Published online Mar 16, 2022. doi: 10.12998/wjcc.v10.i8.2404
Peer-review started: July 16, 2021
First decision: October 16, 2021
Revised: October 20, 2021
Accepted: January 19, 2022
Article in press: January 19, 2022
Published online: March 16, 2022
Processing time: 237 Days and 10.4 Hours
Millions of people have died of coronavirus disease 2019 (COVID-19) due to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, and retrospective studies of the disease in local regions are necessary.
To characterize the epidemiological features and dynamic changes in blood biochemical indices for SARS-CoV-2-infected patients in Hebi, a representative city with a large floating population in North China.
From January 25 to February 10, 2020, the clinical data of patients who tested positive for SARS-CoV-2 by quantitative real-time polymerase chain reaction in Hebi city (China) were evaluated at admission, and laboratory data for hema
Sixteen confirmed COVID-19 patients developed pneumonia but were cured after adequate treatment. Fever and fatigue were the common symptoms. The most common laboratory abnormalities of patients at admission were leukopenia, eosinopenia, decreased percentage of eosinophils, elevated high sensitivity C-reactive protein and fibrinogen levels, hypoalbuminemia, mildly increased aspartate transferase activity and levels of bilirubin, and increased levels of β2-microglobulin. Importantly, aggravated liver dysfunction was detected in most patients, which may be partially attributed to virus infection as well as medicinal treatment.
This study provides several potential diagnostic markers and dynamic biochemical indices of disease progression to better prevent, diagnose and treat COVID-19 infection.
Core Tip: Coronavirus disease 2019 (COVID-19) is still spreading across the world since the outbreak in 2020, and has caused millions of deaths. Although severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) mass vaccination has effectively inhibited this epidemic, the emergence of new mutant strains of SARS-CoV-2 is still challenging the current prevention and treatment of COVID-19. In this retrospective study, we aimed to characterize the epidemiological features and evaluate the dynamic changes in blood biochemical indices for COVID-19 patients in Hebi, a representative city with a large floating population in North China.
- Citation: Nie XB, Shi BS, Zhang L, Niu WL, Xue T, Li LQ, Wei XY, Wang YD, Chen WD, Hou RF. Epidemiological features and dynamic changes in blood biochemical indices for COVID-19 patients in Hebi. World J Clin Cases 2022; 10(8): 2404-2419
- URL: https://www.wjgnet.com/2307-8960/full/v10/i8/2404.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i8.2404
Currently, coronavirus disease 2019 (COVID-19) with an unknown source of infection has spread rapidly worldwide. According to the latest data from Johns Hopkins University (https://coronavirus.jhu.edu/map.html), as of October 20, 2021, more than 241 million people have tested positive for COVID-19, bringing the total number of deaths involving the coronavirus to more than 4.9 million, and the numbers are still increasing sharply with over two million new cases weekly. Although COVID-19 infection is self-limiting in approximately 80% of cases, male individuals, elderly individuals and those with comorbidities such as diabetes mellitus, hypertension, or underlying cardiorespiratory illness are at a higher risk of becoming severely ill[1-3]. According to previous reports, the genome of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has 89% nucleotide identity with human SARS-CoV-2 and 50% identity with that of human Middle East respiratory syndrome coronavirus, and all three coronaviruses can cause severe respiratory illness[4,5]. COVID-19 infection is mainly characterized by respiratory illness with flu-like symptoms such as fever, cough and shortness of breath, whereas severe infection can cause death due to diffuse alveolar damage and progressive respiratory failure[6]. Angiotensin-converting enzyme 2 (ACE2), the human cell receptor of SARS-CoV-2, whose expression is dominant in alveolar type 2 cells, is also expressed in organs such as the liver, kidney, heart and brain; thus, SARS-CoV-2-infected patients are often observed to have systemic damage with widespread inflammation[7-10]. Preventive measures and vaccination are the best ways to protect people from this infectious disease until effective medicines are developed.
Hebi is one of the agricultural and developing cities in North China, with more than 20% of its population (1.63 million) working outside year round and returning to their home villages on holidays. Therefore, the imported case of COVID-19 infection quickly emerged the day after the Chinese Lunar New Year holiday started on January 24. Next, the number of newly confirmed cases had undergone two rapid growth periods and soon disappeared after mandatory social isolation had been issued by the local government. Although several studies have demonstrated the epidemic, clinical, pathological features and prognosis of COVID-19 infection[6,11-13], data on epidemic areas such as Hebi are still lacking. Notably, we identified several valuable diagnostic markers and obtained dynamic indicators of disease progression by comparing the data on blood biochemical indices and computed tomography (CT) imaging of SARS-CoV-2-infected patients at admission, upon hospitalization and before discharge. We hope our study will still guide the subsequent prevention, diagnosis and personalized treatment of this disease.
We conducted a retrospective study of the clinical characteristics of 16 patients who were enrolled in People’s Hospital of Hebi, China between January 25 and February 10, 2020. All patients were diagnosed with viral pneumonia and were confirmed to be infected with SARS-CoV-2 according to the guidelines from the World Health Organization (interim) and National Health Commission of the People’s Republic of China (Trial Version 5)[14]. To confirm COVID-19 infection, nasopharyngeal swab and oropharyngeal swab samples were collected from each patient and tested to detect virus titers using quantitative real-time polymerase chain reaction (RT-PCR) to identify the ORF1ab and N genes of SARS-CoV-2. A positive PCR test was defined if the cycle threshold (Ct) value was less than 37. Virus detection was first performed by a local infectious hospital and further confirmed by Hebi’s Centers for Disease Control and Prevention (CDC). Specimens were considered negative if the Ct value from repeated tests was undetectable.
