Published online Jan 6, 2023. doi: 10.12998/wjcc.v11.i1.218
Peer-review started: October 16, 2022
First decision: November 4, 2022
Revised: November 10, 2022
Accepted: December 16, 2022
Article in press: December 16, 2022
Published online: January 6, 2023
Processing time: 80 Days and 18.5 Hours
Immune checkpoint inhibitor (ICI)-induced rheumatic immune-related adverse events (irAEs) have been infrequently reported, and the treatment of severe or refractory arthritis as irAEs has not been established yet.
The patient was a 67-year-old man with a history of well-controlled foot psoriasis who presented with polyarthralgia. He had received pembrolizumab for metastatic gastric adenocarcinoma 2 mo previously. Physical examination revealed erythematous swelling in the distal interphalangeal joints, left shoulder, and both knees. He had plaque psoriasis with psoriatic nail dystrophy and dactylitis in the distal joints of the fingers and toes. Inflammatory markers including C-reactive protein and erythrocyte sedimentation rate were elevated but rheumatoid factor and anticyclic citrullinated peptide antibody were negative. The patient was diagnosed with psoriatic arthritis (PsA) and started on methylprednisolone 1 mg/kg/day after pembrolizumab discontinuation. However, despite 1 wk of methylprednisolone treatment, PsA worsened; hence, leflunomide and methotrexate were started. After 4 wk of steroid treatment, PsA worsened and improved repeatedly with steroid tapering. Therefore, the therapy was intensified to include etanercept, a tumor necrosis factor inhibitor, which ultimately resulted in adequate PsA control.
This is the first report of ICI-induced PsA in a gastric cancer patient. Some rheumatic irAEs with refractory severe arthritis may require disease-modifying anti-rheumatic drugs and long-term management.
Core Tip: Although immune checkpoint inhibitors are being used increasingly and widely, a fair percentage of patients treated with these drugs experience immune-related adverse events (irAEs). Arthralgia and skin lesions are among the most frequent irAEs. However, rheumatic irAEs are underreported and/or misclassified and have been poorly characterized. These rheumatic irAEs are different in simple arthralgia or cutaneous irAEs that they may be requiring long-term management and disease-modifying anti-rheumatic drugs in severe or refractory case. We report a first case of pembrolizumab-induced psoriatic arthritis that was treated with tumor necrosis factor-α inhibitors in a patient with gastric cancer and discuss the treatment course.
- Citation: Kim S, Sun JH, Kim H, Kim HK, Yang Y, Lee JS, Choi IA, Han HS. Pembrolizumab-induced psoriatic arthritis treated with disease-modifying anti-rheumatic drugs in a patient with gastric cancer: A case report. World J Clin Cases 2023; 11(1): 218-224
- URL: https://www.wjgnet.com/2307-8960/full/v11/i1/218.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i1.218
Immune checkpoint inhibitors (ICIs) are novel biological agents that are used to treat various malignant tumors by blocking cytotoxic T-lymphocyte antigen and programmed cell death 1 (PD-1) or its ligand, which limits T cell cytotoxicity to tumors. Although ICIs have revolutionized cancer treatment, currently, 10%-60% of ICI-treated patients experience immune-related adverse events (irAEs)[1]. These irAEs can result in inflammatory organ damage by excessive activation of the immune system, including that of the lungs, liver, skin, thyroid, and gastrointestinal tract[1]. Arthralgia and skin lesions are the most frequent musculoskeletal and dermatologic irAEs[1]. However, rheumatic irAEs are underreported and/or misclassified as simple musculoskeletal or dermatologic irAEs in clinical trials and have been poorly characterized due to their clinical variability. Moreover, treatment strategies for rheumatic irAEs have not been established yet and are still controversial. A few case series have suggested that biologic disease-modifying anti-rheumatic drugs (s), including tumor necrosis factor-α (TNF-α) inhibitors or interleukin-6 receptor antagonists, are effective[2-6].
Herein, we report a case of pembrolizumab-induced psoriatic arthritis (PsA) that was treated with TNF-α inhibitors in a patient with gastric cancer. We present the following case in accordance with the CARE reporting checklist.
A 67-year-old man presented with a 2-wk history of progressively worsening polyarthralgia and nail dystrophy.
