Published online Mar 26, 2022. doi: 10.12998/wjcc.v10.i9.2751
Peer-review started: August 11, 2021
First decision: October 20, 2021
Revised: November 30, 2021
Accepted: February 12, 2022
Article in press: February 12, 2022
Published online: March 26, 2022
Processing time: 223 Days and 10.5 Hours
The exact definition of Acute kidney injury (AKI) for patients with traumatic brain injury (TBI) is unknown.
To compare the power of the “Risk, Injury, Failure, Loss of kidney function, and End-stage kidney disease” (RIFLE), Acute Kidney Injury Network (AKIN), Creatinine kinetics (CK), and Kidney Disease Improving Global Outcomes (KDIGO) to determine AKI incidence/stage and their association with the in-hospital mortality rate of patients with TBI.
This retrospective study collected the data of patients admitted to the intensive care unit for neurotrauma from 2001 to 2012, and 1648 patients were included. The subjects in this study were assessed for the presence and stage of AKI using RIFLE, AKIN, CK, and KDIGO. In addition, the propensity score matching method was used.
Among the 1648 patients, 291 (17.7%) had AKI, according to KDIGO. The highest incidence of AKI was found by KDIGO (17.7%), followed by AKIN (17.1%), RIFLE (12.7%), and CK (11.5%) (P = 0.97). Concordance between KDIGO and RIFLE/AKIN/CK was 99.3%/99.1%/99.3% for stage 0, 36.0%/91.5%/44.5% for stage 1, 35.9%/90.6%/11.3% for stage 2, and 47.4%/89.5%/36.8% for stage 3. The in-hospital mortality rates increased with the AKI stage in all four definitions. The severity of AKI by all definitions and stages was not associated with in-hospital mortality in the multivariable analyses (all P > 0.05).
Differences are seen in AKI diagnosis and in-hospital mortality among the four AKI definitions or stages. This study revealed that KDIGO is the best method to define AKI in patients with TBI.
Core Tip: Because the exact definition of Acute Kidney Injury (AKI) for patients with Traumatic brain injury (TBI) is unknown, this study compared the power of four different AKI diagnose criteria to determine AKI incidence/stage and their association with the in-hospital mortality rate of patients with TBI.
- Citation: Huang ZY, Liu Y, Huang HF, Huang SH, Wang JX, Tian JF, Zeng WX, Lv RG, Jiang S, Gao JL, Gao Y, Yu XX. Acute kidney injury in traumatic brain injury intensive care unit patients. World J Clin Cases 2022; 10(9): 2751-2763
- URL: https://www.wjgnet.com/2307-8960/full/v10/i9/2751.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i9.2751
Traumatic brain injury (TBI) is a debilitating condition that can be exacerbated by the co-occurrence of acute kidney injury (AKI), which is a clinical syndrome characterized by the abrupt loss of the kidney's excretory function and is often combined with oliguria. The development of AKI usually occurs over the course of hours to days[1]. AKI is observed in 9% of the patients with TBI, and 42% of those patients die in the hospital[2]. Thus, early identification and subsequent clinical intervention of AKI in TBI patients are critical to survival[3].
Nevertheless, it is difficult to determine the true incidence and outcomes of AKI due to the use of different validation criteria[4-6]. The reported incidence of AKI varies greatly, ranging from 15% to 74.2%[7-10]. Moreover, serum creatinine (SCr) and urine output (UO) in patients with TBI are greatly impacted by muscle injury or breakdown secondary to decreased perfusion pressure and the use of osmotic diuretics like mannitol[4-6]. Therefore, sensitive and reliable criteria for AKI are needed for diagnosis and staging, especially in TBI patients.
Since 2004, at least four different AKI definitions and criteria have been proposed. The “Risk, Injury, Failure, Loss of kidney function, and End-stage kidney disease” (RIFLE) classification was the first validated tool for AKI identification[11]. It is based on SCr levels and UO and defines three severity classes of AKI (risk, injury, and failure) and two outcome classes (Loss of kidney function and End-stage kidney disease). Following that, the Acute Kidney Injury Network (AKIN) criteria were proposed in 2007 as a modification of the RIFLE criteria[12]. The AKIN is based on evidence that suggests that even small increases in SCr are associated with a poor outcome. It is also based on SCr and UO and defines three stages (1, 2, and 3). Following the evidence for small changes in SCr and outcomes, the creatinine kinetics (CK) model was proposed by Waikar and Bonventre[13], who defined AKI based on the absolute changes in baseline SCr levels over 24-48 h. In 2012, an updated consensus definition of AKI was further proposed by the Kidney Disease Improving Global Outcomes (KDIGO) group to reconcile the subtle differences in the RIFLE and AKIN criteria and to establish a common definition known as the KDIGO criteria[14]. Nevertheless, currently, there are no widely accepted criteria to determine the severity of AKI for patients with TBI in the intensive care unit (ICU)[2,7,15], and the power of the criteria above among TBI patients’ needs further exploration.
Therefore, the present study aimed to explore the compatibility among the RIFLE, AKIN, CK, and KDIGO definitions, and to compare the power of these criteria in determining the incidence and stage of AKI and explore the association between severity of AKI by all definitions/stages and in-hospital mortality of patients admitted to ICU for TBI.
This was a retrospective study of patients admitted to the ICU for neurotrauma from 2001 to 2012. The exclusion criteria were: (1) Discharged within 24 h; or (2) < 18 years of age; or (3) missing data; or (4) history of end-stage renal disease (ESRD). The patients were included in the AKI and non-AKI groups according to whether they were diagnosed with AKI based on the KDIGO criteria[16].
The data for this study were extracted from the Medical Information Mart for Intensive Care (MIMIC-III, https://mimic.physionet.org/about/mimic/). It is a large public single-center database[17] that contains information relating to patients admitted to the critical care units at Beth Israel Deaconess Medical Center during 2001-2012. The presence of TBI was defined by diagnostic code, ICD-9, in MIMIC-III[18].
This retrospective study was approved by the Ethics Committee for Human Research of Shenzhen Hospital, Southern Medical University (No. NYS2YYEC20180009), which waived the requirement for informed consent from subjects.
Demographics and clinical data were retrieved for all patients, including sex, age, ethnicity, category diagnosis at ICU admission, Elixhauser score, simplified acute physiology score (SAPS II), SOFA score, Glasgow Coma Scale (GCS) score[19], serum creatinine concentration (SCr), including peak SCr and SCr at admission, previous treatment (craniotomy, transfusion and the use of antiplatelet drugs, anticoagulants, vancomycin, angiotensin receptor blocker/angiotensin-converting enzyme inhibitor (ARB/ACE-I) and aminoglycosides), length of stay (in days), UO, APACHE II classification, and in-hospital, 30-d, and 1-year mortality rates. Comorbidity was defined and calculated using the ICD-9-CM codes based on Elixhauser’s algorithm[20]. Patients presenting with shock upon admission, organ failure, and multiple organ failure (MOF) were selected according to definitions previously published[21].
