Published online Apr 16, 2023. doi: 10.12998/wjcc.v11.i11.2386
Peer-review started: December 26, 2022
First decision: February 8, 2023
Revised: February 23, 2023
Accepted: March 14, 2023
Article in press: March 14, 2023
Published online: April 16, 2023
Processing time: 100 Days and 23.2 Hours
Programmed cell death (PCD) is mediated by specific genes that encode signals. It can balance cell survival and death. Pyroptosis is a type of inflammatory, caspase-dependent PCD mediated by gasdermin proteins, which function in pore formation, cell expansion, and plasma membrane rupture, followed by the release of intracellular contents. Pyroptosis is mediated by caspase-1/3/4/5/11 and is primarily divided into the classical pathway, which is dependent on caspase-1, and the non-classical pathway, which is dependent on caspase-4/5/11. Inflammasomes play a vital role in these processes. The various components of the pyroptosis pathway are related to the occurrence, invasion, and metastasis of tumors. Research on pyroptosis has revealed new options for tumor treatment. This article summarizes the recent research progress on the molecular mechanism of pyroptosis, the relationship between the various components of the pyroptosis pathway and cancer, and the applications and prospects of pyroptosis in anticancer therapy.
Core Tip: Pyroptosis is a type of inflammatory caspase-dependent programmed cell death mediated by gasdermin proteins, which function in pore formation, cell swelling, and cell membrane rupture. This review discusses the classical and non-classical pathways of pyroptosis and the recent research progress on the molecular mechanisms of pyroptosis. We also review the relationship between various pyroptosis pathway components and different cancers, including nucleotide-binding oligomerization domain-like receptor thermal protein domain associated protein 3 inflammasomes, interleukin (IL)-18, IL-1β, and absent in melanoma 2. The potential applications of this form of cell death as a cancer treatment approach are also explored.
- Citation: Liu SW, Song WJ, Ma GK, Wang H, Yang L. Pyroptosis and its role in cancer. World J Clin Cases 2023; 11(11): 2386-2395
- URL: https://www.wjgnet.com/2307-8960/full/v11/i11/2386.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i11.2386
The dynamic balance among cell death, proliferation, and differentiation sustains individual development, homeostasis, and pathological processes in humans. Many disease states are associated with cell death. Programmed cell death (PCD) is regulated by specific cellular mechanisms, and some signaling pathways are activated in these processes[1]. Autophagy, apoptosis, and programmed necrosis are the three main types of PCD[2], and together, they may affect the fate of cancer cells. Apoptosis is a type of PCD that involves cell self-destruction controlled by genes. In apoptosis, the cell membrane remains intact, and inflammation usually does not occur[3]. Necrosis is a passive cell death process caused by pathological stimuli. The permeability of the cell membrane of necrotic cells increases, which causes the cells to swell and finally break down and release their contents. This leads to an inflammatory reaction[3]. Pyroptosis is a form of programmed necrosis, which is PCD induced by gasdermin-mediated.
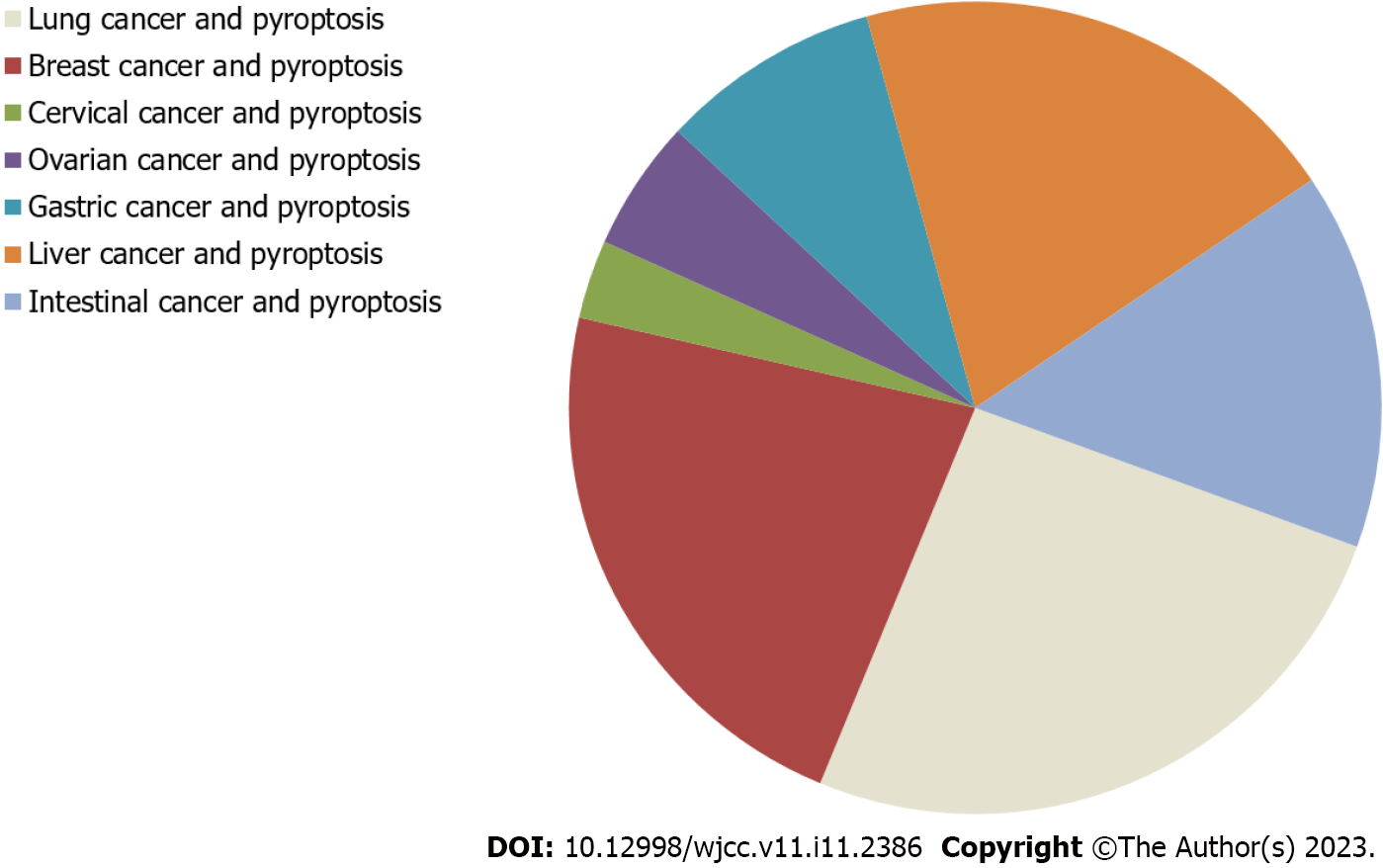
Pyroptosis was first described in myeloid cells infected by pathogens in 1992[4-6]. It is believed that by clearing intracellular replication niches and improving the defensive responses of the host, pyroptosis plays a vital role in clearing various bacterial and viral infections[7]. The activation of pyroptosis may promote cell death and exert anticancer effects[8]. Pyroptosis has attracted increased attention because it is related to innate immunity and disease. The research status of the relationship between pyroptosis and various cancers is shown in Figure 1, according to publications in PubMed. Emerging evidence has demonstrated the importance of pyroptosis in cancer. Recently, more and more studies have shown that pyroptosis has become a new research topic in cancer, because it may affect the process of cancer. In this review, we outline the molecular mechanism of pyroptosis and highlight its differences from apoptosis. The importance of the various components of the pyroptosis pathway in cancer and its application prospects in antitumor therapy are also discussed.