The patient was diagnosed with advanced gastric adenocarcinoma with distant lymph nodes (LNs) metastases was referred to our department in March 2020. Esophagogastroduodenoscopy revealed a 3-cm sized ulcerofungating mass in the pre-pyloric antrum. Histopathological examination of the endoscopic biopsy specimen revealed moderately differentiated adenocarcinoma, negative results for human epidermal growth factor receptor 2 (immunohistochemistry 1+), Epstein-Barr virus, and deficient DNA mismatch repair (dMMR) (retained nuclear expression of MSH2 and MSH6 and loss of MLH-1 and PMS-1). Abdominal computed tomography showed multiple enlarged LNs in the para-aortic and aortocaval areas. The patient was treated with capecitabine and oxaliplatin as the first-line palliative chemotherapy for 11 mo, followed by ramucirumab and paclitaxel as second-line palliative chemotherapy. After 2 mo of treatment with ramucirumab and paclitaxel, the tumor response indicated progressive disease. In June 2021, pembrolizumab (200 mg in a 3-wk cycle) was initiated as third-line palliative chemotherapy, given that the tumors showed dMMR.
He had a history of foot psoriasis, which was well controlled without treatment.
He had no personal and family history.
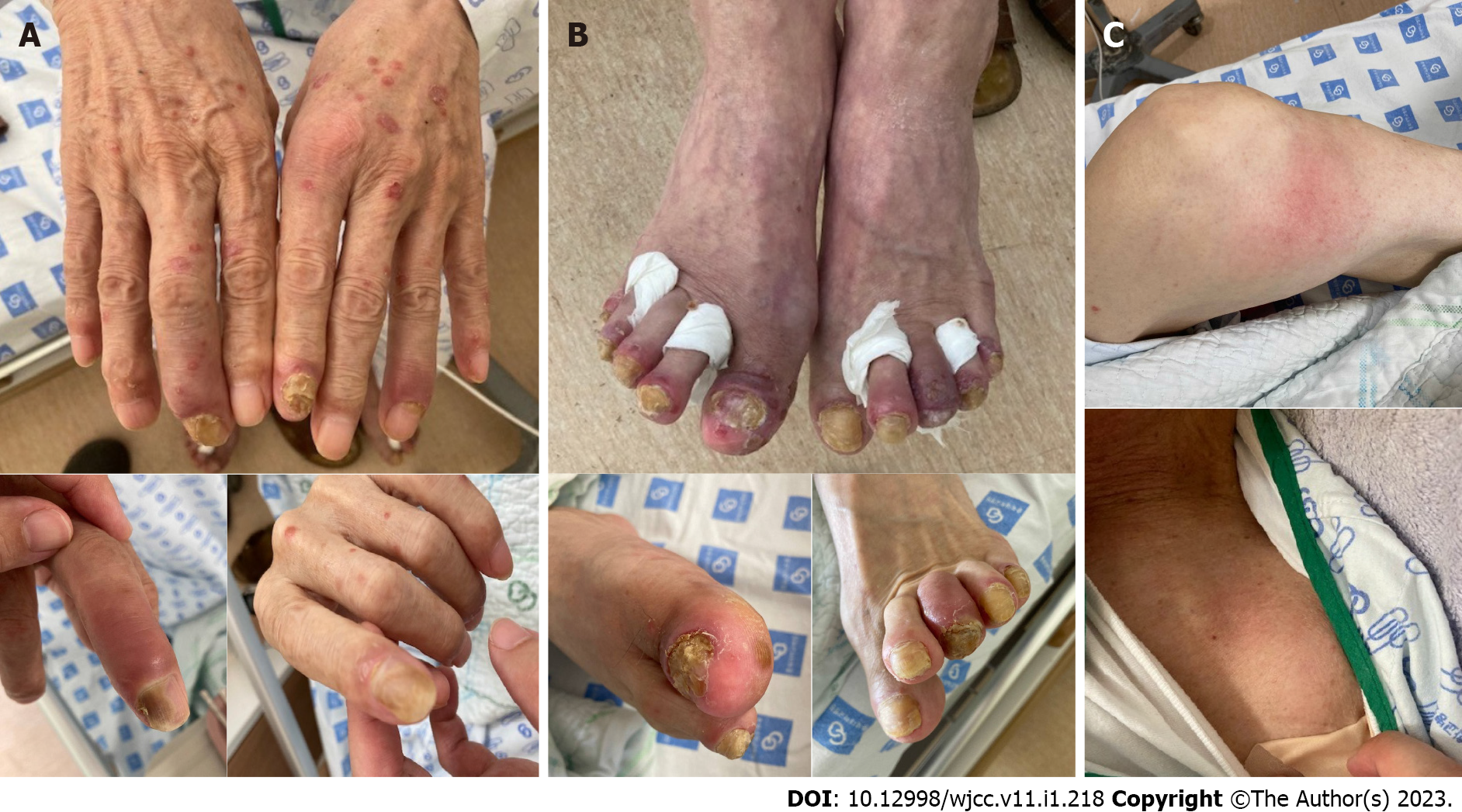
The patient was afebrile with stable vital signs. Physical examination revealed erythematous swelling in the distal interphalangeal joints, left shoulder, and both knees (Figure 1). He had plaque psoriasis with psoriatic nail dystrophy and dactylitis in the distal joints of the fingers and toes (Figure 1).