If the patient's weight value was missing, the patient's height was used to estimate the weight[22]. Based on baseline SCr, the two groups in this study were assessed for the presence and stage of AKI using RIFLE[11], AKIN[12], CK[13], and KDIGO[16]. Baseline SCr was calculated according to the theoretical baseline SCr value for a given patient, assuming normal GFR[11].
The matching factors for propensity score matching (PSM) were ethnicity, age, sex, Elixhaouser score, SAPS II, SOFA, GCS, craniotomy, max creatinine, creatinine at admission, use of antiplatelet drugs, use of anticoagulants, shock, use of vancomycin, use of ARB/ACE-I, use of aminoglycosides, transfusion, red blood cell, plasma, and UO. The matching ratio was 1:1. Statistical analyses were performed using STATA 12.0 (StataCorp LP, College Station, TX, United States). The continuous data were tested for normal distribution using the Kolmogorov-Smirnov test. Those with a normal distribution were presented as means ± SD and analyzed using Student’s t-test; otherwise, they were presented as medians [interquartile ranges (IQR)] and analyzed using the Mann-Whitney U-test. The categorical data were presented as numbers (percentages) and analyzed using the chi-square test or Fisher’s exact test. Univariable and multivariable (enter) logistic regression analyses were performed to explore the association between in-hospital mortality (dependent variable) and the AKI stages diagnosed by CK, RIFLE, AKIN, and KDIGO. In-hospital, 30-d, and 1-year mortality rates were analyzed using the Kaplan-Meier method and the log-rank test. The observed proportional agreement was used to examine the compatibility between the different scoring systems. The Marascuilo procedure was used for multiple comparisons. Two-sided P values < 0.05 were considered statistically significant.
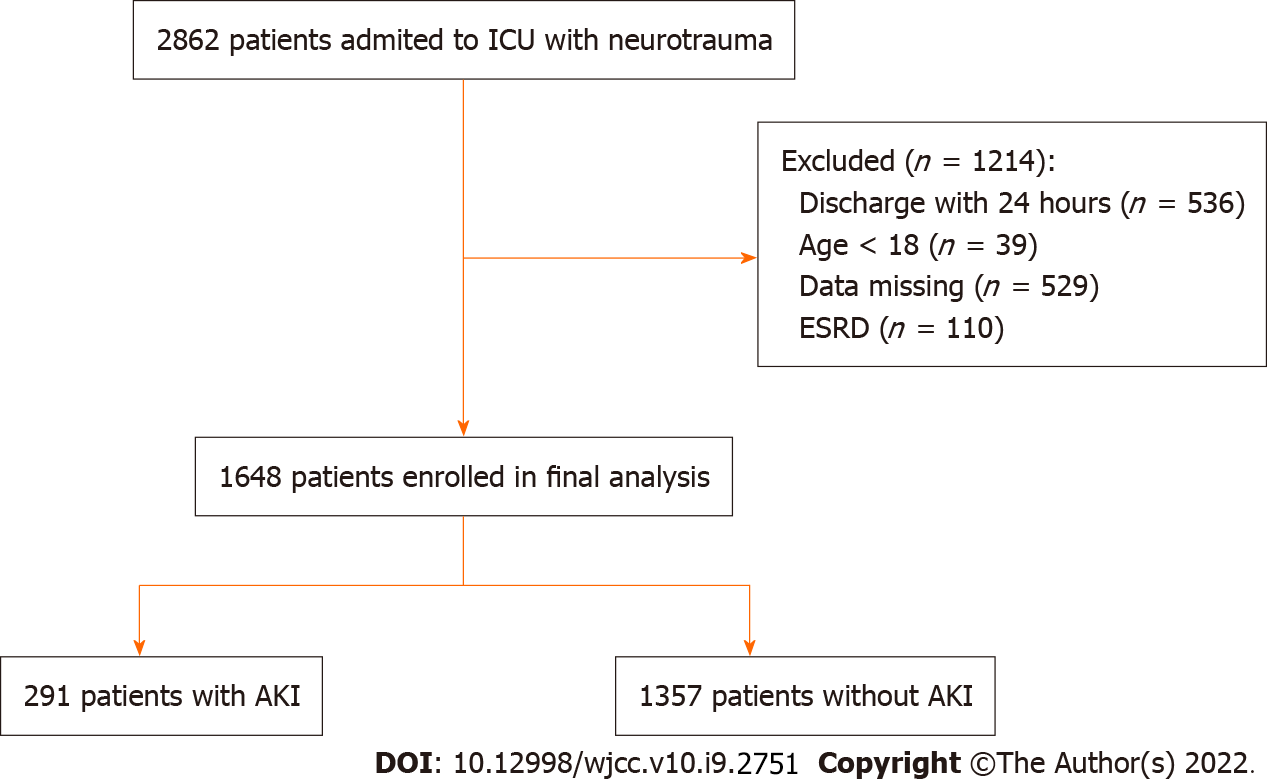
From the 2862 patients retrieved from the MIMCS-III database, 1214 were excluded (536 for being discharged within 24 h, 39 for being < 18 years of age, 529 with missing data, and 110 for being with ESRD), and 1648 were examined for the presence of AKI. Of those patients with TBI, 291 (17.7%) had AKI according to the KDIGO criteria (Figure 1). After PSM, the mean age of the patient cohort was 55.3 ± 23.9 years. Patients with AKI had higher SAPS II (36.6 ± 15.9 vs 33.1 ± 13.4, P = 0.004) and SOFA (4.8 ± 3.1 vs 4.0 ± 2.7, P = 0.001) scores, compared with patients without AKI. Moreover, patients with AKI had a higher frequency of shock (35.7% vs 25.1%, P = 0.007) and had more transfusions of red blood cells (RBC) (377.3 ± 1433.2 vs 174.1 ± 623.1 mL, P = 0.027) (Table 1). Table 2 shows the characteristics of the study population before PSM.
