Published online Mar 26, 2022. doi: 10.12998/wjcc.v10.i9.2743
Peer-review started: August 24, 2021
First decision: November 17, 2021
Revised: November 19, 2021
Accepted: February 15, 2022
Article in press: February 15, 2022
Published online: March 26, 2022
Processing time: 210 Days and 1.9 Hours
Pneumocystis jirovecii pneumonia (PJP) is an infectious disease common in immunocompromised hosts. However, the currently, the clinical characteristics of non-HIV patients with PJP infection have not been fully elucidated.
To explore efficacy of trimethoprim–sulfamethoxazole (TMP-SMX) and caspofungin for treatment of non-human immunodeficiency virus (HIV)-infected PJP patients.
A retrospective study enrolled 22 patients with non-HIV-infected PJP treated with TMP-SMX and caspofungin from 2019 to 2021. Clinical manifestations, treatment and prognosis of the patients were analyzed.
Five patients presented with comorbidity of autoimmune diseases, seven with lung cancer, four with lymphoma, two with organ transplantation and four with membranous nephropathy associated with use of immunosuppressive agents. The main clinical manifestations of patients were fever, dry cough, and progressive dyspnea. All patients presented with acute onset and respiratory failure. The most common imaging manifestation was ground glass opacity around the hilar, mainly in the upper lobe. All patients were diagnosed using next-generation sequencing, and were treated with a combination of TMP-SMX and caspofungin. Among them, 17 patients received short-term adjuvant glucocorticoid therapy. All patients recovered well and were discharged from hospital.
Non-HIV-infected PJP have rapid disease progression, high risk of respiratory failure, and high mortality. Combination of TMP-SMX and caspofungin can effectively treat severe non-HIV-infected PJP patients with respiratory failure.
Core tip: Pneumocystis jirovecii pneumonia (PJP) is common in immunocompromised hosts. However, the clinical characteristics of non-human immunodeficiency virus (HIV) infected PJP patients have not been fully elucidated. This was a retrospective study of non-HIV-infected PJP treated with trimethoprim– sulfamethoxazole (TMP-SMX) and caspofungin. Clinical manifestations, treatment and prognosis of the patients were evaluated. Non-HIV-infected PJP patients are characterized by rapid disease progression, high risk of respiratory failure, and high mortality. Early diagnosis and treatment can improve survival in non-HIV-infected PJP patients. The findings of the current study showed that combination of TMP-SMX and caspofungin is effective for non-HIV-infected PJP patients with respiratory failure.
- Citation: Wu HH, Fang SY, Chen YX, Feng LF. Treatment of Pneumocystis jirovecii pneumonia in non-human immunodeficiency virus-infected patients using a combination of trimethoprim-sulfamethoxazole and caspofungin. World J Clin Cases 2022; 10(9): 2743-2750
- URL: https://www.wjgnet.com/2307-8960/full/v10/i9/2743.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i9.2743
Approximately 75% of patients infected with human immunodeficiency virus (HIV) positive present with Pneumocystis jirovecii pneumonia (PJP) during the early stages of the infection[1]. Technological advancements in medicine have led to development of effective therapies that have significantly reduced the incidence of PJP in patients with acquired immunodeficiency syndrome (AIDS). For example, the CD4+ T lymphocyte counts have been shown to be accurate in identifying patients at high clinical risk of developing PJP[2]. In addition, prophylaxis interventions have been designed for patients at risk of PJP[3] and sensitive immunofluorescence detection methods are now available for timely detection. The development of combined antiretroviral therapy drugs has significantly reduced incidence of PJP and improved prognosis of HIV-positive PJP (HIV + PJP) patients[4]. Clinically, the number of non-HIV-infected PJP patients has been on the rise annually owing to the increased use of immunosuppressants and organ transplantations[5,6]. The mortality levels of non-HIV-infected PJP patients ranges between 30% and 60%, whereas the mortality of HIV + PJP patients ranges between 10% and 20%[7]. In comparison, management measures for non-HIV-infected PJP are not standardized as is the case for HIV + PJP patients. Moreover, the clinical attention for non-HIV-infected patients is poorer compared with that of HIV + PJP patients.