Before pembrolizumab administration, the neutrophil-to-lymphocyte ratio (NLR, absolute neutrophil count divided by absolute lymphocyte count) and prognostic nutritional index (PNI, 10 × serum albumin value, g/dL + 0.005 × total lymphocyte count/mm3) were 1.1 and 50.5, respectively. Two months after the initiation of pembrolizumab, laboratory testing revealed a normal white blood cell count of 7970/µL with 72.6% neutrophils and 17.9% lymphocytes, an elevated erythrocyte sedimentation rate (ESR) of 89.0 mm/h, and a serum high-sensitivity C-reactive protein (hs-CRP) level of 2.3 mg/L. Immunological serum tests were negative for rheumatoid factor (RF) and anticyclic citrullinated peptide antibody. Synovial fluid analysis of knee joint revealed a yellow appearance, decreased viscosity, and a cell count of 26,678 cells/mm3 with 81.2% neutrophils, indicating inflammatory arthritis. Gram staining was negative, no crystals were detected by microscopy, and cultures for common pathogens and mycobacteria were negative.
Based on the clinical evaluation and laboratory results, the patient was diagnosed with PsA as severe rheumatic irAEs [Common Terminology Criteria for Adverse Events (CTCAE) grade 3]. Disease activity score by calculating disease activity in psoriatic arthritis (DAPSA) (tender joint counts, swollen joints count, CRP, patient’s assessment of disease activity and pain) was 59.3.
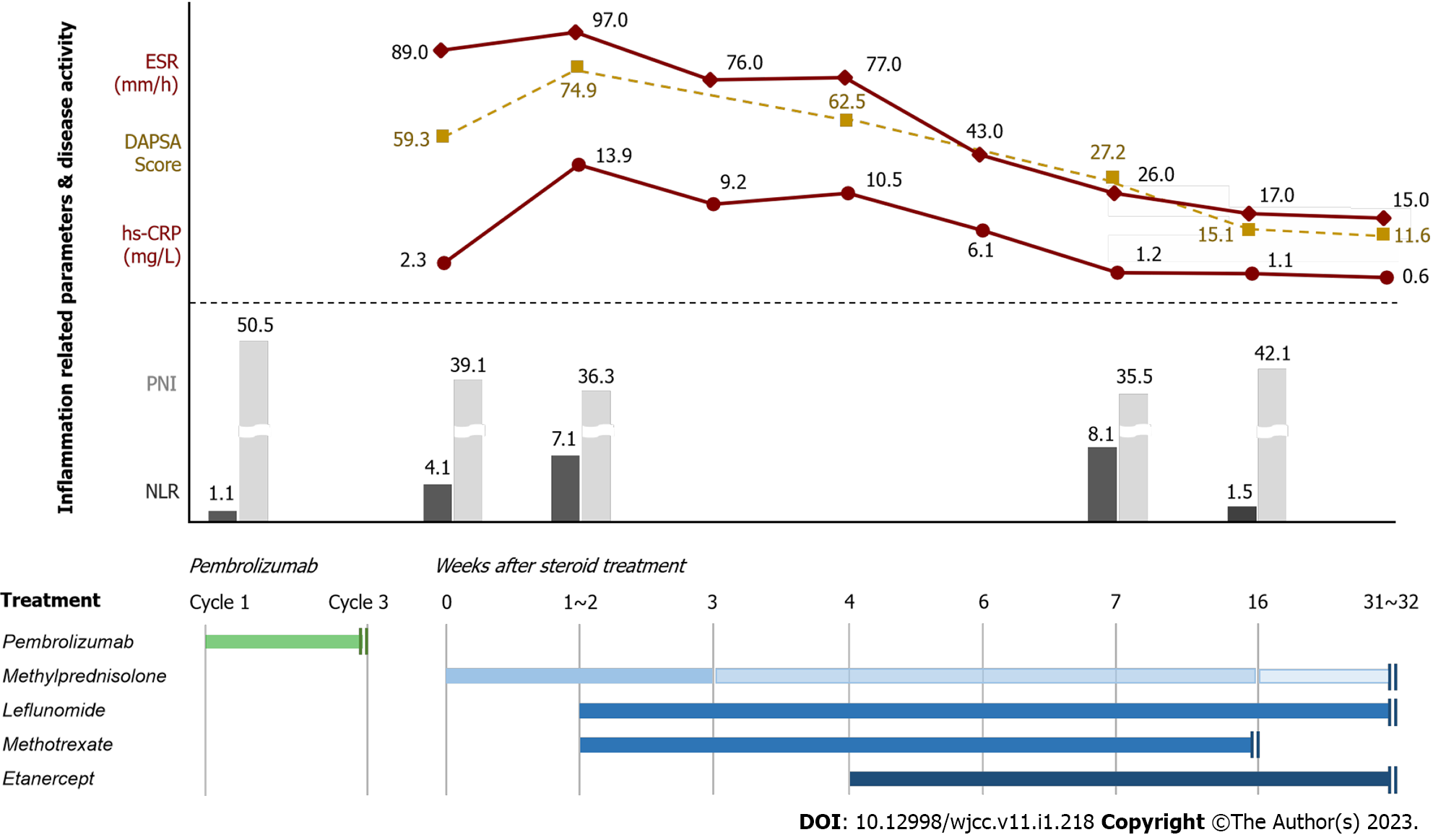