| Characteristics | Total patients (n = 582) | Non-AKI (n = 291) | AKI (n = 291) | P value |
| Ethnicity | 0.195 | |||
| White | 415 (71.3) | 206 (70.8) | 209 (71.8) | |
| Hispanic/Latino | 8 (1.4) | 7 (2.4) | 1 (0.3) | |
| African American | 22 (3.8) | 13 (4.5) | 9 (3.1) | |
| Asian | 3 (0.5) | 2 (0.7) | 1 (0.3) | |
| Other/unknown | 134 (23.0) | 63 (21.7) | 71 (24.4) | |
| Age (yr) | 55.3 ± 23.9 | 54.7 ± 24.7 | 55.8 ± 23.1 | 0.595 |
| Sex | 0.925 | |||
| Female | 152 (26.1) | 75 (25.8) | 77 (26.5) | |
| Male | 430 (73.9) | 216 (74.2) | 214 (73.5) | |
| Elixhauser score | 8.1 ± 11.5 | 7.4 ± 10.9 | 8.7 ± 12.1 | 0.187 |
| SAPS II | 34.8 ± 14.8 | 33.1 ± 13.4 | 36.6 ± 15.9 | 0.004 |
| SOFA | 4.4 ± 2.9 | 4.0 ± 2.7 | 4.8 ± 3.1 | 0.001 |
| GCS | 12.8 ± 3.2 | 12.8 ± 3.2 | 12.9 ± 3.3 | 0.778 |
| Craniotomy | 84 (14.4) | 42 (14.4) | 42 (14.4) | > 0.99 |
| Peak SCr (µmol/L) | 1.46 ± 0.72 | 1.22 ± 0.34 | 1.70 ± 0.90 | < 0.001 |
| SCr at admission (µmol/L) | 1.19 ± 0.46 | 1.08 ± 0.27 | 1.29 ± 0.57 | < 0.001 |
| Use of antiplatelet drugs | 40 (6.87) | 16 (5.50) | 24 (8.25) | 0.251 |
| Use of anticoagulants | 13 (2.2) | 5 (1.7) | 8 (2.8) | 0.575 |
| Use of vancomycin | 178 (30.6) | 80 (27.5) | 98 (33.7) | 0.126 |
| Use of ARB/ACE-I | 34 (5.8) | 19 (6.5) | 15 (5.2) | 0.596 |
| Use of aminoglycosides | 39 (6.7) | 16 (5.5) | 23 (7.9) | 0.320 |
| Transfusion (mL) | 553 ± 1589 | 400 ± 1077 | 645 ± 1967 | 0.063 |
| Red blood cell (mL) | 276 ± 1109 | 174 ± 623 | 377 ± 1433 | 0.027 |
| Plasma (mL) | 232 ± 690 | 214 ± 646 | 250 ± 733 | 0.526 |
| Shock | 177 (30.4) | 73 (25.1) | 104 (35.7) | 0.007 |
| UO (mL) | 0.68 ± 0.60 | 0.70 ± 0.44 | 0.67 ± 0.73 | 0.468 |
| Characteristics | Total patients (n = 1648) | Non-AKI (n = 1357) | AKI (n = 291) | P value |
| Ethnicity | 0.070 | |||
| White | 1220 (74.0) | 1011 (74.5) | 209 (71.8) | |
| Hispanic/Latino | 20 (1.2) | 19 (1.4) | 1 (0.3) | |
| African American | 55 (3.3) | 46 (3.4) | 9 (3.1) | |
| Asian | 23 (1.4) | 22 (1.6) | 1 (0.3) | |
| Other/unknown | 330 (20.0) | 259 (18.8) | 71 (28.4) | |
| Age (yr) | 58.5 ± 22.5 | 59.0 ± 22.4 | 55.8 ± 22.1 | 0.025 |
| Sex | 0.001 | |||
| Female | 603 (36.6) | 526 (38.8) | 77 (26.5) | |
| Male | 1045 (63.4) | 831 (61.2) | 214 (73.5) | |
| Elixhauser score | 6.4 ± 10.2 | 5.9 ± 9.6 | 8.7 ± 12.1 | < 0.001 |
| SAPS II | 31.9 ± 13.0 | 30.9 ± 12.0 | 36.6 ± 15.9 | < 0.001 |
| SOFA | 3.3 ± 2.5 | 2.9 ± 2.2 | 4.8 ± 3.1 | < 0.001 |
| GCS | 13.1 ± 2.9 | 13.1 ± 2.8 | 12.9 ± 3.3 | 0.096 |
| Craniotomy | 344 (20.9) | 302 (22.3) | 42 (14.4) | 0.020 |
| Peak SCr (µmol/L) | 1.09 ± 0.53 | 0.96 ± 0.27 | 1.70 ± 0.90 | < 0.001 |
| SCr at admission (µmol/L) | 0.95 ± 0.36 | 0.88 ± 0.23 | 1.29 ± 0.57 | < 0.001 |
| Use of antiplatelet drugs | 138 (8.4) | 114 (8.4) | 24 (8.3) | 0.932 |
| Use of anticoagulants | 31 (1.9) | 23 (1.7) | 8 (2.8) | 0.235 |
| Use of vancomycin | 408 (24.8) | 310 (22.8) | 98 (33.7) | < 0.001 |
| Use of ARB/ACE-I | 84 (5.1) | 69 (5.1) | 15 (5.2) | 0.961 |
| Use of aminoglycosides | 80 (4.9) | 57 (4.2) | 23 (7.9) | 0.008 |
| Transfusion (mL) | 309 ± 1082 | 237 ± 752 | 645 ± 1967 | 0.001 |
| Red blood cell (mL) | 141 ± 710 | 93 ± 398 | 377 ± 1433 | 0.001 |
| Plasma (mL) | 158 ± 596 | 138 ± 560 | 250 ± 733 | 0.004 |
| Shock | 460 (27.9) | 356 (26.2) | 104 (35.7) | < 0.001 |
| UO (mL) | 0.80 ± 1.06 | 0.83 ± 1.12 | 0.67 ± 0.73 | 0.016 |
The patients with AKI were divided according to KDIGO stage 1 (n = 200), 2 (n = 53), and 3 (n = 38) (Table 3). The patients with KDIGO stage 2 were older than in the two other groups (P = 0.001) and had a higher proportion of females (P < 0.001). The Elixhauser score, SAPS II, and SOFA scores were higher in the stage 3 group compared with the two other groups (all P < 0.001). The proportion of shock was higher in stage 3 (P = 0.004), the use of vancomycin was higher in stage 1 (P < 0.001), the use of aminoglycosides was higher in stages 2 and 3 (P = 0.040), and transfusions were higher in stage 3 patients (all P ≤ 0.01).