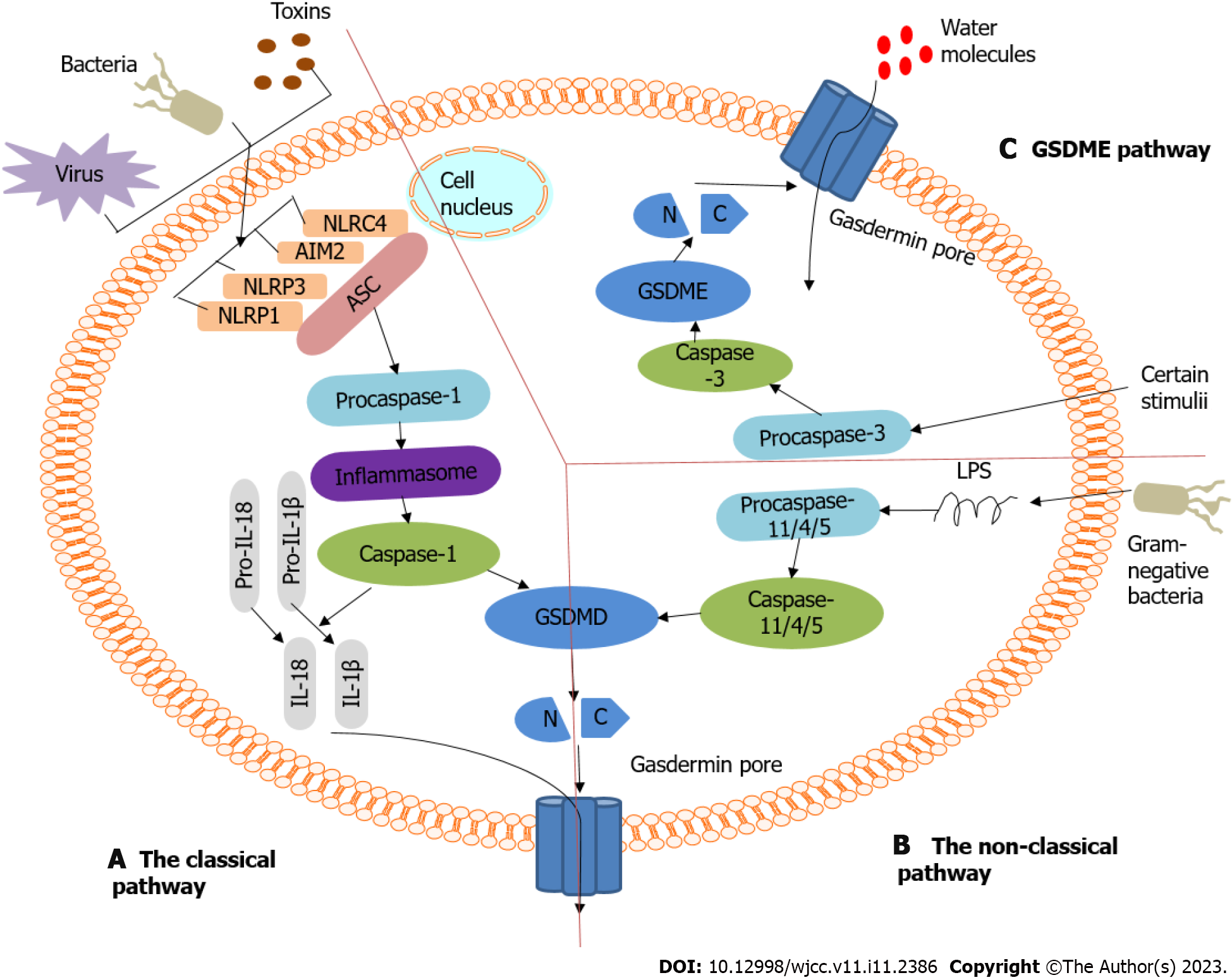
Pyroptosis is also called gasdermin-mediated PCD. The gasdermin family has regulatory functions in normal cell proliferation and differentiation; it includes gasdermin A (GSDMA), gasdermin B (GSDMB), gasdermin C (GSDMC), gasdermin D (GSDMD), gasdermin E (GSDME) (also known as DFNA5, [Deafness, Autosomal Dominant Nonsyndromic Sensorineural 5]), and DFNB59 (Autosomal Recessive Deafness Type 59 Protein)[9-13]. Among them, GSDMD and GSDME have been extensively studied in pyroptosis. These proteins have inherent necrotic activity in their gasdermin-N domain, which is usually masked by their gasdermin-C domain[14-16]. Pyroptosis is activated by various stimuli and inflammatory caspases, which induce the cleavage of proteins in the gasdermin family and release its N-terminal effector and C-terminal inhibitory domains. The necrotic gasdermin-N domain is then transferred to the plasma membrane, forming oligomers[14-19]. These oligomers form transmembrane pores that disrupt the osmotic potential, which results in rapid plasma membrane rupture, causing the cells to release their intracellular contents and pro-inflammatory mediators, such as interleukin (IL)-1β and IL-18[20].