The aim of this retrospective study was to explore the characteristics of non-HIV-infected PJP treated with trimethoprim–sulfamethoxazole (TMP-SMX) and caspofungin. The findings of this study are expected to improve our understanding of the occurrence of PJP in non-HIV-infected patients, and thus prevent misdiagnosis and reduce mortality rate of non-HIV-infected PJP patients.
A retrospective case review of 22 non-HIV-infected PJP patients admitted to Dongyang Hospital Affiliated to Wenzhou Medical University, a tertiary hospital in Zhejiang, China was carried out between October 2019 and April 2021. Patient data including symptoms, laboratory results, dynamic and comprehensive computed tomography data, and clinical course of the disease were extracted from electronic medical records. Information on treatment, response to treatment, outcomes, and any follow-up data were also collected. The study protocol was approved by the Ethics Committee of the Dongyang Hospital Affiliated to Wenzhou Medical University, (No. 2021-YX-127). All data were anonymized prior to analysis.
In total, four women and 18 men diagnosed as non-HIV-infected PJP were enrolled in the study. The median age of these patients was 61.18 ± 16.51 (range 17–84) years (Table 1). All patients tested positive for Pneumocystis jiroveciiPneumocystis jirovecii DNA fragments as determined using metagenomic next-generation sequencing (mNGS). Systematic screening did not identify any other respiratory pathogens at the time of hospital admission. All patients presented with dry cough and dyspnea, whereas 13 patients had fever. The median time from onset of illness to admission was 5.68 ± 3.66 (range 1–15) d. On physical examination, the patients presented with heterogeneous and nonspecific clinical signs. Among the comorbidities found were autoimmune disease (n = 5), non-Hodgkin’s lymphoma (n = 4), lung cancer (n = 7; all underwent chest radiotherapy), organ transplantation (n = 2), and membranous nephropathy (n = 4). All patients developed respiratory failure and received oxygen therapy (oxygen therapy, administered nasally for 11 patients, mask oxygen therapy for 10 patients, and high flow nasal cannula therapy for 1 patient). No patient received invasive mechanical ventilation.
| Characteristics | Patients, n (%) | Median value (range) |
| Demographics | ||
| Male/female | 18/4 | |
| Age, median (range, yr) | 61.18 ± 16.51 | |
| Underlying disease | 22/22 | |
| Clinical manifestations | ||
| Fever | 13/22 | |
| Cough | 22/22 | |
| Dyspnea | 22/22 | |
| Laboratory tests | ||
| WBC (normal 4 × 109–10 × 109/L) | 9.69 ± 6.22 | |
| Percentage of neutrophils | 0.82 ± 0.14 | |
| CRP (normal 0–8 mg/L) | 79.69 ± 64.21 | |
| PCT (normal 0–0.5 ng/mL) | 0.23 ± 0.21 | |
| LDH (normal 120–250 U/L) | 408.23 ± 117.87 | |
| Oxygenation index | 209.55 ± 35.03 | |
| CD4+ T lymphocyte counts | 0.81 ± 0.52 | |
| Imaging | ||
| Diffuse ground glass opacity | 19/22 (86.36%) | |
| Streak like dense shadow | 3/22 (13.64%) | |
| Complete CT recovery in survivors | 22/22 (100.0%) |
Clinical examination carried out at admission showed that patients had a mean white blood cell count of 9.69 ± 6.22 × 109/L and with a proportion of neutrophils of 0.82 ± 0.14%. In addition, C-reactive protein level was 79.69 ± 64.21 mg/L, lactate dehydrogenase (LDH) level was 408.23 ± 117.87 U/L, and procalcitonin level was 0.23 ± 0.21 ng/mL.