| Characteristics | Stage 1 (n = 200) | Stage 2 (n = 53) | Stage 3 (n = 38) | P value |
| Ethnicity | 0.512 | |||
| White | 141 (70.5) | 39 (73.6) | 29 (76.3) | |
| Hispanic/Latino | 1 (0.5) | 0 | 0 | |
| African American | 5 (2.5) | 3 (5.7) | 1 (2.6) | |
| Asian | 1 (0.5) | 0 | 0 | |
| Other/unknown | 52 (26.0) | 11 (20.8) | 8 (21.1) | |
| Age (yr) | 53.4 ± 23.1 | 64.6 ± 20.4 | 55.8 ± 23.9 | 0.001 |
| Sex | < 0.001 | |||
| Female | 42 (21.0) | 23 (43.4) | 12 (31.6) | |
| Male | 158 (79.0) | 30 (56.6) | 26 (68.4) | |
| Elixhauser score | 8.2 ± 12.5 | 8.9 ± 9.9 | 11.1 ± 12.9 | < 0.001 |
| SAPS II | 35.1 ± 16.0 | 39.2 ± 12.8 | 40.6 ± 18.1 | < 0.001 |
| SOFA | 4.6 ± 2.9 | 4.6 ± 2.9 | 6.3 ± 4.0 | < 0.001 |
| GCS | 12.9 ± 3.3 | 12.9 ± 3.2 | 12.7 ± 3.8 | 0.582 |
| Craniotomy | 28 (14.0) | 7 (13.2) | 7 (18.4) | 0.025 |
| Max creatinine (µmol/L) | 1.49 ± 0.44 | 1.70 ± 1.03 | 2.81 ± 1.54 | < 0.001 |
| Creatinine at admission (µmol/L) | 1.24 ± 0.34 | 1.22 ± 0.57 | 1.67 ± 1.14 | < 0.001 |
| Use of antiplatelet drugs | 94 (9.8) | 20 (13.2) | 10 (13.0) | 0.890 |
| Use of anticoagulant | 21 (2.2) | 8 (5.3) | 5 (6.49) | 0.281 |
| Use of vancomycin | 74 (37.0) | 14 (26.4) | 10 (26.32) | < 0.001 |
| Use of ARB/ACE-I | 9 (4.5) | 5 (9.4) | 1 (2.63) | 0.443 |
| Use of aminoglycosides | 14 (7.0) | 5 (9.4) | 4 (10.53) | 0.040 |
| Transfusion (mL) | 537 ± 1081 | 500 ± 1210 | 1412 ± 4613 | < 0.001 |
| Red blood cell (mL) | 291 ± 692 | 274 ± 920 | 974 ± 3451 | < 0.001 |
| Plasma (mL) | 231 ± 666 | 213 ± 606 | 400 ± 1133 | 0.010 |
| Shock | 460 (26.9) | 356 (26.2) | 104 (35.74) | 0.004 |
| UO (mL) | 0.70 ± 0.80 | 0.62 ± 0.47 | 0.53 ± 0.58 | 0.080 |
The incidence of AKI and stages determined by each classification method were examined. The highest incidence of AKI was found by KDIGO (17.7%), followed by AKIN (17.1%), RIFLE (12.7%), and CK (11.5%). There were no differences in the incidence of AKI among the four definitions (P = 0.967) (Table 4).
| Stages, n (%) | KDIGO | AKIN | RIFLE | CK | P value |
| Stage 0 | 1357 (82.3) | 1365 (82.9) | 1439 (87.3) | 1458 (88.5) | 0.967 |
| Stage 1 | 200 (12.1) | 199 (12.1) | 118 (7.2) | 103 (6.3) | 0.370 |
| Stage 2 | 53 (3.2) | 49 (3.0) | 57 (3.5) | 51 (3.1) | 0.998 |
| Stage 3 | 38 (2.3) | 34 (2.1) | 34 (2.1) | 35 (2.1) | > 0.99 |
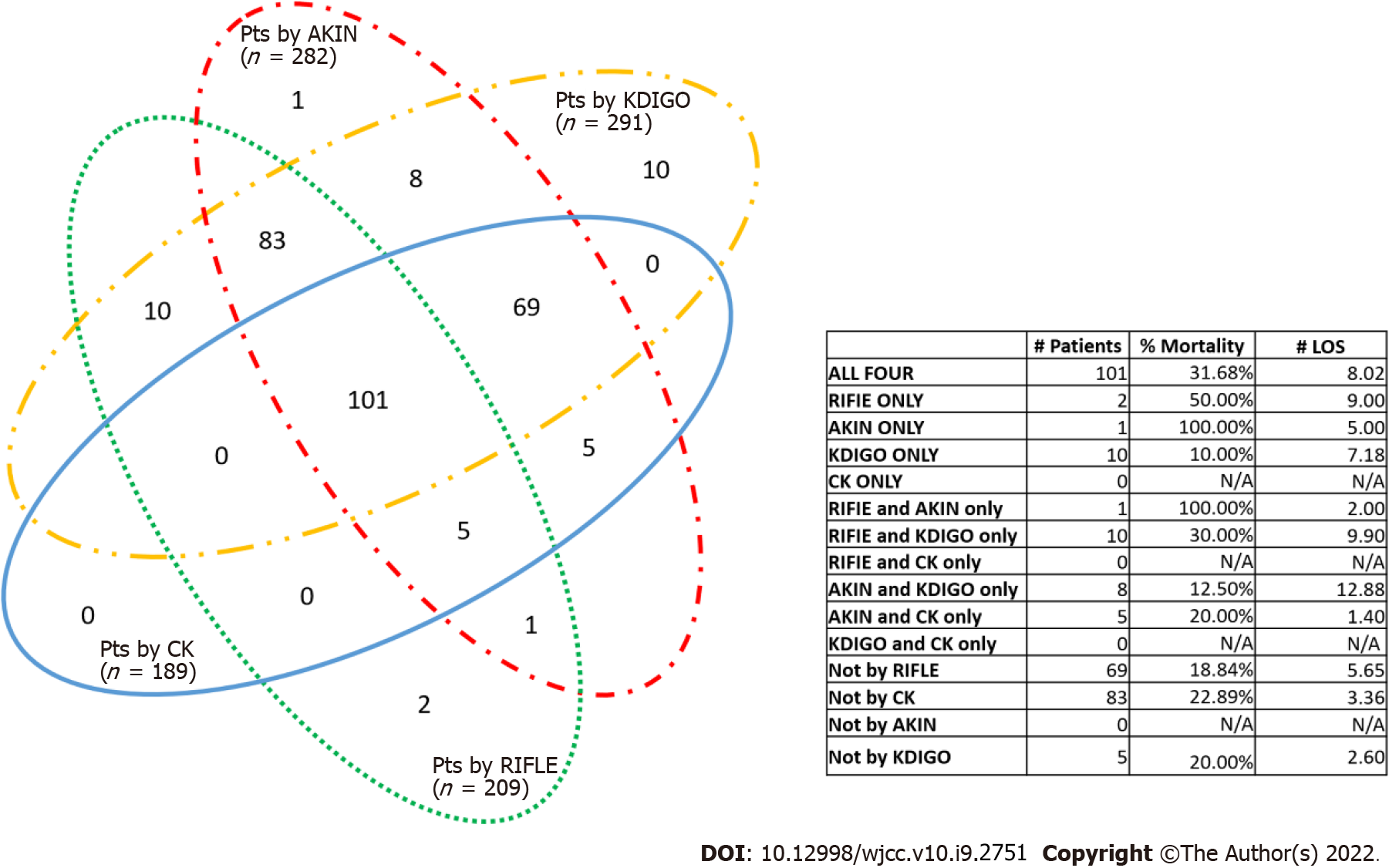
The identification of AKI overlaps across all the definitions (Figure 2). KDIGO identified the most AKI patients, and CK identified the least. Ten patients were identified as AKI only by KDIGO, while two and one were identified only by RIFLE and AKIN, respectively. KDIGO and AKIN failed to identify 14 and 22 AKI patients, respectively, while CK failed to identify 115 cases. For patients identified by AKIN and KDIGO only, the patients’ length of stay was the longest among all other combinations (12.9 d).