Of the proteins in the gasdermin family, GSDMA, GSDMB, and GSDMC proteins possess a pore-forming gasdermin-N domain. However, they are not cleaved to form functional pores in response to physiological or pathological stimuli[14,21]. Only GSDMD and GSDME are cleaved by caspases between their gasdermin-N and gasdermin-C domains to form membrane pores[11,14,21-23]. Typically, GSDMD, the downstream effector of inflammasome activation, is cleaved by inflammatory caspases (caspase-1/4/5/11) to induce pyroptosis. However, GSDME is cleaved by an apoptotic caspase (caspase-3), which causes pyroptotic death[14]. Different molecular patterns are activated depending on the specific signaling pathway and cell type to induce pyroptosis[24]. The role of each component in the gasdermin family is described in Supplementary Table 1.
Pyroptosis belongs to the inflammatory cell death pathway. According to its activation mechanism, pyroptosis can be divided into the classical pathway, which is dependent on caspase-1, and the non-classical pathway, which is dependent on caspase-4, 5, and 11. These pathways are shown in Figure 2. Both pathways are formed by the cleavage of GSDMD, which forms a free N-terminal peptide; this peptide induces cells to form pores and rupture, which causes the release of cytoplasmic components. Both pathways can simultaneously induce the cleavage of IL-1β and IL-18 precursors to form mature IL-1β and IL-18. The difference between the two is whether caspase-1 is directly activated. (1) The classical pathway of pyroptosis[21,25]: Inflammasomes are multimolecular complexes that contain pattern-recognition receptors (PRRs). The PRR series usually contains Toll-like receptors, intracellular nucleotide-binding oligomerization domain (NOD)-like receptors, and absent in melanoma-like receptors. PRRs can recognize pathogen-associated and damage-associated molecular patterns, and can sense the presence of risk factors, such as infection and injury. PRRs can also recruit the adaptor protein containing the caspase recruitment domain [apoptosis-associated speck-like protein containing a caspase recruitment domain (ASC)] and activate caspase-1 through the adaptor protein ASC and pro-caspase-1 binding. On the one hand, the activated caspase-1 cleaves GSDMD to form the GSDMD-N and GSDMD-C domains. The GSDMD-N domain combines with phospholipid proteins on the cell membrane to form holes, after which contents are released, and pyroptosis is induced. On the other hand, the activated caspase-1 cleaves IL-1β and IL-18 precursors to form active IL-1β and IL-18, which are released outside the cell, causing inflammation; and (2) Non-classical pathways that depend on caspase-4, 5, and 11[21,25]. Taking the inflammatory stimulating factor lipopolysaccharide (LPS) as an example, it directly enters the cytoplasm, although not through the receptor, where it activates other caspases, such as caspase-4, 5, and 11, which cleave GSDMD to induce pyroptosis. A new pathway known to cause pyroptosis is caspase-3/GSDME[11,26]. Caspase-3 can be activated by death receptors and mitochondrial pathways. Mature caspase-3 cleaves GSDME to produce GSDME N-fragments. It participates in pore formation in the plasma membrane, resulting in cell swelling and pyroptosis.
Pyroptosis is an important natural immune response of the body. It plays a vital role in antagonizing infection and endogenous danger signals. Pyroptosis is widely involved in the occurrence and development of infectious, nervous system-related, and atherosclerotic diseases. In-depth research on pyroptosis has implicated its role in the occurrence, development, and outcome of related diseases and has provided new ideas for clinical prevention and treatment. In recent years, research interest in pyroptosis has increased significantly as it has successful attracted the attention of scientists and has become a popular research topic. For tumors, pyroptosis is a double-edged sword. On the one hand, as an innate immune mechanism, pyroptosis can inhibit the occurrence and development of tumors. On the other hand, as a mechanism of pro-inflammatory cell death, pyroptosis provides a suitable microenvironment for tumor growth. The key components of the pyroptosis pathway, inflammasomes, gasdermin proteins, and pro-inflammatory cytokines, are all related to tumor occurrence, invasion, and metastasis[27].
Many molecules that participate in the pyroptosis process are closely related to the occurrence and development of lung cancer. Studies have confirmed that the expression level of GSDMD in non-small cell lung cancer is significantly higher than in surrounding lung tissues. Moreover, the GSDMD expression is related to the tumor size, tumor–node–metastasis stage, and high aggressive characteristics[28]. In addition, GSDMD is considered to be an independent prognostic marker of lung adenocarcinoma[28]. Studies have found that GSDMD in gastric cancer was downregulated and led to the occurrence and spread of this cancer type[29]. The low level of GSDMD in gastric cancer cells may be associated with the acceleration of the cell cycle S/G2 transition; GSDMD also inhibits the signal transducer and activator of transcription 3, extracellular signal-regulated kinase (ERK), and phosphatidylinositol 3-kinase/protein kinase B (PI3K/AKT) in gastric cancer. These data indicate the tumor-suppressive effect of GSDMD in gastric cancer. However, whether GSDMD plays a tumor-suppressive or cancer-promoting role in breast and colorectal cancers and other cancers is unknown. Therefore, this will be the focus of future research.
In lung cancer, the deletion of the DFNA5/GSDME gene promotes drug resistance, whereas the overexpression of DFNA5/GSDME can result in increased drug sensitivity[30]. Both cisplatin and paclitaxel can induce pyroptosis through caspase-3/GSDME activation. However, in A549 Lung cancer cells, cisplatin is more effective than paclitaxel in triggering pyroptosis[31]. According to several studies, the expression of DFNA5/GSDME in hepatocellular carcinoma (HCC) cells was significantly reduced compared to that in normal cells. Furthermore, the upregulation of the DFNA5/GSDME expression inhibited cell proliferation, which suggests that DFNA5/GSDME may be an anticancer gene[10,32]. Studies have found that GSDME knockout can significantly inhibit breast cancer (BC) cell pyroptosis and reduce the sensitivity of cancer cells to paclitaxel. In addition, GSDME methylation can increase the risk of BC lymph node metastasis, which suggests that GSDME exerts anticancer effects. Recent studies have suggested that chemotherapeutics can convert caspase-3-dependent apoptosis into pyroptosis via DFNA5/GSDME, which may be downregulated because of promoter methylation[33]. Treatment with decitabine can induce DFNA5/GSDME up-regulation in cancer cells, which causes pyroptosis and increases the sensitivity of these cells to chemotherapeutics[11,34].