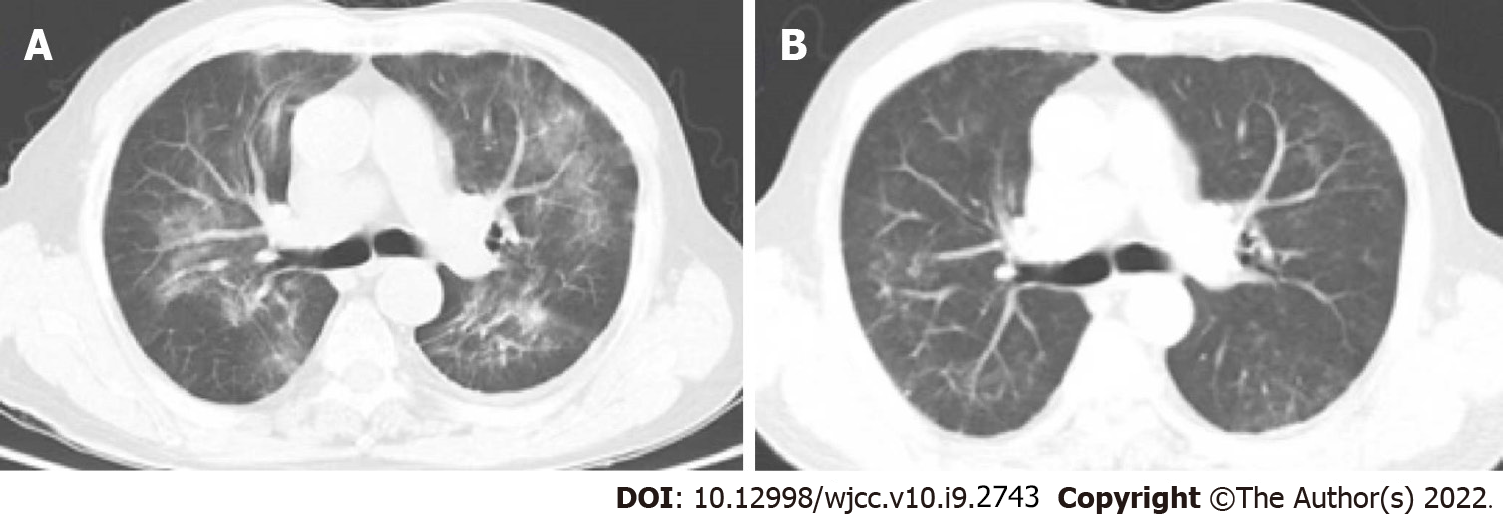
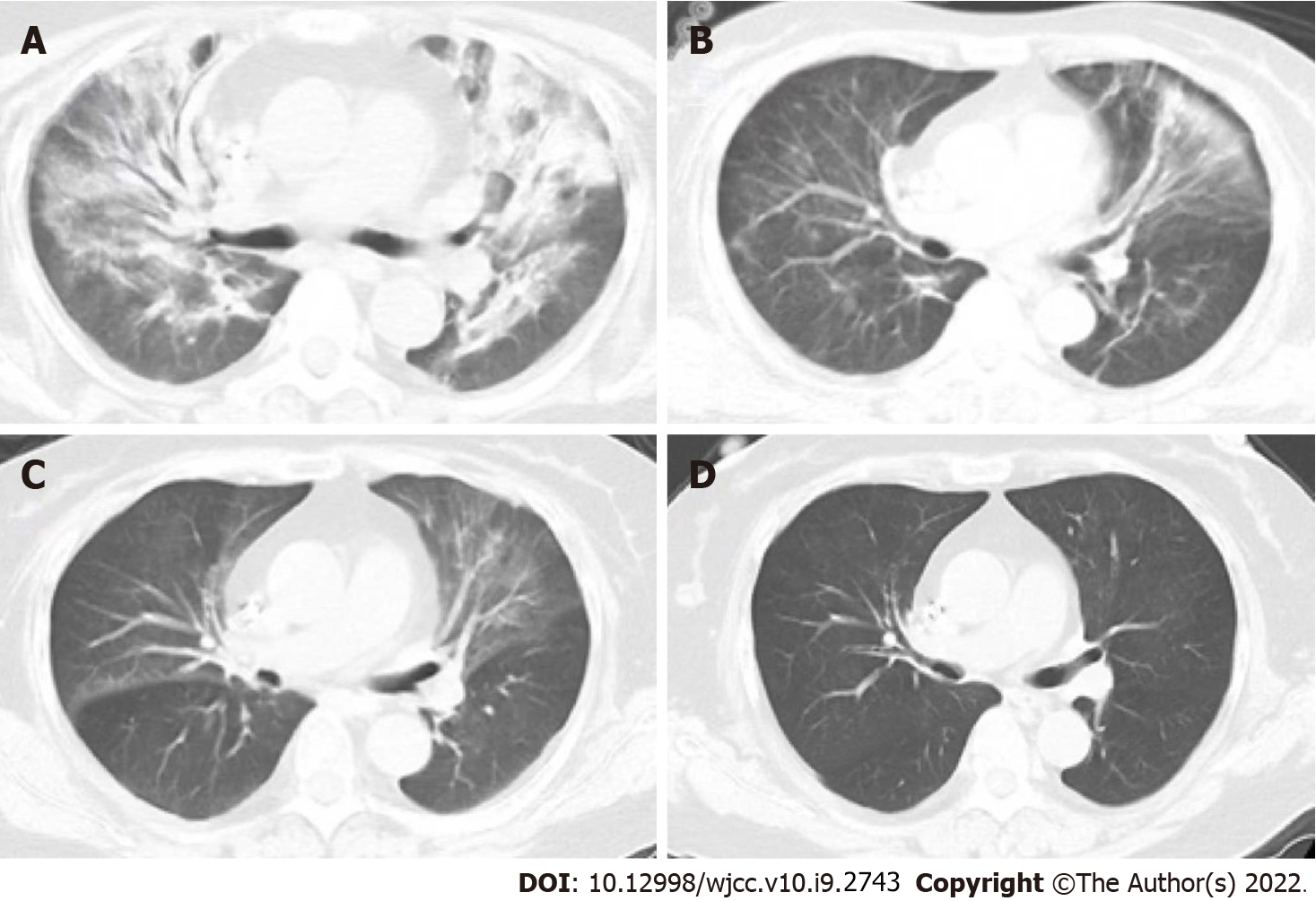
Lung imaging revealed lesions in the upper lobe of lung in the early stage. With progression of the disease, the lesions spread to the lower lobe of the lung. The most common imaging manifestation was widespread ground glass opacity, which had a mosaic or diffuses distribution, mainly in the upper lobe of the lung (Figures 1 and 2).
Analysis of medical records showed that most of the patients were treated with antibiotics such as cephalosporin prior to admission. However, their symptoms gradually deteriorated leading to development of progressive dyspnea. After hospital admission, patients underwent bronchofibroscopy and alveolar lavage fluid was collected for mNGS analysis.
mNGS analysis was carried out within 48–72 h from the time of sample collection to the time results were reported. After confirmation of PJP, treatment was changed to TMP-SMX (TMP, 15–20 mg/kg/d; SMX, 75–100 mg/kg/d), administered for at least 2 wk, as reported previously[8]. Patients received TMP-SMX combined with caspofungin (through intravenous administration of 70 mg QD on the first day and 50 mg QD as the maintenance dose afterwards) for those with respiratory failure or renal insufficiency. In addition, moderate to severe non-HIV-infected PJP patients received glucocorticoid treatment (n = 17) for 1 wk.
After initiation of therapy, the body temperature gradually returned to normal within 3 d, and their respiratory function improved. Further analysis revealed that the level of inflammatory indices in blood significantly decreased, and the lung lesions gradually disappeared following treatment. All patients had fully recovered before discharge.
The incidence of PJP is high among immunocompromised HIV-negative patients. This calls for development of novel diagnostic, treatment, and prevention strategies for non-HIV-infected PJP[9-11]. Non-HIV-infected PJP patients have an acute onset, rapid disease progression, poor prognosis, and higher mortality rates compared with HIV-infected PJP patients[12-16].