The concordance of AKI diagnosis and staging were further evaluated between KDIGO and the other classifications, using KDIGO as the diagnostic standard (Table 5). Compared with KDIGO, RIFLE correctly staged 1348/1357 (99.3%) stage 0 patients, 72/200 (36.0%) stage 1 patients, 19/53 (35.9%) stage 2 patients, and 18/38 (47.4%) stage 3 patients. Compared with KDIGO, AKIN correctly staged 1344/1357 (99.1%) stage 0 patients, 183/200 (91.5%) stage 1 patients, 48/53 (90.6%) stage 2 patients, and 34/38 (89.5%) stage 3 patients. Compared with KDIGO, CK correctly staged 1346/1357 (99.3%) stage 0 patients, 89/200 (44.5%) stage 1 patients, 6/53 (11.3%) stage 2 patients, and 14/38 (36.8%) stage 3 patients. Concordance was 88.4% between KDIGO and RIFLE, 97.6% between KDIGO and AKIN, and 88.3% between KDIGO and CK.
| KDIGO definition | Compared criteria | AKI Stage by RIFLE, AKIN, or CK | |||
| Stage 0, n (%) | R/Stage1, n (%) | I/Stage 2, n (%) | F/Stage 3, n (%) | ||
| Stage 0 | RIFLE | 1348 (99.3) | 9 (0.1) | 0 | 0 |
| Stage 1 | 126 (63.0) | 72 (36.0) | 2 (1.0) | 0 | |
| Stage 2 | 31 (58.5) | 2 (3.8) | 19 (35.9) | 1 (1.9) | |
| Stage 3 | 14 (36.8) | 2 (5.3) | 4 (10.5) | 18 (47.4) | |
| Stage 0 | AKIN | 1344 (99.1) | 12 (0.9) | 0 | 0 |
| Stage 1 | 17 (8.5) | 183 (91.5) | 0 | 0 | |
| Stage 2 | 2 (3.8) | 3 (5.7) | 48 (90.6) | 0 | |
| Stage 3 | 2 (5.3) | 1 (2.6) | 1 (2.6) | 34 (89.5) | |
| Stage 0 | CK | 1346 (99.3) | 5 (0.4) | 2 (0.2) | 3 (0.2) |
| Stage 1 | 66 (33.0) | 89 (44.5) | 40 (20.0) | 5 (2.5) | |
| Stage 2 | 31 (58.5) | 3 (5.7) | 6 (11.3) | 13 (24.5) | |
| Stage 3 | 15 (39.5) | 6 (15.8) | 3 (7.9) | 14 (36.8) | |
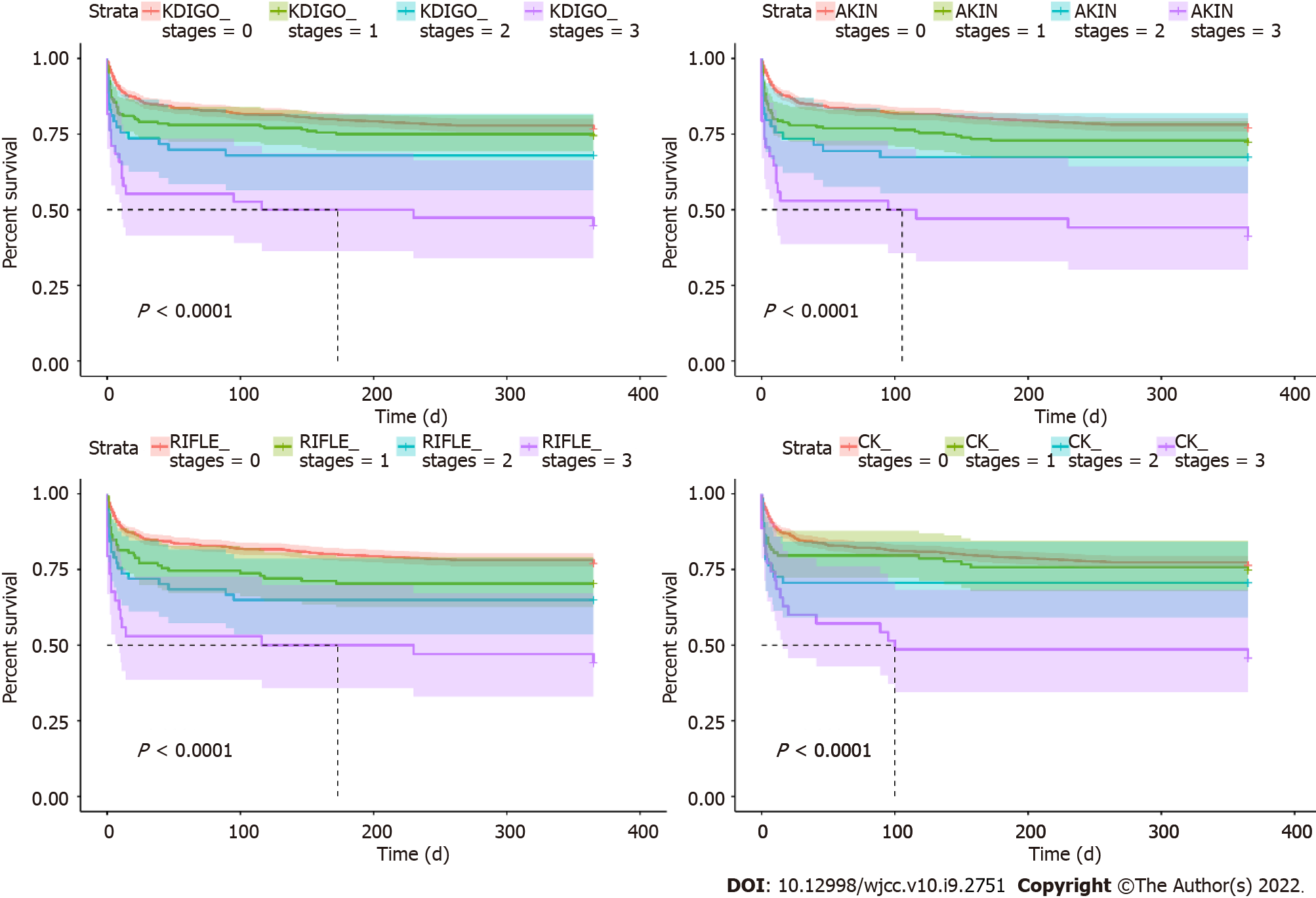
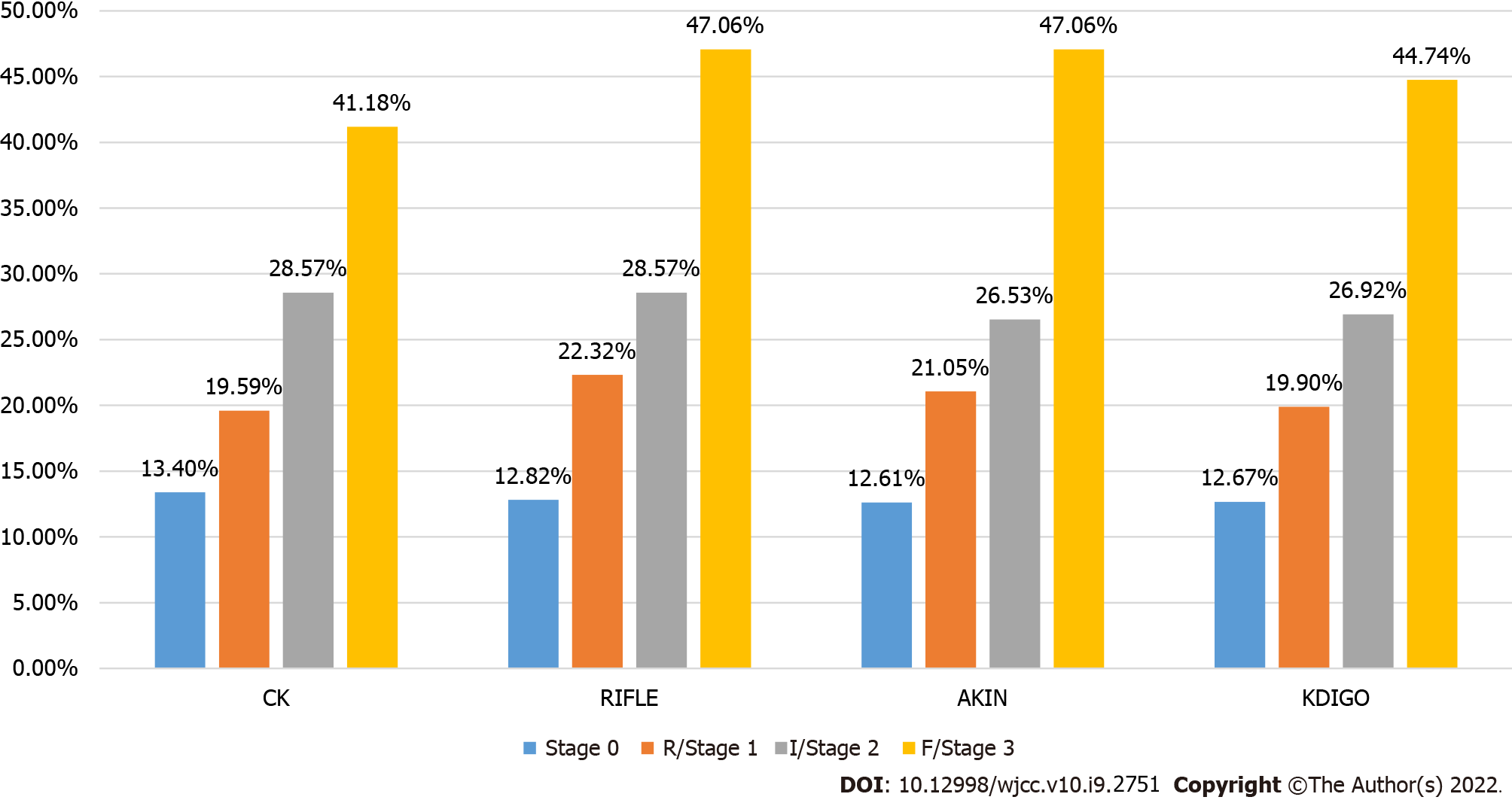
Regardless of AKI determination criteria, the in-hospital mortality was higher for those with AKI than those without. Moreover, the in-hospital mortality increased with the AKI stage (all P < 0.001) (Figures 3 and 4).
When staged according to KDIGO, ventilation time increased with AKI stage (P = 0.03), and ICU stay and hospitalization were longer for any-stage AKI compared to non-AKI (all P < 0.05). In-hospital mortality (P = 0.001), 30-d mortality (P < 0.001), and 1-year mortality (P < 0.001) increased with the AKI stage (Table 6).
| Indicators | Non-AKI | AKI | P value | ||
| Stage 0 n = 291 | Stage 1 n = 200 | Stage 2 n = 53 | Stage 3 n = 38 | ||
| Ventilation (h) | 105.5 ± 132.4 | 135.4 ± 149.8 | 138.7 ± 206.7 | 149.1 ± 207.1 | 0.029 |