Studies have reported that stimulating the formation of NOD-like receptor protein 3 (NLRP3) inflammasomes in A549 lung cancer cells with LPS and adenosine triphosphate can activate AKT, ERK1/2, and cyclic adenosine monophosphate response element binding protein. Moreover, it can upregulate the transcription factor Snail and downregulate E-cadherin, which confirms that the NLRP3 inflammasome can promote lung cancer cell proliferation and migration[35]. In BC, the production of NLRP3 inflammasomes and IL-1β promote the infiltration of bone marrow cells, such as tumor-associated macrophages and myeloid-derived suppressor cells, which provide an inflammatory microenvironment that promotes BC progression[36]. Furthermore, NLRP3 inflammasomes in fibroblasts are associated with progression and metastasis[37]. The NLRP3 inflammasome appears to be an effector that promotes lymphatic system metastasis and BC development[38]. Various components involved in pyroptosis are closely related to digestive system tumors. Studies have found that the NLRP3 expression in HCC is significantly downregulated or even absent and that its expression is negatively correlated with the clinical stage and pathological grade. This suggests that the NLRP3 inflammasome participates in developing HCC[39]. Furthermore, the NLRP3 inflammasome participates in the innate immune response to cervical cancer, and its expression is widely present in tumor cells[40,41]. The NLRP3 inflammasome activation can be achieved through lysosomal rupture, hemi-ion channels, and reactive oxygen species (ROS). In cervical cancer, the NLRP3 inflammasome is mainly activated by ROS to induce pyroptosis. In most of the cited reports, evidence on the role of NLRP3 in tumors is still in the preliminary stage, and further confirmation is needed to determine the potential therapeutic role of NLRP3 inflammasomes in human malignancies.
IL-18 plays an immunomodulatory role in the occurrence of esophageal squamous cell carcinoma (ESCC)[42,43]. IL-18 can induce CD8+ T cells and natural killer cells to produce interferon-γ, improve anticancer immunity, and inhibit cancer cell proliferation and metastasis[42]. Exogenous IL-18 is expected to be a new approach for treating ESCC. In one study, the vascular endothelial growth factor stimulated the production, processing, and secretion of IL-18 in gastric cancer cells. IL-18 promotes cell migration through actin polymerization and tensin down-regulation. Therefore, IL-18 may amplify the angiogenesis, migration, and progression of gastric cancer cells[44,45]. In addition to the angiogenic and invasive properties of IL-18, this cytokine can also induce the expression of protease inhibitor 9 and granzyme B inhibitor in gastric cancer cells, which reduces their sensitivity to lymphocyte-mediated cytotoxicity[46]. Recent data indicate that the NLRP3 inflammatory body inhibitor thymoquinone and resveratrol inhibit the metastases of murine melanoma cells by inhibiting the IL-18-mediated vascular cell adhesion molecule 1 expression and IL-18 secretion[47,48]. Different studies have reported the association between polymorphisms in the IL-18 gene promoter (-137 G>C and -607 C>A) and the development of different human cancers. A meta-analysis showed that the -137 G>C polymorphism is associated with an increased risk of nasopharyngeal carcinoma (NPC) in Asian populations but not in Caucasian populations[49]. Another meta-analysis showed that the -607 C>A polymorphism is connected with an increase in the overall cancer risk, especially for esophageal cancer and NPC, in Asian populations[50]. Recently, the discovery of IL-18-binding protein (IL-18BP) as a physiological inhibitor of IL-18 has suggested that this cytokine may be an attractive target. Its advantages and disadvantages in treating various diseases are currently being investigated[51,52]. In some types of tumors, the tumor-promoting effect of IL-18 is dominant, and IL-18BP may be beneficial. Therefore, any potential IL-18 treatment should be considered with caution.
IL-1β can promote epithelial–mesenchymal transition (EMT) in ESCC, colorectal carcinoma (CRC), and HCC, and it can promote the migration and invasiveness of cancer cells[53-56]. Multiple studies have found that IL-1β is a crucial cytokine related to BC. IL-1β can induce the EMT process in BC, increase tumor malignancy, and increase the resistance of BC to cisplatin by upregulating resistance-related genes. IL-1β can also promote the expression of the oncogene baculoviral inhibitor of apoptosis repeat-containing 3 to decrease the resistance of BC to doxorubicin[56]. Moreover, other studies have reported that IL-1β can induce tamoxifen resistance in BC by downregulating estrogen receptor-α[57]. In response to the cancer-promoting effect of IL-1β, Tulotta et al[53] used anakinra and kanazumab to block the IL-1β signaling pathway. They found that the body’s anticancer immunity was enhanced, the number of cancer cells entering the circulation was decreased, and the metastasis of BC was suppressed. In the future, it is hoped that antitumor drugs that target IL-1β will become novel cancer therapies.