The population of HIV-negative patients susceptible to Pneumocystis jirovecii includes patients who have undergone transplantation, hemato-oncological patients, and those taking immunosuppressive drugs for autoimmune diseases. Approximately 20% of non-HIV-infected PJP patients are those with autoimmune diseases, mainly nodular polyarteritis, granuloma with polyarteritis, dermatomyo
The main symptoms of non-HIV-infected PJP patients include fever, dyspnea and dry cough. Moreover, hypoxia and respiratory failure are more common in non-HIV-infected PJP patients compared with HIV-infected PJP patients. However, frequency of LDH elevation is lower, the onset and disease progression are more rapid (often within a few days) in non-HIV-infected PJP than in HIV-infected PJP. For non-HIV-infected PJP patients, physical examination is nonspecific, and lung auscultation is usually normal, but often inconsistent for those with severe clinical manifestations[21]. Computed tomography (CT) scans show ground-glass opacity (GGO), mainly presenting with a central distribution, is a common radiological pattern for PJP patients. These manifestations were observed in patients included in the current study (Figure 1). GGOs may present as a mosaic-like distribution or diffuse distribution, more commonly occurring in the upper lobes of the lungs[22]. In the advanced stages of the disease, CT scans show lung consolidation. Paving stone-like manifestations are observed in 6%–18% of patients[23]. Hollowness and pleural effusion are rare, and if they occur, they often indicate existence of other diseases[23]. Approximately 3%–6% of non-HIV-infected PJP patients may present with lung cyst-like changes of different shapes, sizes, and wall thicknesses, implying that they are susceptible to spontaneous pneumothorax[23]. GGOs in non-HIV-infected PJP patients spread faster compared than in HIV-infected PJP patients.