| ICU duration (d) | 4.7 ± 5.8 | 6.5 ± 7.4 | 5.6 ± 9.1 | 6.3 ± 9.2 | 0.001 |
| Hospitalization (d) | 10.8 ± 11.0 | 13.9 ± 14.6 | 12.6 ± 13.1 | 12.4 ± 10.2 | 0.008 |
| Hospital mortality | 185 (12.9) | 38 (19.0) | 14 (26.4) | 17 (44.7) | 0.001 |
| 30-d mortality | 202 (14.9) | 42 (21.0) | 14 (26.4) | 17 (44.7) | < 0.001 |
| 1-yr mortality | 316 (23.3) | 51 (25.5) | 17 (32.1) | 21 (55.3) | < 0.001 |
The association between severity of AKI by all definitions/stages and in-hospital mortality was tested. As shown in Table 7, the severity of AKI by all definitions and stages was associated with in-hospital mortality in the univariable analyses (all P < 0.05), except for stage 1 by CK (P > 0.05), but the associations were no longer significant in the multivariable analyses (all P > 0.05).
| Variables | Univariable analysis | Multivariable analysis | |||
| Odds ratio (95%CI) | P value | Odds ratio (95%CI) | P value | ||
| KDIGO | Stage 1 | 1.64 (1.11-2.41) | 0.013 | 0.70 (0.27-1.83) | 0.635 |
| Stage 2 | 2.51 (1.33-4.71) | 0.004 | 0.15 (0.00-4.76) | 0.242 | |
| Stage 3 | 5.65 (2.92-10.93) | < 0.001 | 0.10 (0.00-7.83) | 0.705 | |
| RIFLE | Stage 1 | 1.86 (1.16, 2.96) | 0.010 | 0.97 (0.39-2.43) | 0.879 |
| Stage 2 | 2.70 (1.48-4.90) | 0.001 | 3.26 (0.18-59.43) | 0.166 | |
| Stage 3 | 6.14 (3.08-12.25) | < 0.001 | 1.90 (0.07-49.94) | 0.466 | |
| AKIN | Stage 1 | 1.77 (1.21-2.59) | 0.004 | 1.32 (0.39-4.40) | 0.933 |
| Stage 2 | 2.54 (1.32-4.88) | 0.005 | 3.23 (0.33-31.98) | 0.552 | |
| Stage 3 | 6.25 (3.13-12.49) | < 0.001 | 16.88 (0.74-349.18) | 0.403 | |
| CK | Stage 1 | 1.49 (0.89-2.51) | 0.132 | 1.34 (0.44-4.83) | 0.500 |
| Stage 2 | 2.49 (1.32-4.70) | 0.005 | 1.07 (0.35-3.25) | 0.579 | |
| Stage 3 | 4.40 (2.20-8.79) | < 0.001 | 0.72 (0.17-2.98) | 0.615 | |
For the diagnosis and staging of AKI, at least four different AKI criteria, RIFLE, AKIN, CK, and KDIGO, have been proposed. However, the power of these criteria among TBI patients needs further exploration. This study revealed that differences were seen in AKI diagnosis among the four AKI criteria. The highest incidence of AKI was found by KDIGO (17.7%), followed by AKIN (17.1%), RIFLE (12.7%), and CK (11.5%). Concordance to KDIGO was the lowest for CK, followed by RIFLE and AKIN. The in-hospital mortality rates increased with the AKI stage in all four definitions, but the severity of AKI by all definitions and stages was not associated with in-hospital mortality in the multivariable analyses.