The rheumatology department was consulted, and methylprednisolone (1 mg/kg/day) and non-steroidal anti-inflammatory drug (celecoxib 200 mg bid daily) were administered after pembrolizumab discontinuation. However, despite 1-2 wk of methylprednisolone treatment, his polyarthralgia and joint swelling worsened and ESR, hs-CRP level, and DAPSA score were elevated to 97.0 mm/h, 13.9 mg/L, and 74.9, respectively. Subsequently, he was treated with 10 mg of leflunomide daily and 10 mg of methotrexate weekly. After 4 wk of steroid treatment, PsA worsened and improved repeatedly with steroid tapering. Moreover, ESR, hs-CRP, and DAPSA score were still elevated at 77.0 mm/h, 10.5 mg/L, and 62.5, respectively. Therefore, the therapy was intensified with etanercept 50 mg weekly. After 2 wk of etanercept treatment, ESR, hs-CRP level, and DAPSA score decreased to 26.0 mm/h, 1.2 mg/L, and 27.2, respectively, and PsA-related symptoms improved. Leflunomide and etanercept were continued, methylprednisolone was tapered, and methotrexate was discontinued 3 mo after etanercept administration. The treatment course for PsA and the changes in inflammation-related peripheral blood parameters and disease activity are shown in Figure 3.
Although tumor response after three cycles of pembrolizumab treatment was stable disease, pembrolizumab was discontinued permanently. The disease progressed 4 mo after discontinuation of pembrolizumab, and the progression-free survival for pembrolizumab was 5.5 mo. In November 2021, the patient was treated with irinotecan for 4 mo as forth-line palliative chemotherapy, after which palliative systemic therapy was discontinued. The patient’s mild polyarthralgia and nail dystrophy (CTCAE grade 1) persisted, and low-dose methylprednisolone and etanercept were maintained until March 2022.