Pneumocystis jirovecii cannot be cultured in the laboratory. Therefore, this infection has been traditionally diagnosed based on microscopic observation of the organisms in respiratory specimens. However, due to the lower pathogen burden in non-HIV-infected PJP patients compared with HIV-infected PJP patients, specimens from non-HIV-infected PJP patients have lower microscopic sensitivity[24,25]. A combination of different diagnostic methods, such as microscopy, polymerase chain reaction, and (1,3)-β-D-glucan detection have been previously adopted to increase the diagnosis rate. Silver staining can detect cysts with high specificity. However, the sensitivity of this method is not satisfactory. PJP patients have a higher number of trophozoites than cysts, thus Giemsa and Diff-Quik stains used for the detection of trophozoites should have high sensitivity. Moreover, the results depend on the skill and experience of the examiner. Advances in technology have led to development of mNGS which can unbiasedly detect various pathogenic microorganisms in clinical samples. This approach ensures rapid screening of pathogens, and provides timely identification of pathogens. Therefore, it is currently applied in the detection of pathogens that cause infectious diseases. Bronchoalveolar lavage fluid (BALF) is the preferred specimen, as BALF provides a higher diagnosis rate, and can be used to identify other pathogens, such as tuberculosis, histoplasmosis and cytomegalovirus infection. Induced sputum testing can be used as an alternative method if the patient cannot tolerate bronchoscopy, however, its diagnostic sensitivity is lower compared to that of BALF (55%–90%)[26,27].
Current guidelines and published reviews state that the first-line treatment for non-HIV-infected PJP is TMP-SMX[7,8,28]. However, prolonged use of TMP-SMX may increase risk of drug resistance and side effects. Some patients cannot tolerate high doses of this treatment however, low-dose therapy is well-tolerated and has fewer side effects[29]. In the current study, one patient developed renal impairment during treatment, but renal function was restored when the dosage was reduced. Therefore, the dose of TMP-SMX should be adjusted based on the renal function of the patient. If the patient is taking immunosuppressants during treatment, the dose of immunosuppressants should be reduced, suspended, or stopped. However, the dose should be individualized. Previous studies have shown that caspofungin alone or in combination with TMP-SMX is effective for HIV-infected PJP patients, and caspofungin can also be used as a remedial drug[30,31]. In the current study, all patients were successfully treated with caspofungin combined with TMP-SMX. This finding indicates that caspofungin can cure PJP; however, these findings should be validated in large-sample clinical studies. According to current guidelines, standard treatment should be used for 2–3 wk[8]. The duration should be appropriately extended until respiratory function is stable for non-HIV-infected PJP patients with severe immunosuppression, high pneumocystis load, and slow clinical improvement[8,28]. Non-HIV-infected PJP patients present with severe inflammation and some studies recommend use of adjuvant glucocorticoid to control it[31]. In the current study, 17 patients underwent adjuvant treatment of glucocorticoids for 7 d with good results. This indicates that patients with moderate to severe disease should be put under adjunctive corticosteroids to improve survival.
Non-HIV-infected PJP patients manifest with rapid disease progression, respiratory failure, and high mortality. Physicians should be aware that PJP is an important infectious complication in hemato-oncological and post-transplant patients, including who undergo solid organ transplant, allogenic or autologous hematopoietic stem cell transplantation. It is also common in patients taking immunosuppressive drugs. For non-HIV-infected PJP patients with respiratory failure, or patients with renal insufficiency, TMP-SMX combined with caspofungin can alleviate symptoms and accelerate recovery. Several challenges are encountered in the treatment of non-HIV-infected PJP patients, such as difficulty of determining the timing and duration of adjunctive treatment using glucocorticoids and identification of patients susceptible to pneumocystis jirovecii infection. Therefore, future studies should explore methods to circumvent these challenges.