The diagnosis of AKI in patients with TBI has significant clinical relevance, given the requirements for prompt medical intervention for AKI patients. Similar to the results of a previous study[23], this study suggested that the incidence of AKI varied depending on the criteria used, which may lead to confusion during criteria selection and may negatively affect the efficiency of clinical treatment. Some studies showed that KDIGO is more sensitive than AKIN and RIFLE in AKI diagnosis in patients with myocardial infarction and acute decompensated heart failure[9,10]. In a study comparing KDIGO and CK in diagnosing AKI in trauma patients, KDIGO was shown to be more sensitive, and CK was found to be superior to KDIGO only in patients with pre-existing chronic kidney disease (CKD)[7]. In the present study, the KDIGO classification identified the highest incidence of AKI and was more able to detect than RIFLE, CK, and AKIN. Although there were no significant differences in the proportions of patients with AKI according to the different criteria, misclassification was observed, particularly with the CK and RIFLE definitions.
More specifically, the present study showed the highest incidence of AKI was found by KDIGO (17.7%), followed by AKIN (17.1%), RIFLE (12.7%), and CK (11.5%) among patients with TBI patients. The reason for the difference may be the selection of the baseline SCr to be used for evaluating AKI. For example, the AKIN criteria consider the lowest SCr measurement during the ICU stay as the baseline SCr level, and it probably overestimates the AKI incidence, which can be as high as 74.2%-85.0%[24-27]. However, when baseline SCr was estimated using the MDRD equation, AKI incidence was reported to be 11.6%-23% based on the RIFLE or AKIN criteria[7,10]. The selection of baseline SCr could also affect the incidence of AKI in the general population when following the KDIGO criteria. A possible explanation for this finding is the temporary overhydration during hospitalization. The creatinine concentration extrapolated by the MDRD equation (with a GFR of 75 mL/min) might be more accurate, although it should be used with caution. Other influencing factors include, but are not limited to, UO and population heterogeneity, and further studies are needed in the future.
The KDIGO classification was rarely compared to other AKI definitions regarding its prognostic power in TBI patients. According to Tsai et al[24], the KDIGO classification has a relatively higher discriminatory power (0.840 ± 0.032) in predicting in-hospital mortality than the RIFLE (0.826 ± 0.033) and AKIN (0.836 ±0.032) classifications. Zeng et al[28] showed that the incidence of AKI changed with the definition but that all definitions were associated with in-hospital mortality. In the present study, KDIGO did not improve the predictive performance of in-hospital mortality, i.e., the in-hospital mortality increased with the increasing stage in all four definitions. On the other hand, the associations disappeared for all four definitions in the multivariable regression analyses after adjusting for ethnicity, age, sex, Elixhauser score, SAPS II, SOFA, GCS, craniotomy, max creatinine, creatinine at admission, use of antiplatelet drugs, anticoagulant, vancomycin, ARB/ACE-I and aminoglycosides, transfusion, red blood cell, plasma, and shock. Therefore, the results mean that one or multiple factors included in the adjusted analyses are a stronger predictor of mortality than AKI in patients with TBI. A study by Ulger et al[25] showed that the in-hospital mortality for stage 2 and 3 AKI in AKIN, RIFLE, and KDIGO was nearly the same. These discrepancies might be attributable to the baseline creatine estimation based on the MDRD formula[15]. Besides, clinically, death attributable to AKI is rare in TBI patients, which may explain the lack of association between the four definitions and in-hospital mortality. Osmotic therapy during ICU stay appears to affect the mortality due to AKI[29]. A recent study suggested that the AKI stage was associated with mortality in patients with TBI, but not AKI duration or AKI burden; in addition, most deaths occurred during the first 3 d of ICU stay[30]. The use of renoprotective measures affects the mortality due to AKI in patients with TBI[31].
There are some limitations to this research. First, as a retrospective, a single-center study from a single academic hospital, the generalizability of these findings is questionable. Incidence estimates, mortality rates, and procedures vary greatly among hospitals and countries. Second, baseline creatinine was calculated as a theoretical baseline SCr for a given patient assuming a normal GFR, which may overestimate or underestimate the incidence of AKI to some extent. Third, the patients were identified using the administrative codes entered in the database, which is subject to bias regarding the use of the incorrect code by the physicians and administrative personnel[17].
In conclusion, this study indicates that in patients with TBI, differences are seen in AKI diagnosis among the four AKI definitions or stages, and concordance varies, as well as the in-hospital mortality. Thus, more universal AKI criteria are needed in patients with TBI. Besides, the severity of AKI was not associated with in-hospital mortality rates when using any of the four definitions.
Early identification and subsequent clinical intervention of acute kidney injury (AKI) in traumatic brain injury (TBI) patients are critical to survival.
The exact definition of AKI for patients with TBI is unknown.
We aimed to compare four AKI diagnostic criteria to determine AKI incidence/stage and their association with the in-hospital mortality rate of patients with TBI.
The subjects in this study were assessed for the presence and stage of AKI using four different AKI diagnostic criteria.
The in-hospital mortality rates increased with the AKI stage in all four definitions. The severity of AKI by all definitions and stages was not associated with in-hospital mortality in the multivariable analyses (all P > 0.05).
This study revealed that Kidney Disease Improving Global Outcomes (KDIGO) is the best method to define AKI in patients with TBI.