To our knowledge, this is the first case of PsA in a patient treated with an ICI for gastric cancer. Thus far, PsA has been reported in a few patients with melanoma and lung cancer[7-9].
The classification criteria for psoriatic arthritis consist of inflammatory articular disease with the minimum of 3 points from the following items: evidence of psoriasis (current, personal history or familial history), psoriatic nail dystrophy, a negative test for RF, dactylitis, and juxta-articular new bone formation[10]. In the case of our patient, although there was no juxta-articular new bone formation, all other criteria were met for the diagnosis of PsA. PsA is a heterogeneous chronic inflammatory disease with a wide clinical spectrum and a variable clinical course that affects the skin and musculoskeletal system. A recent study on the clinical patterns of rheumatic irAEs reported that patients with PsA patterns had a significantly higher risk of persistent arthritis[6]. In our case, PsA-related symptoms persisted for more than 8 mo. Therefore, it is necessary to distinguish between simple arthralgia and inflammatory arthritis as rheumatic irAEs when a patient who has received ICI presents with arthralgia.
The biological drivers of irAEs are poorly characterized, and currently, there is no method available in clinical practice to identify patients at high risk of experiencing these events. Several recent studies have found that a low NLR (< 5) and high PNI (≥ 45) in patients with non-small cell lung cancer treated with anti-PD-1 therapy are significantly associated with better survival and the onset of irAEs[11-13]. In our case, NLR and PNI before pembrolizumab treatment were 1.1 and 50.5, respectively. The patient was responsive to pembrolizumab treatment (stable disease) and developed severe PsA as an irAE. Although the predictive role of these inflammation-related biomarkers (NLR and PNI) was preliminary, pretreatment blood biomarker levels may correlate with the onset of irAEs in patients receiving ICIs.
Our patient had severe (grade 3) and refractory (corticosteroid was ineffective) arthritis requiring conventional and biologic DMARD therapies. The National Comprehensive Cancer Network and European Society for Medical Oncology guidelines recommend classifying and treating immune-related inflammatory arthritis according to its severity[14,15]. For severe arthritis that limits instrumental active daily living, immunotherapy must be withheld, and methylprednisolone should be used at a dosage of 1 mg/kg/day. If there was no improvement by week 2, rheumatologists should be consulted for the consideration of additional DMARDs, depending on the clinical phenotype of inflammatory arthritis. Recent reviews of ICI-induced arthritis have reported that many patients respond to steroid treatment; however, a large subset requires additional therapies, such as conventional or biologic DMARDs[3-6]. Corticosteroids are the mainstay of treatment for immune-related inflammatory arthritis; however, in refractory cases, it is recommended to consider either conventional or biological DMARDs. In our case, the patient achieved remission with the initiation of the TNF-α inhibitor therapy.
In conclusion, rheumatic irAEs are different from simple arthralgia or cutaneous AEs, which may require long-term management. Moreover, in severe or refractory cases, it is recommended to consider either synthetic or biological DMARDs. Because ICIs are being used with increasing frequency in patients with gastric cancer, there is a need for increased awareness of these rheumatic irAEs among oncologists and rheumatologists, and coordinated multidisciplinary management is critical.