Incidence of non-human immunodeficiency virus (HIV)-infected Pneumocystis jirovecii pneumonia (PJP) patients has been increasing annually owing to increased use of immunosuppressants and organ transplantation. In addition, management of non-HIV-infected PJP patients is not standardized as it is the case for HIV-infected PJP patients, and the clinical attention is poor compared with that of HIV-infected PJP patients.
The aim of this retrospective study was to explore the characteristics of non-HIV-infected PJP treated with trimethoprim–sulfamethoxazole (TMP-SMX) and caspofungin.
The findings of the current study will improve understanding of PJP in non-HIV-infected patients, and reduce misdiagnosis rate and mortality rate of non-HIV-infected PJP patients.
A retrospective case review of 22 non-HIV-infected PJP patients admitted to Dongyang Hospital Affiliated to Wenzhou Medical University was conducted between October 2019 and April 2021. Patient data on symptoms, laboratory results, dynamic and comprehensive computed tomography, and clinical course of the disease were extracted from electronic medical records. Additional data on treatment, response to treatment, outcomes, and any relevant follow-up data were also collected.
A total of 22 cases of non-HIV-infected PJP were included in the current study. Clinical manifestations of patients mainly included fever, dry cough, and progressive dyspnea. All patients presented with an acute onset and respiratory failure. The most common imaging manifestation was ground glass opacity around the hilar, mainly located in the upper lobe. All patients underwent diagnosis using next-generation sequencing, and were all treated with TMP-SMX and caspofungin. Seventeen patients received short-term adjuvant glucocorticoid therapy. All patients recovered and were discharged from hospital.
Non-HIV-infected PJP patients are characterized by rapid disease progression, high risk of respiratory failure, and high mortality. The findings of the current study showed that a combination of TMP-SMX and caspofungin is an effective treatment option for severe non-HIV-infected PJP patients with respiratory failure.
The timing and duration of adjunctive treatment using glucocorticoids and identification of patients susceptible to Pneumocystis jirovecii infection at an early stage to start effective prophylaxis drugs.
| 1. | Fauci AS, Macher AM, Longo DL, Lane HC, Rook AH, Masur H, Gelmann EP. NIH conference. Acquired immunodeficiency syndrome: epidemiologic, clinical, immunologic, and therapeutic considerations. Ann Intern Med. 1984;100:92-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 600] [Cited by in RCA: 540] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 2. | Masur H, Ognibene FP, Yarchoan R, Shelhamer JH, Baird BF, Travis W, Suffredini AF, Deyton L, Kovacs JA, Falloon J. CD4 counts as predictors of opportunistic pneumonias in human immunodeficiency virus (HIV) infection. Ann Intern Med. 1989;111:223-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 344] [Cited by in RCA: 319] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 3. | Recommendations for prophylaxis against Pneumocystis carinii pneumonia for adults and adolescents infected with human immunodeficiency virus. MMWR Recomm Rep. 1992;41:1-11. [PubMed] |
| 4. | Kovacs JA, Ng VL, Masur H, Leoung G, Hadley WK, Evans G, Lane HC, Ognibene FP, Shelhamer J, Parrillo JE. Diagnosis of Pneumocystis carinii pneumonia: improved detection in sputum with use of monoclonal antibodies. N Engl J Med. 1988;318:589-593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 214] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 5. | Varthalitis I, Aoun M, Daneau D, Meunier F. Pneumocystis carinii pneumonia in patients with cancer. An increasing incidence. Cancer. 1993;71:481-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 6. | Arend SM, Kroon FP, van't Wout JW. Pneumocystis carinii pneumonia in patients without AIDS, 1980 through 1993. An analysis of 78 cases. Arch Intern Med. 1995;155:2436-2441. [PubMed] |
| 7. | van Well G, van Furth M. Pneumocystis pneumonia. N Engl J Med. 2004;351:1262-1263. [PubMed] |