In the future, it is necessary to increase the sample size for prospective studies to further explore.
| 1. | Koza Y. Acute kidney injury: current concepts and new insights. J Inj Violence Res. 2016;8:58-62. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 52] [Article Influence: 5.2] [Reference Citation Analysis (4)] |
| 2. | Moore EM, Bellomo R, Nichol A, Harley N, Macisaac C, Cooper DJ. The incidence of acute kidney injury in patients with traumatic brain injury. Ren Fail. 2010;32:1060-1065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 93] [Article Influence: 6.2] [Reference Citation Analysis (1)] |
| 3. | Freeman WD, Wadei HM. A brain-kidney connection: the delicate interplay of brain and kidney physiology. Neurocrit Care. 2015;22:173-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 4. | Machado MN, Nakazone MA, Maia LN. Acute kidney injury based on KDIGO (Kidney Disease Improving Global Outcomes) criteria in patients with elevated baseline serum creatinine undergoing cardiac surgery. Rev Bras Cir Cardiovasc. 2014;29:299-307. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 5. | Barasch J, Zager R, Bonventre JV. Acute kidney injury: a problem of definition. Lancet. 2017;389:779-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 73] [Article Influence: 8.1] [Reference Citation Analysis (1)] |
| 6. | Zappitelli M, Parikh CR, Akcan-Arikan A, Washburn KK, Moffett BS, Goldstein SL. Ascertainment and epidemiology of acute kidney injury varies with definition interpretation. Clin J Am Soc Nephrol. 2008;3:948-954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 270] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 7. | Li N, Zhao WG, Zhang WF. Acute kidney injury in patients with severe traumatic brain injury: implementation of the acute kidney injury network stage system. Neurocrit Care. 2011;14:377-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 68] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 8. | Avila RCN, Fernandez A, Filippi M. The incidence of acute kidney injury in patients with traumatic brain injury. Int Care Med Exp. 2015;3:A263. |
| 9. | Zygun DA, Kortbeek JB, Fick GH, Laupland KB, Doig CJ. Non-neurologic organ dysfunction in severe traumatic brain injury. Crit Care Med. 2005;33:654-660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 205] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 10. | Bagshaw SM, George C, Gibney RT, Bellomo R. A multi-center evaluation of early acute kidney injury in critically ill trauma patients. Ren Fail. 2008;30:581-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 118] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 11. | Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P; Acute Dialysis Quality Initiative workgroup. Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8:R204-R212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4448] [Cited by in RCA: 4786] [Article Influence: 217.5] [Reference Citation Analysis (0)] |
| 12. | Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, Levin A; Acute Kidney Injury Network. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4846] [Cited by in RCA: 5064] [Article Influence: 266.5] [Reference Citation Analysis (0)] |
| 13. | Waikar SS, Bonventre JV. Creatinine kinetics and the definition of acute kidney injury. J Am Soc Nephrol. 2009;20:672-679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 435] [Cited by in RCA: 489] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 14. | KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney Int Suppl. 2012;2:1-138. |
| 15. | Davenport A. Clinical guidelines for the protection of kidney function and prevention of acute kidney injury in the intensive care unit: common sense rather than magic bullets? Intensive Care Med. 2010;36:379-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 16. | Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012;120:c179-c184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1436] [Cited by in RCA: 3706] [Article Influence: 264.7] [Reference Citation Analysis (0)] |
| 17. | Johnson AE, Pollard TJ, Shen L, Lehman LW, Feng M, Ghassemi M, Moody B, Szolovits P, Celi LA, Mark RG. MIMIC-III, a freely accessible critical care database. Sci Data. 2016;3:160035. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2345] [Cited by in RCA: 3317] [Article Influence: 331.7] [Reference Citation Analysis (0)] |
| 18. | Carlson KF, Barnes JE, Hagel EM, Taylor BC, Cifu DX, Sayer NA. Sensitivity and specificity of traumatic brain injury diagnosis codes in United States Department of Veterans Affairs administrative data. Brain Inj. 2013;27:640-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 19. | Sternbach GL. The Glasgow coma scale. J Emerg Med. 2000;19:67-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 174] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 20. | Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36:8-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6456] [Cited by in RCA: 8077] [Article Influence: 288.5] [Reference Citation Analysis (0)] |
| 21. | Vincent JL, Nielsen ND, Shapiro NI, Gerbasi ME, Grossman A, Doroff R, Zeng F, Young PJ, Russell JA. Mean arterial pressure and mortality in patients with distributive shock: a retrospective analysis of the MIMIC-III database. Ann Intensive Care. 2018;8:107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 111] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 22. | Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11183] [Cited by in RCA: 11952] [Article Influence: 442.7] [Reference Citation Analysis (0)] |
| 23. | Izawa J, Uchino S, Fujii T, Arii T, Fukushima T, Kawano S. Thorough evaluation for the new acute kidney injury criteria by Kidney Disease Improving Global Outcomes. Crit Care Med. 2013;17:P410. |
| 24. | Tsai TY, Chien H, Tsai FC, Pan HC, Yang HY, Lee SY, Hsu HH, Fang JT, Yang CW, Chen YC. Comparison of RIFLE, AKIN, and KDIGO classifications for assessing prognosis of patients on extracorporeal membrane oxygenation. J Formos Med Assoc. 2017;116:844-851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 44] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 25. | Ülger F, Pehlivanlar Küçük M, Küçük AO, İlkaya NK, Murat N, Bilgiç B, Abanoz H. Evaluation of acute kidney injury (AKI) with RIFLE, AKIN, CK, and KDIGO in critically ill trauma patients. Eur J Trauma Emerg Surg. 2018;44:597-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 26. | Pickering JW, Endre ZH. Back-calculating baseline creatinine with MDRD misclassifies acute kidney injury in the intensive care unit. Clin J Am Soc Nephrol. 2010;5:1165-1173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 130] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 27. | Sigurdsson MI, Vesteinsdottir IO, Sigvaldason K, Helgadottir S, Indridason OS, Sigurdsson GH. Acute kidney injury in intensive care units according to RIFLE classification: a population-based study. Acta Anaesthesiol Scand. 2012;56:1291-1297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 28. | Zeng X, McMahon GM, Brunelli SM, Bates DW, Waikar SS. Incidence, outcomes, and comparisons across definitions of AKI in hospitalized individuals. Clin J Am Soc Nephrol. 2014;9:12-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 316] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 29. | Robba C, Banzato E, Rebora P, Iaquaniello C, Huang CY, Wiegers EJA, Meyfroidt G, Citerio G; Collaborative European NeuroTrauma Effectiveness Research in Traumatic Brain Injury (CENTER-TBI) ICU Participants and Investigators. Acute Kidney Injury in Traumatic Brain Injury Patients: Results From the Collaborative European NeuroTrauma Effectiveness Research in Traumatic Brain Injury Study. Crit Care Med. 2021;49:112-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 30. | Wang R, Zhang J, Xu J, He M. Incidence and Burden of Acute Kidney Injury among Traumatic Brain-Injury Patients. Risk Manag Healthc Policy. 2021;14:4571-4580. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 31. | Ramtinfar S, Chabok SY, Chari AJ, Reihanian Z, Leili EK, Alizadeh A. Kidney disease improving global outcome for predicting acute kidney injury in traumatic brain injury patients. J Acute Med. 2016;6:90-94. |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Neurosciences
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Esposito P, Hassan EM S-Editor: Xing YX L-Editor: Filipodia P-Editor: Xing YX