| 1. | Postow MA, Sidlow R, Hellmann MD. Immune-Related Adverse Events Associated with Immune Checkpoint Blockade. N Engl J Med. 2018;378:158-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2308] [Cited by in RCA: 3454] [Article Influence: 431.8] [Reference Citation Analysis (0)] |
| 2. | Lidar M, Giat E, Garelick D, Horowitz Y, Amital H, Steinberg-Silman Y, Schachter J, Shapira-Frommer R, Markel G. Rheumatic manifestations among cancer patients treated with immune checkpoint inhibitors. Autoimmun Rev. 2018;17:284-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 140] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 3. | Roberts J, Ennis D, Hudson M, Ye C, Saltman A, Himmel M, Rottapel R, Pope J, Hoa S, Tisseverasinghe A, Fifi-Mah A, Maltez N, Jamal S. Rheumatic immune-related adverse events associated with cancer immunotherapy: A nationwide multi-center cohort. Autoimmun Rev. 2020;19:102595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 56] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 4. | Ghosh N, Bass AR. Rheumatic Complications of Immune Checkpoint Inhibitors. Rheum Dis Clin North Am. 2022;48:411-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 5. | Chatzidionysiou K, Liapi M, Tsakonas G, Gunnarsson I, Catrina A. Treatment of rheumatic immune-related adverse events due to cancer immunotherapy with immune checkpoint inhibitors-is it time for a paradigm shift? Clin Rheumatol. 2021;40:1687-1695. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 6. | Gómez-Puerta JA, Lobo-Prat D, Perez-García C, Ponce A, Frade-Sosa B, Millán Arciniegas AM, Ojeda F, Ruiz-Esquide V, Corominas H. Clinical Patterns and Follow-Up of Inflammatory Arthritis and Other Immune-Related Adverse Events Induced by Checkpoint Inhibitors. A Multicenter Study. Front Med (Lausanne). 2022;9:888377. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 7. | Ruiz-Bañobre J, Pérez-Pampín E, García-González J, Gómez-Caamaño A, Barón-Duarte FJ, López-López R, Vázquez-Rivera F. Development of psoriatic arthritis during nivolumab therapy for metastatic non-small cell lung cancer, clinical outcome analysis and review of the literature. Lung Cancer. 2017;108:217-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 8. | Jatwani K, Kaur H, Chugh K, Jatwani S. Nivolumab-Induced Psoriatic Arthritis in a Patient With Advanced Small Cell Lung Cancer. J Clin Rheumatol. 2021;27:e162-e163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 9. | Halle BR, Betof Warner A, Zaman FY, Haydon A, Bhave P, Dewan AK, Ye F, Irlmeier R, Mehta P, Kurtansky NR, Lacouture ME, Hassel JC, Choi JS, Sosman JA, Chandra S, Otto TS, Sullivan R, Mooradian MJ, Chen ST, Dimitriou F, Long G, Carlino M, Menzies A, Johnson DB, Rotemberg VM. Immune checkpoint inhibitors in patients with pre-existing psoriasis: safety and efficacy. J Immunother Cancer. 2021;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 50] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 10. | Taylor W, Gladman D, Helliwell P, Marchesoni A, Mease P, Mielants H; CASPAR Study Group. Classification criteria for psoriatic arthritis: development of new criteria from a large international study. Arthritis Rheum. 2006;54:2665-2673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2083] [Cited by in RCA: 2626] [Article Influence: 131.3] [Reference Citation Analysis (0)] |
| 11. | Liu W, Liu Y, Ma F, Sun B, Wang Y, Luo J, Liu M, Luo Z. Peripheral Blood Markers Associated with Immune-Related Adverse Effects in Patients Who Had Advanced Non-Small Cell Lung Cancer Treated with PD-1 Inhibitors. Cancer Manag Res. 2021;13:765-771. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 12. | Matsukane R, Watanabe H, Minami H, Hata K, Suetsugu K, Tsuji T, Masuda S, Okamoto I, Nakagawa T, Ito T, Eto M, Mori M, Nakanishi Y, Egashira N. Continuous monitoring of neutrophils to lymphocytes ratio for estimating the onset, severity, and subsequent prognosis of immune related adverse events. Sci Rep. 2021;11:1324. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 57] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 13. | Peng L, Wang Y, Liu F, Qiu X, Zhang X, Fang C, Qian X, Li Y. Peripheral blood markers predictive of outcome and immune-related adverse events in advanced non-small cell lung cancer treated with PD-1 inhibitors. Cancer Immunol Immunother. 2020;69:1813-1822. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 196] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 14. | National Comprehensive Cancer Network. Clinical Practive Guidelines in Oncology for Management of Immunotherapy-Related Toxicities. [Internet] [accessed 1 August 2022]. Available from: https://www.nccn.org/professionals/physician_gls/pdf/immunotherapy.pdf. |
| 15. | Haanen JBAG, Carbonnel F, Robert C, Kerr KM, Peters S, Larkin J, Jordan K; ESMO Guidelines Committee. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29:iv264-iv266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 337] [Article Influence: 42.1] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Atanasova EG, Bulgaria; Tovoli F, Italy S-Editor: Liu GL L-Editor: A P-Editor: Liu GL