| 8. | Limper AH, Knox KS, Sarosi GA, Ampel NM, Bennett JE, Catanzaro A, Davies SF, Dismukes WE, Hage CA, Marr KA, Mody CH, Perfect JR, Stevens DA; American Thoracic Society Fungal Working Group. An official American Thoracic Society statement: Treatment of fungal infections in adult pulmonary and critical care patients. Am J Respir Crit Care Med. 2011;183:96-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 363] [Cited by in RCA: 407] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 9. | McKinnell JA, Cannella AP, Kunz DF, Hook EW 3rd, Moser SA, Miller LG, Baddley JW, Pappas PG. Pneumocystis pneumonia in hospitalized patients: a detailed examination of symptoms, management, and outcomes in human immunodeficiency virus (HIV)-infected and HIV-uninfected persons. Transpl Infect Dis. 2012;14:510-518.. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 59] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 10. | Bienvenu AL, Traore K, Plekhanova I, Bouchrik M, Bossard C, Picot S. Pneumocystis pneumonia suspected cases in 604 non-HIV and HIV patients. Int J Infect Dis. 2016;46:11-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 103] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 11. | Avino LJ, Naylor SM, Roecker AM. Pneumocystis jirovecii Pneumonia in the Non-HIV-Infected Population. Ann Pharmacother. 2016;50:673-679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 95] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 12. | Roux A, Canet E, Valade S, Gangneux-Robert F, Hamane S, Lafabrie A, Maubon D, Debourgogne A, Le Gal S, Dalle F, Leterrier M, Toubas D, Pomares C, Bellanger AP, Bonhomme J, Berry A, Durand-Joly I, Magne D, Pons D, Hennequin C, Maury E, Roux P, Azoulay É. Pneumocystis jirovecii pneumonia in patients with or without AIDS, France. Emerg Infect Dis. 2014;20:1490-1497. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 169] [Cited by in RCA: 250] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 13. | Reid AB, Chen SC, Worth LJ. Pneumocystis jirovecii pneumonia in non-HIV-infected patients: new risks and diagnostic tools. Curr Opin Infect Dis. 2011;24:534-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 102] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 14. | Matsumura Y, Shindo Y, Iinuma Y, Yamamoto M, Shirano M, Matsushima A, Nagao M, Ito Y, Takakura S, Hasegawa Y, Ichiyama S. Clinical characteristics of Pneumocystis pneumonia in non-HIV patients and prognostic factors including microbiological genotypes. BMC Infect Dis. 2011;11:76. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 76] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 15. | Li MC, Lee NY, Lee CC, Lee HC, Chang CM, Ko WC. Pneumocystis jiroveci pneumonia in immunocompromised patients: delayed diagnosis and poor outcomes in non-HIV-infected individuals. J Microbiol Immunol Infect. 2014;47:42-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 104] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 16. | Yu Q, Jia P, Su L, Zhao H, Que C. Outcomes and prognostic factors of non-HIV patients with pneumocystis jirovecii pneumonia and pulmonary CMV co-infection: A Retrospective Cohort Study. BMC Infect Dis. 2017;17:392. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 50] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 17. | Harigai M, Koike R, Miyasaka N; Pneumocystis Pneumonia under Anti-Tumor Necrosis Factor Therapy (PAT) Study Group. Pneumocystis pneumonia associated with infliximab in Japan. N Engl J Med. 2007;357:1874-1876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 134] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 18. | Yates M, Watts RA, Bajema IM, Cid MC, Crestani B, Hauser T, Hellmich B, Holle JU, Laudien M, Little MA, Luqmani RA, Mahr A, Merkel PA, Mills J, Mooney J, Segelmark M, Tesar V, Westman K, Vaglio A, Yalçındağ N, Jayne DR, Mukhtyar C. EULAR/ERA-EDTA recommendations for the management of ANCA-associated vasculitis. Ann Rheum Dis. 2016;75:1583-1594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 920] [Cited by in RCA: 799] [Article Influence: 79.9] [Reference Citation Analysis (0)] |