AIM2 is a cytoplasmic sensor that recognizes double-stranded DNA (dsDNA) released during cellular perturbation and pathogenic assault[58]. Upon binding to dsDNA, AIM2 assembles a multiprotein complex termed the inflammasome, which drives IL-1β and IL-18 secretion and pyroptosis[59]. Several studies observed a decreased AIM2 expression in HCC tissues but not in normal tissues. The AIM2 expression was negatively correlated with tumor progression[60,61]. Additionally, AIM2 deficiency enhanced EMT and fibronectin-1 expression, which may be related to HCC metastasis[60]. In human papillomavirus-infected cervical cancer cells, AIM2 can exert a tumor-inhibitory effect by stimulating pyroptosis[62]. The AIM2 gene contains a microsatellite instability site, leading to frequent gene mutations in CRC and small intestine cancer[63,64]. Two independent studies have shown that AIM2 can inhibit CRC development[65,66]; AIM2 inhibits the proliferation of colonic stem cells and facilitates cell death by inhibiting the PI3K/AKT signaling pathway[65,66]. In addition, studies have shown that AIM2 inhibits the proliferation of colon cancer cells in the G2/M phase by inducing cell cycle arrest[67]. Furthermore, the release of IL-18 mediated by the AIM2 inflammasome triggers the up-regulation of IL-22 binding protein and antimicrobial peptides that regulate intestinal homeostasis[68]. A high AIM2 expression is associated with the increased survival rate of patients with Epstein–Barr virus-associated NPC. The function of AIM2 in NPC may involve IL-1β and the recruitment of immunostimulatory neutrophils into tumor masses, which can mediate antitumor activity[69]. Depending on cancer, AIM2 plays different roles; for example, AIM2 functions as a tumor suppressor in CRC and HCC but as a tumor promoter in skin carcinoma[70]. The AIM2 expression is moderate in skin squamous cell carcinoma, whereas its expression is low or absent in normal skin. The knockdown of AIM2 also leads to the reduced invasiveness of skin squamous cell carcinoma cells. It can inhibit the growth and vascularization of skin squamous cell carcinoma in vivo[70]. Further research on AIM2 will help us better understand the role of AIM2 in cancer and to develop new antitumor drugs.
Pyroptosis is a type of inflammatory PCD characterized by cell swelling and lysis that is mediated by various inflammasomes, which can discern danger signals and activate the secretion of pro-inflammatory cytokines (such as IL-18 and IL-1β). Pyroptosis can regulate cell proliferation, infiltration, migration, chemotherapy resistance, and other malignant phenotypes through various cell signaling pathways, thereby affecting tumor progression. The various components of the pyroptosis pathway are involved in almost all aspects of tumor development. They play either a tumor-suppressive or a pro-tumorigenic role. Therefore, research on the characteristics and mechanisms of pyroptosis and its relationship with cancer can provide novel ideas and effective drug targets for disease prevention and treatment.
| 1. | Andón FT, Fadeel B. Programmed cell death: molecular mechanisms and implications for safety assessment of nanomaterials. Acc Chem Res. 2013;46:733-742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 187] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 2. | Ouyang L, Shi Z, Zhao S, Wang FT, Zhou TT, Liu B, Bao JK. Programmed cell death pathways in cancer: a review of apoptosis, autophagy and programmed necrosis. Cell Prolif. 2012;45:487-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 938] [Cited by in RCA: 1077] [Article Influence: 76.9] [Reference Citation Analysis (9)] |
| 3. | Fink SL, Cookson BT. Apoptosis, pyroptosis, and necrosis: mechanistic description of dead and dying eukaryotic cells. Infect Immun. 2005;73:1907-1916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1330] [Cited by in RCA: 1576] [Article Influence: 75.0] [Reference Citation Analysis (0)] |
| 4. | Wallach D, Kang TB, Dillon CP, Green DR. Programmed necrosis in inflammation: Toward identification of the effector molecules. Science. 2016;352:aaf2154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 430] [Article Influence: 43.0] [Reference Citation Analysis (0)] |
| 5. | Shi J, Gao W, Shao F. Pyroptosis: Gasdermin-Mediated Programmed Necrotic Cell Death. Trends Biochem Sci. 2017;42:245-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1123] [Cited by in RCA: 2388] [Article Influence: 238.8] [Reference Citation Analysis (0)] |
| 6. | Zychlinsky A, Prevost MC, Sansonetti PJ. Shigella flexneri induces apoptosis in infected macrophages. Nature. 1992;358:167-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 731] [Cited by in RCA: 942] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 7. | Jorgensen I, Miao EA. Pyroptotic cell death defends against intracellular pathogens. Immunol Rev. 2015;265:130-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 531] [Cited by in RCA: 835] [Article Influence: 83.5] [Reference Citation Analysis (0)] |
| 8. | Li L, Li Y, Bai Y. Role of GSDMB in Pyroptosis and Cancer. Cancer Manag Res. 2020;12:3033-3043. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 83] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 9. | Burgener SS, Leborgne NGF, Snipas SJ, Salvesen GS, Bird PI, Benarafa C. Cathepsin G Inhibition by Serpinb1 and Serpinb6 Prevents Programmed Necrosis in Neutrophils and Monocytes and Reduces GSDMD-Driven Inflammation. Cell Rep. 2019;27:3646-3656.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 213] [Article Influence: 35.5] [Reference Citation Analysis (0)] |
| 10. | Wang CJ, Tang L, Shen DW, Wang C, Yuan QY, Gao W, Wang YK, Xu RH, Zhang H. The expression and regulation of DFNA5 in human hepatocellular carcinoma DFNA5 in hepatocellular carcinoma. Mol Biol Rep. 2013;40:6525-6531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 67] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 11. | Wang Y, Gao W, Shi X, Ding J, Liu W, He H, Wang K, Shao F. Chemotherapy drugs induce pyroptosis through caspase-3 cleavage of a gasdermin. Nature. 2017;547:99-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 994] [Cited by in RCA: 2380] [Article Influence: 264.4] [Reference Citation Analysis (0)] |
