin M, Rode A, Sebagh M, Mabrut JY. Gallbladder adenomyomatosis: Diagnosis and management. J Visc Surg. 2017;154:345-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 54] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 4. | Nishimura A, Shirai Y, Hatakeyama K. Segmental adenomyomatosis of the gallbladder predisposes to cholecystolithiasis. J Hepatobiliary Pancreat Surg. 2004;11:342-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 42] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 5. | Li T, Apte U. Bile Acid Metabolism and Signaling in Cholestasis, Inflammation, and Cancer. Adv Pharmacol. 2015;74:263-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 241] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 6. | Ootani T, Shirai Y, Tsukada K, Muto T. Relationship between gallbladder carcinoma and the segmental type of adenomyomatosis of the gallbladder. Cancer. 1992;69:2647-2652. [PubMed] [DOI] [Full Text] |
| 7. | Tulchinsky M, Ciak BW, Delbeke D, Hilson A, Holes-Lewis KA, Stabin MG, Ziessman HA; Society of Nuclear Medicine. SNM practice guideline for hepatobiliary scintigraphy 4.0. J Nucl Med Technol. 2010;38:210-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 74] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 8. | Snyder E, Kashyap S, Lopez PP. Hepatobiliary Iminodiacetic Acid Scan. 2022 Jul 25. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. [PubMed] |
| 9. | Mahid SS, Jafri NS, Brangers BC, Minor KS, Hornung CA, Galandiuk S. Meta-analysis of cholecystectomy in symptomatic patients with positive hepatobiliary iminodiacetic acid scan results without gallstones. Arch Surg. 2009;144:180-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 32] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 10. | Gudsoorkar VS, Oglat A, Jain A, Raza A, Quigley EMM. Systematic review with meta-analysis: cholecystectomy for biliary dyskinesia-what can the gallbladder ejection fraction tell us? Aliment Pharmacol Ther. 2019;49:654-663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 11. | Yap L, Wycherley AG, Morphett AD, Toouli J. Acalculous biliary pain: cholecystectomy alleviates symptoms in patients with abnormal cholescintigraphy. Gastroenterology. 1991;101:786-793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 184] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 12. | Aguirre JR, Bohler RO, Guraieb S. Hyperplastic cholecystoses; a new contribution to the unitarian theory. Am J Roentgenol Radium Ther Nucl Med. 1969;107:1-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 13. | Kramer EL, Rumancik WM, Harkavy L, Tiu S, Banner HJ, Sanger JJ. Hepatobiliary scintigraphy of the compartmentalized gallbladder. AJR Am J Roentgenol. 1985;145:1205-1206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (1)] |
| 14. | Krishnamurthy GT, Krishnamurthy S, Milleson T, Brown PH, Urstadt DS. Segmentation of the gallbladder: effect on bile entry and exit and its clinical relevance in a patient with abdominal pain. Nucl Med Commun. 2007;28:109-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 15. | Chuang SC, Hsi E, Lee KT. Genetics of gallstone disease. Adv Clin Chem. 2013;60:143-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 16. | Shabanzadeh DM, Sørensen LT, Jørgensen T. A Prediction Rule for Risk Stratification of Incidentally Discovered Gallstones: Results From a Large Cohort Study. Gastroenterology. 2016;150:156-167 e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 79] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 17. | Festi D, Reggiani ML, Attili AF, Loria P, Pazzi P, Scaioli E, Capodicasa S, Romano F, Roda E, Colecchia A. Natural history of gallstone disease: Expectant management or active treatment? J Gastroenterol Hepatol. 2010;25:719-724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 112] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 18. | Lee SK, Kim MH. Natural history of gallstone; an important and old issue, but still debatable. J Gastroenterol Hepatol. 2010;25:651-652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 19. | Friedman GD, Raviola CA, Fireman B. Prognosis of gallstones with mild or no symptoms: 25 years of follow-up in a health maintenance organization. J Clin Epidemiol. 1989;42:127-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 136] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 20. | Leitzmann MF, Rimm EB, Willett WC, Spiegelman D, Grodstein F, Stampfer MJ, Colditz GA, Giovannucci E. Recreational physical activity and the risk of cholecystectomy in women. N Engl J Med. 1999;341:777-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 127] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 21. | Leitzmann MF, Giovannucci EL, Rimm EB, Stampfer MJ, Spiegelman D, Wing AL, Willett WC. The relation of physical activity to risk for symptomatic gallstone disease in men. Ann Intern Med. 1998;128:417-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 119] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 23. | Afzal M, Ali SM, Khairy AT, Khattabi M, Shahid F, Ramzee AF, Ahmed K. Hepatobiliary scintigraphy and its clinical implications: mini review. International Surgery Journal 2021; 8: 766-770. [DOI] [Full Text] |
| 24. | Ziessman HA. Hepatobiliary scintigraphy in 2014. J Nucl Med. 2014;55:967-975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 25. | Richmond BK, DiBaise J, Ziessman H. Utilization of cholecystokinin cholescintigraphy in clinical practice. J Am Coll Surg. 2013;217:317-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 26. | Won KY, Kim C. Bicameral Gallbladder With Chronic Cholecystitis on Hepatobiliary Scintigraphy. Clin Nucl Med. 2021;46:78-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 27. | DeBruin A, Ellefson K, Johnson C. Comparison of fatty meal interventional agents to CCK for GBEF studies. Soc Nuclear Med. 2021;62 (suppl 1):157. |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C, C
Grade D (Fair): D, D
Grade E (Poor): 0
P-Reviewer: Gupta R, India; Limaiem F, Tunisia; Wani I, India S-Editor: Li L L-Editor: Filipodia P-Editor: Li L