| 19. | Martin-Garrido I, Carmona EM, Specks U, Limper AH. Pneumocystis pneumonia in patients treated with rituximab. Chest. 2013;144:258-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 145] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 20. | Grubbs JA, Baddley JW. Pneumocystis jirovecii pneumonia in patients receiving tumor-necrosis-factor-inhibitor therapy: implications for chemoprophylaxis. Curr Rheumatol Rep. 2014;16:445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 21. | Thomas CF Jr, Limper AH. Pneumocystis pneumonia. N Engl J Med. 2004;350:2487-2498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 761] [Cited by in RCA: 748] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 22. | Kovacs JA, Hiemenz JW, Macher AM, Stover D, Murray HW, Shelhamer J, Lane HC, Urmacher C, Honig C, Longo DL. Pneumocystis carinii pneumonia: a comparison between patients with the acquired immunodeficiency syndrome and patients with other immunodeficiencies. Ann Intern Med. 1984;100:663-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 585] [Cited by in RCA: 529] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 23. | Vogel MN, Vatlach M, Weissgerber P, Goeppert B, Claussen CD, Hetzel J, Horger M. HRCT-features of Pneumocystis jiroveci pneumonia and their evolution before and after treatment in non-HIV immunocompromised patients. Eur J Radiol. 2012;81:1315-1320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 60] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 24. | Alanio A, Hauser PM, Lagrou K, Melchers WJ, Helweg-Larsen J, Matos O, Cesaro S, Maschmeyer G, Einsele H, Donnelly JP, Cordonnier C, Maertens J, Bretagne S; 5th European Conference on Infections in Leukemia (ECIL-5), a joint venture of The European Group for Blood and Marrow Transplantation (EBMT), The European Organization for Research and Treatment of Cancer (EORTC), the Immunocompromised Host Society (ICHS) and The European LeukemiaNet (ELN). ECIL guidelines for the diagnosis of Pneumocystis jirovecii pneumonia in patients with haematological malignancies and stem cell transplant recipients. J Antimicrob Chemother. 2016;71:2386-2396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 200] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 25. | Alanio A, Desoubeaux G, Sarfati C, Hamane S, Bergeron A, Azoulay E, Molina JM, Derouin F, Menotti J. Real-time PCR assay-based strategy for differentiation between active Pneumocystis jirovecii pneumonia and colonization in immunocompromised patients. Clin Microbiol Infect. 2011;17:1531-1537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 168] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 26. | Limper AH, Offord KP, Smith TF, Martin WJ 2nd. Pneumocystis carinii pneumonia. Differences in lung parasite number and inflammation in patients with and without AIDS. Am Rev Respir Dis. 1989;140:1204-1209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 364] [Cited by in RCA: 384] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 27. | Pagano L, Fianchi L, Mele L, Girmenia C, Offidani M, Ricci P, Mitra ME, Picardi M, Caramatti C, Piccaluga P, Nosari A, Buelli M, Allione B, Cortelezzi A, Fabbiano F, Milone G, Invernizzi R, Martino B, Masini L, Todeschini G, Cappucci MA, Russo D, Corvatta L, Martino P, Del Favero A. Pneumocystis carinii pneumonia in patients with malignant haematological diseases: 10 years' experience of infection in GIMEMA centres. Br J Haematol. 2002;117:379-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 96] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 28. | Tasaka S, Tokuda H. Pneumocystis jirovecii pneumonia in non-HIV-infected patients in the era of novel immunosuppressive therapies. J Infect Chemother. 2012;18:793-806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 86] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 29. | Nakashima K, Aoshima M, Nakashita T, Hara M, Otsuki A, Noma S, Misawa M, Otsuka Y, Motojima S. Low-dose trimethoprim-sulfamethoxazole treatment for pneumocystis pneumonia in non-human immunodeficiency virus-infected immunocompromised patients: A single-center retrospective observational cohort study. J Microbiol Immunol Infect. 2018;51:810-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 30. | Mu XD, Que CL, He B, Wang GF, Li HC. Caspofungin in salvage treatment of severe pneumocystis pneumonia: case report and literature review. Chin Med J (Engl). 2009;122:996-999. [PubMed] |
| 31. | Hof H, Schnülle P. Pneumocystis jiroveci pneumonia in a patient with Wegener's granulomatosis treated efficiently with caspofungin. Mycoses. 2008;51:65-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 34] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Infectious diseases
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Brat K S-Editor: Zhang H L-Editor: Kerr C P-Editor: Zhang H