| 12. | Tamura M, Tanaka S, Fujii T, Aoki A, Komiyama H, Ezawa K, Sumiyama K, Sagai T, Shiroishi T. Members of a novel gene family, Gsdm, are expressed exclusively in the epithelium of the skin and gastrointestinal tract in a highly tissue-specific manner. Genomics. 2007;89:618-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 253] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 13. | Wei Q, Zhu R, Zhu J, Zhao R, Li M. E2-Induced Activation of the NLRP3 Inflammasome Triggers Pyroptosis and Inhibits Autophagy in HCC Cells. Oncol Res. 2019;27:827-834. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 141] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 14. | Ding J, Wang K, Liu W, She Y, Sun Q, Shi J, Sun H, Wang DC, Shao F. Pore-forming activity and structural autoinhibition of the gasdermin family. Nature. 2016;535:111-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1158] [Cited by in RCA: 2075] [Article Influence: 207.5] [Reference Citation Analysis (0)] |
| 15. | Rogers C, Fernandes-Alnemri T, Mayes L, Alnemri D, Cingolani G, Alnemri ES. Cleavage of DFNA5 by caspase-3 during apoptosis mediates progression to secondary necrotic/pyroptotic cell death. Nat Commun. 2017;8:14128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 578] [Cited by in RCA: 1187] [Article Influence: 131.9] [Reference Citation Analysis (0)] |
| 16. | Liu X, Zhang Z, Ruan J, Pan Y, Magupalli VG, Wu H, Lieberman J. Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature. 2016;535:153-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2315] [Cited by in RCA: 2537] [Article Influence: 253.7] [Reference Citation Analysis (0)] |
| 17. | Aglietti RA, Estevez A, Gupta A, Ramirez MG, Liu PS, Kayagaki N, Ciferri C, Dixit VM, Dueber EC. GsdmD p30 elicited by caspase-11 during pyroptosis forms pores in membranes. Proc Natl Acad Sci U S A. 2016;113:7858-7863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 481] [Cited by in RCA: 749] [Article Influence: 74.9] [Reference Citation Analysis (0)] |
| 18. | Russo HM, Rathkey J, Boyd-Tressler A, Katsnelson MA, Abbott DW, Dubyak GR. Active Caspase-1 Induces Plasma Membrane Pores That Precede Pyroptotic Lysis and Are Blocked by Lanthanides. J Immunol. 2016;197:1353-1367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 162] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 19. | Sborgi L, Rühl S, Mulvihill E, Pipercevic J, Heilig R, Stahlberg H, Farady CJ, Müller DJ, Broz P, Hiller S. GSDMD membrane pore formation constitutes the mechanism of pyroptotic cell death. EMBO J. 2016;35:1766-1778. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 551] [Cited by in RCA: 947] [Article Influence: 94.7] [Reference Citation Analysis (0)] |
| 20. | Pizato N, Luzete BC, Kiffer LFMV, Corrêa LH, de Oliveira Santos I, Assumpção JAF, Ito MK, Magalhães KG. Omega-3 docosahexaenoic acid induces pyroptosis cell death in triple-negative breast cancer cells. Sci Rep. 2018;8:1952. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 145] [Cited by in RCA: 165] [Article Influence: 20.6] [Reference Citation Analysis (1)] |
| 21. | Shi J, Zhao Y, Wang K, Shi X, Wang Y, Huang H, Zhuang Y, Cai T, Wang F, Shao F. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature. 2015;526:660-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2573] [Cited by in RCA: 5038] [Article Influence: 458.0] [Reference Citation Analysis (0)] |
| 22. | He WT, Wan H, Hu L, Chen P, Wang X, Huang Z, Yang ZH, Zhong CQ, Han J. Gasdermin D is an executor of pyroptosis and required for interleukin-1β secretion. Cell Res. 2015;25:1285-1298. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1061] [Cited by in RCA: 2004] [Article Influence: 182.2] [Reference Citation Analysis (0)] |
| 23. | Kayagaki N, Stowe IB, Lee BL, O'Rourke K, Anderson K, Warming S, Cuellar T, Haley B, Roose-Girma M, Phung QT, Liu PS, Lill JR, Li H, Wu J, Kummerfeld S, Zhang J, Lee WP, Snipas SJ, Salvesen GS, Morris LX, Fitzgerald L, Zhang Y, Bertram EM, Goodnow CC, Dixit VM. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature. 2015;526:666-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1796] [Cited by in RCA: 2926] [Article Influence: 266.0] [Reference Citation Analysis (1)] |
| 24. | Wimmer K, Sachet M, Oehler R. Circulating biomarkers of cell death. Clin Chim Acta. 2020;500:87-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 25. | Ding J, Wang K, Liu W, She Y, Sun Q, Shi J, Sun H, Wang DC, Shao F. Erratum: Pore-forming activity and structural autoinhibition of the gasdermin family. Nature. 2016;540:150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 54] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 26. | Zhang Z, Zhang H, Li D, Zhou X, Qin Q, Zhang Q. Caspase-3-mediated GSDME induced Pyroptosis in breast cancer cells through the ROS/JNK signalling pathway. J Cell Mol Med. 2021;25:8159-8168. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 140] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 27. | Kolb R, Liu GH, Janowski AM, Sutterwala FS, Zhang W. Inflammasomes in cancer: a double-edged sword. Protein Cell. 2014;5:12-20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 162] [Cited by in RCA: 211] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 28. | Gao J, Qiu X, Xi G, Liu H, Zhang F, Lv T, Song Y. Downregulation of GSDMD attenuates tumor proliferation via the intrinsic mitochondrial apoptotic pathway and inhibition of EGFR/Akt signaling and predicts a good prognosis in nonsmall cell lung cancer. Oncol Rep. 2018;40:1971-1984. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 168] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 29. | Wang WJ, Chen D, Jiang MZ, Xu B, Li XW, Chu Y, Zhang YJ, Mao R, Liang J, Fan DM. Downregulation of gasdermin D promotes gastric cancer proliferation by regulating cell cycle-related proteins. J Dig Dis. 2018;19:74-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 148] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 30. | Lu H, Zhang S, Wu J, Chen M, Cai MC, Fu Y, Li W, Wang J, Zhao X, Yu Z, Ma P, Zhuang G. Molecular Targeted Therapies Elicit Concurrent Apoptotic and GSDME-Dependent Pyroptotic Tumor Cell Death. Clin Cancer Res. 2018;24:6066-6077. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 195] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 31. | Zhang CC, Li CG, Wang YF, Xu LH, He XH, Zeng QZ, Zeng CY, Mai FY, Hu B, Ouyang DY. Chemotherapeutic paclitaxel and cisplatin differentially induce pyroptosis in A549 lung cancer cells via caspase-3/GSDME activation. Apoptosis. 2019;24:312-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 344] [Article Influence: 49.1] [Reference Citation Analysis (0)] |
| 32. | Sharma D, Malik A, Guy CS, Karki R, Vogel P, Kanneganti TD. Pyrin Inflammasome Regulates Tight Junction Integrity to Restrict Colitis and Tumorigenesis. Gastroenterology. 2018;154:948-964.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 132] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 33. | Wang Y, Yin B, Li D, Wang G, Han X, Sun X. GSDME mediates caspase-3-dependent pyroptosis in gastric cancer. Biochem Biophys Res Commun. 2018;495:1418-1425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 244] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 34. | Feng S, Fox D, Man SM. Mechanisms of Gasdermin Family Members in Inflammasome Signaling and Cell Death. J Mol Biol. 2018;430:3068-3080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 284] [Article Influence: 35.5] [Reference Citation Analysis (0)] |
| 35. | Wang Y, Kong H, Zeng X, Liu W, Wang Z, Yan X, Wang H, Xie W. Activation of NLRP3 inflammasome enhances the proliferation and migration of A549 lung cancer cells. Oncol Rep. 2016;35:2053-2064. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 156] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 36. | Guo B, Fu S, Zhang J, Liu B, Li Z. Targeting inflammasome/IL-1 pathways for cancer immunotherapy. Sci Rep. 2016;6:36107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 139] [Cited by in RCA: 228] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 37. | Ershaid N, Sharon Y, Doron H, Raz Y, Shani O, Cohen N, Monteran L, Leider-Trejo L, Ben-Shmuel A, Yassin M, Gerlic M, Ben-Baruch A, Pasmanik-Chor M, Apte R, Erez N. NLRP3 inflammasome in fibroblasts links tissue damage with inflammation in breast cancer progression and metastasis. Nat Commun. 2019;10:4375. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 112] [Cited by in RCA: 247] [Article Influence: 35.3] [Reference Citation Analysis (0)] |
| 38. | Weichand B, Popp R, Dziumbla S, Mora J, Strack E, Elwakeel E, Frank AC, Scholich K, Pierre S, Syed SN, Olesch C, Ringleb J, Ören B, Döring C, Savai R, Jung M, von Knethen A, Levkau B, Fleming I, Weigert A, Brüne B. S1PR1 on tumor-associated macrophages promotes lymphangiogenesis and metastasis via NLRP3/IL-1β. J Exp Med. 2017;214:2695-2713. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 142] [Cited by in RCA: 231] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 39. | Wei Q, Mu K, Li T, Zhang Y, Yang Z, Jia X, Zhao W, Huai W, Guo P, Han L. Deregulation of the NLRP3 inflammasome in hepatic parenchymal cells during liver cancer progression. Lab Invest. 2014;94:52-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 228] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 40. | Zhang H, Li L, Liu L. FcγRI (CD64) contributes to the severity of immune inflammation through regulating NF-κB/NLRP3 inflammasome pathway. Life Sci. 2018;207:296-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 41. | Hoseini Z, Sepahvand F, Rashidi B, Sahebkar A, Masoudifar A, Mirzaei H. NLRP3 inflammasome: Its regulation and involvement in atherosclerosis. J Cell Physiol. 2018;233:2116-2132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 257] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 42. | Li J, Qiu G, Fang B, Dai X, Cai J. Deficiency of IL-18 Aggravates Esophageal Carcinoma Through Inhibiting IFN-γ Production by CD8(+)T Cells and NK Cells. Inflammation. 2018;41:667-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 43. | Wei YS, Lan Y, Liu YG, Tang H, Tang RG, Wang JC. Interleukin-18 gene promoter polymorphisms and the risk of esophageal squamous cell carcinoma. Acta Oncol. 2007;46:1090-1096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 33] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 44. | Kim KE, Song H, Kim TS, Yoon D, Kim CW, Bang SI, Hur DY, Park H, Cho DH. Interleukin-18 is a critical factor for vascular endothelial growth factor-enhanced migration in human gastric cancer cell lines. Oncogene. 2007;26:1468-1476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 65] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 45. | Kim KE, Song H, Hahm C, Yoon SY, Park S, Lee HR, Hur DY, Kim T, Kim CH, Bang SI, Bang JW, Park H, Cho DH. Expression of ADAM33 is a novel regulatory mechanism in IL-18-secreted process in gastric cancer. J Immunol. 2009;182:3548-3555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 46. | Majima T, Ichikura T, Chochi K, Kawabata T, Tsujimoto H, Sugasawa H, Kuranaga N, Takayama E, Kinoshita M, Hiraide H, Seki S, Mochizuki H. Exploitation of interleukin-18 by gastric cancers for their growth and evasion of host immunity. Int J Cancer. 2006;118:388-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 28] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 47. | Ahmad I, Muneer KM, Tamimi IA, Chang ME, Ata MO, Yusuf N. Thymoquinone suppresses metastasis of melanoma cells by inhibition of NLRP3 inflammasome. Toxicol Appl Pharmacol. 2013;270:70-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 119] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 48. | Salado C, Olaso E, Gallot N, Valcarcel M, Egilegor E, Mendoza L, Vidal-Vanaclocha F. Resveratrol prevents inflammation-dependent hepatic melanoma metastasis by inhibiting the secretion and effects of interleukin-18. J Transl Med. 2011;9:59. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 47] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 49. | Yang X, Qiu MT, Hu JW, Jiang F, Li M, Wang J, Zhang Q, Yin R, Xu L. Association of interleukin-18 gene promoter -607 C>A and -137G>C polymorphisms with cancer risk: a meta-analysis of 26 studies. PLoS One. 2013;8:e73671. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 50. | Wang M, Zhu XY, Wang L, Lin Y. The -607C/A polymorphisms in interleukin-18 gene promoter contributes to cancer risk: evidence from a meta-analysis of 22 case-control studies. PLoS One. 2013;8:e76915. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 51. | Esmailbeig M, Ghaderi A. Interleukin-18: a regulator of cancer and autoimmune diseases. Eur Cytokine Netw. 2017;28:127-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 68] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 52. | Fabbi M, Carbotti G, Ferrini S. Context-dependent role of IL-18 in cancer biology and counter-regulation by IL-18BP. J Leukoc Biol. 2015;97:665-675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 101] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 53. | Tulotta C, Lefley DV, Freeman K, Gregory WM, Hanby AM, Heath PR, Nutter F, Wilkinson JM, Spicer-Hadlington AR, Liu X, Bradbury SMJ, Hambley L, Cookson V, Allocca G, Kruithof de Julio M, Coleman RE, Brown JE, Holen I, Ottewell PD. Endogenous Production of IL1B by Breast Cancer Cells Drives Metastasis and Colonization of the Bone Microenvironment. Clin Cancer Res. 2019;25:2769-2782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 137] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 54. | Yu Q, Zhang M, Ying Q, Xie X, Yue S, Tong B, Wei Q, Bai Z, Ma L. Decrease of AIM2 mediated by luteolin contributes to non-small cell lung cancer treatment. Cell Death Dis. 2019;10:218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 71] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 55. | Chen L, Weng B, Li H, Wang H, Li Q, Wei X, Deng H, Wang S, Jiang C, Lin R, Wu J. A thiopyran derivative with low murine toxicity with therapeutic potential on lung cancer acting through a NF-κB mediated apoptosis-to-pyroptosis switch. Apoptosis. 2019;24:74-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 64] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 56. | Storr SJ, Safuan S, Ahmad N, El-Refaee M, Jackson AM, Martin SG. Macrophage-derived interleukin-1beta promotes human breast cancer cell migration and lymphatic adhesion in vitro. Cancer Immunol Immunother. 2017;66:1287-1294. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 57. | Jiménez-Garduño AM, Mendoza-Rodríguez MG, Urrutia-Cabrera D, Domínguez-Robles MC, Pérez-Yépez EA, Ayala-Sumuano JT, Meza I. IL-1β induced methylation of the estrogen receptor ERα gene correlates with EMT and chemoresistance in breast cancer cells. Biochem Biophys Res Commun. 2017;490:780-785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 77] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 58. | Sharma BR, Karki R, Kanneganti TD. Role of AIM2 inflammasome in inflammatory diseases, cancer and infection. Eur J Immunol. 2019;49:1998-2011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 220] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 59. | Man SM, Karki R, Kanneganti TD. AIM2 inflammasome in infection, cancer, and autoimmunity: Role in DNA sensing, inflammation, and innate immunity. Eur J Immunol. 2016;46:269-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 278] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 60. | Chen SL, Liu LL, Lu SX, Luo RZ, Wang CH, Wang H, Cai SH, Yang X, Xie D, Zhang CZ, Yun JP. HBx-mediated decrease of AIM2 contributes to hepatocellular carcinoma metastasis. Mol Oncol. 2017;11:1225-1240. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 84] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 61. | Ma X, Guo P, Qiu Y, Mu K, Zhu L, Zhao W, Li T, Han L. Loss of AIM2 expression promotes hepatocarcinoma progression through activation of mTOR-S6K1 pathway. Oncotarget. 2016;7:36185-36197. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 100] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 62. | So D, Shin HW, Kim J, Lee M, Myeong J, Chun YS, Park JW. Cervical cancer is addicted to SIRT1 disarming the AIM2 antiviral defense. Oncogene. 2018;37:5191-5204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 76] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 63. | Mori Y, Yin J, Rashid A, Leggett BA, Young J, Simms L, Kuehl PM, Langenberg P, Meltzer SJ, Stine OC. Instabilotyping: comprehensive identification of frameshift mutations caused by coding region microsatellite instability. Cancer Res. 2001;61:6046-6049. [PubMed] |
| 64. | Schulmann K, Brasch FE, Kunstmann E, Engel C, Pagenstecher C, Vogelsang H, Krüger S, Vogel T, Knaebel HP, Rüschoff J, Hahn SA, Knebel-Doeberitz MV, Moeslein G, Meltzer SJ, Schackert HK, Tympner C, Mangold E, Schmiegel W; German HNPCC Consortium. HNPCC-associated small bowel cancer: clinical and molecular characteristics. Gastroenterology. 2005;128:590-599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 132] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 65. | Wilson JE, Petrucelli AS, Chen L, Koblansky AA, Truax AD, Oyama Y, Rogers AB, Brickey WJ, Wang Y, Schneider M, Mühlbauer M, Chou WC, Barker BR, Jobin C, Allbritton NL, Ramsden DA, Davis BK, Ting JP. Inflammasome-independent role of AIM2 in suppressing colon tumorigenesis via DNA-PK and Akt. Nat Med. 2015;21:906-913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 250] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 66. | Man SM, Zhu Q, Zhu L, Liu Z, Karki R, Malik A, Sharma D, Li L, Malireddi RK, Gurung P, Neale G, Olsen SR, Carter RA, McGoldrick DJ, Wu G, Finkelstein D, Vogel P, Gilbertson RJ, Kanneganti TD. Critical Role for the DNA Sensor AIM2 in Stem Cell Proliferation and Cancer. Cell. 2015;162:45-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 276] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 67. | Patsos G, Germann A, Gebert J, Dihlmann S. Restoration of absent in melanoma 2 (AIM2) induces G2/M cell cycle arrest and promotes invasion of colorectal cancer cells. Int J Cancer. 2010;126:1838-1849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 75] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 68. | Ratsimandresy RA, Indramohan M, Dorfleutner A, Stehlik C. The AIM2 inflammasome is a central regulator of intestinal homeostasis through the IL-18/IL-22/STAT3 pathway. Cell Mol Immunol. 2017;14:127-142. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 128] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 69. | Chen LC, Wang LJ, Tsang NM, Ojcius DM, Chen CC, Ouyang CN, Hsueh C, Liang Y, Chang KP, Chang YS. Tumour inflammasome-derived IL-1β recruits neutrophils and improves local recurrence-free survival in EBV-induced nasopharyngeal carcinoma. EMBO Mol Med. 2012;4:1276-1293. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 147] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 70. | Farshchian M, Nissinen L, Siljamäki E, Riihilä P, Piipponen M, Kivisaari A, Kallajoki M, Grénman R, Peltonen J, Peltonen S, Quint KD, Bavinck JNB, Kähäri VM. Tumor cell-specific AIM2 regulates growth and invasion of cutaneous squamous cell carcinoma. Oncotarget. 2017;8:45825-45836. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 64] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Arslan M, Turkey; Portillo R, Czech Republic S-Editor: Liu JH L-Editor: A P-Editor: Liu JH