The study was approved by the Ethics Committee of People’s Hospital of Hebi at Henan University, written informed consent was obtained from all patients, and their private information was strictly confidential.
All available medical information, including epidemic characteristics, exposure history, time of onset, medical visit, symptoms and signs, treatment strategies and clinical outcomes, was collected from the 16 confirmed patients infected with SARS-CoV-2 at admission, upon hospitalization and before discharge. Data on CT imaging and laboratory tests at three time periods were also collected, including routine blood examination, blood lipid levels, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP) levels, coagulation function, myocardial enzymes, liver function, renal function and immune function. However, not all patients had complete data for the three time periods, and the number of cases in some figures was less than 16.
No patient or public involvement occurred in the development of the research design or in conducting the study.
Unpaired two-sided Student’s t-test was conducted to evaluate the statistical significance of the difference between groups of data on patients at admission and before discharge (normal distribution), and unpaired two-sided Student’s t-test with Welch’s correction was used for data with abnormal distribution. The error bar for the experiments represents the standard deviation of the mean value (mean ± SD). The data on age and time are presented as medians and interquartile ranges (IQRs). P values less than 0.1 were all presented, and those less than 0.05 were considered statistically significant. All statistical analyses and figures were assessed using SPSS 25.0 and GraphPad Prism 8.0.
Sixteen patients with COVID-19 infection who were discharged from our hospital were enrolled. The basic clinical records are shown in Table 1. The median age was 34 (IQR: 25-53) years, 3 patients (18.7%) were aged older than 60 years, 9 patients (56.3%) were aged younger than 40 years, and 9 patients (56.3%) were female. Regarding potential exposure history, 7 patients (43.8%) were living outside of Hebi and returned to Hebi after the Chinese Lunar New Year holiday started on January 24. Next, the confirmed cases (n = 6, 37.5%) with COVID-19 infection in Hebi had undergone a rapid growth period from January 25 to January 31, 2020, and these cases had exposure in Hubei. The number of newly confirmed cases (n = 10; 62.5%) reached another rapid growth period (February 5 to February 10, 2020).
| Variable | |
| Age (yr) | 34 (25, 53) |
| ≥ 60 | 3 (18.7%) |
| 50-59 | 2 (12.5%) |
| 40-49 | 2 (12.5%) |
| ≤ 39 | 9 (56.3%) |
| Gender | |
| Male | 7 (43.8%) |
| Female | 9 (56.3%) |
| Exposure history | |
| Living in Wuhan | 7 (43.8%) |
| Exposure to infected patients | 8 (50%) |
| Unknown origin | 1 (6.2%) |
| Number of new cases | |
| Jan 25 to Jan 31, 2020 | 6 (37.5%) |
| Feb 5 to Feb 10, 2020 | 10 (62.5%) |
| Time (d) | |
| Interval from onset to admission | 4 (2, 6) |
| Hospital stay | 17 (14, 20) |
| Symptoms | |
| Fever | 13 (81.3%) |
| Fatigue | 10 (62.5%) |
| Dry cough | 13 (37.5%) |
| Cough and expectoration | 13 (37.5%) |
| Anorexia | 4 (25%) |
| Headache | 3 (18.7%) |
| Pharyngalgia | 2 (12.5%) |
| Running nose | 2 (12.5%) |
| Chill | 2 (12.5%) |
| Muscle soreness | 2 (12.5%) |
| Diarrhea | 1 (6.3%) |
| Chest distress | 1 (6.3%) |
| Underlying disease | |
| Hypertension | 2 (12.5%) |
| Diabetes | 2 (12.5%) |
| Cardiovascular disease | 1 (6.3%) |
| Cerebrovascular disease | 1 (6.3%) |
| Bronchitis | 1 (6.3%) |
| Treatments | |
| Antiviral treatment | 16 (100%) |
| Traditional Chinese Medicine | 16 (100%) |
| Recombinant human interferon | 16 (100%) |
| High-flow oxygen therapy | 9 (56.3%) |
| Anticoagulant treatment | 6 (37.5%) |
| Digestant | 5 (31.3%) |
| Antibiotic treatment | 4 (25%) |
| Glucocorticoids | 3 (18.7%) |
| Intensive care unit | 2 (12.5%) |
| Immunoglobulin | 1 (6.3%) |
The median time interval from illness onset to admission was 4 (IQR: 2-6) d. Fever (81.3%), fatigue (62.5%), dry cough (37.5%), cough and expectoration (37.5%), and anorexia (25%) were the most common symptoms of COVID-19 infection. Additionally, headache (18.7%), chills (12.5%), pharyngalgia (12.5%), runny nose (12.5%), muscle soreness (12.5%), chest distress (6.3%), and diarrhea (6.3%) were found in some patients. A few patients had comorbidities such as hypertension (12.5%), diabetes mellitus (12.5%), cardiovascular disease (6.3%), cerebrovascular disease (6.3%) or bronchitis (6.3%) at admission. All patients received antiviral (lopinavir and ritonavir), traditional Chinese medicine (oral administration) and recombinant human interferon (aerosol inhalation) treatments; more than half of the patients (56.3%) required high-flow oxygen therapy; a few patients received anticoagulant (heparin sodium, 37.5%), digestant (31.3%), antibiotic (25%), glucocorticoid (18.7%) and immunoglobulin (6.3%) treatments if necessary; and two (12.5%) patients needed intensive unit care. All patients were cured to discharge after adequate treatment, including one severe case aged 89 years. The median length of hospital stay of all patients was 17 (IQR: 14-20) d.
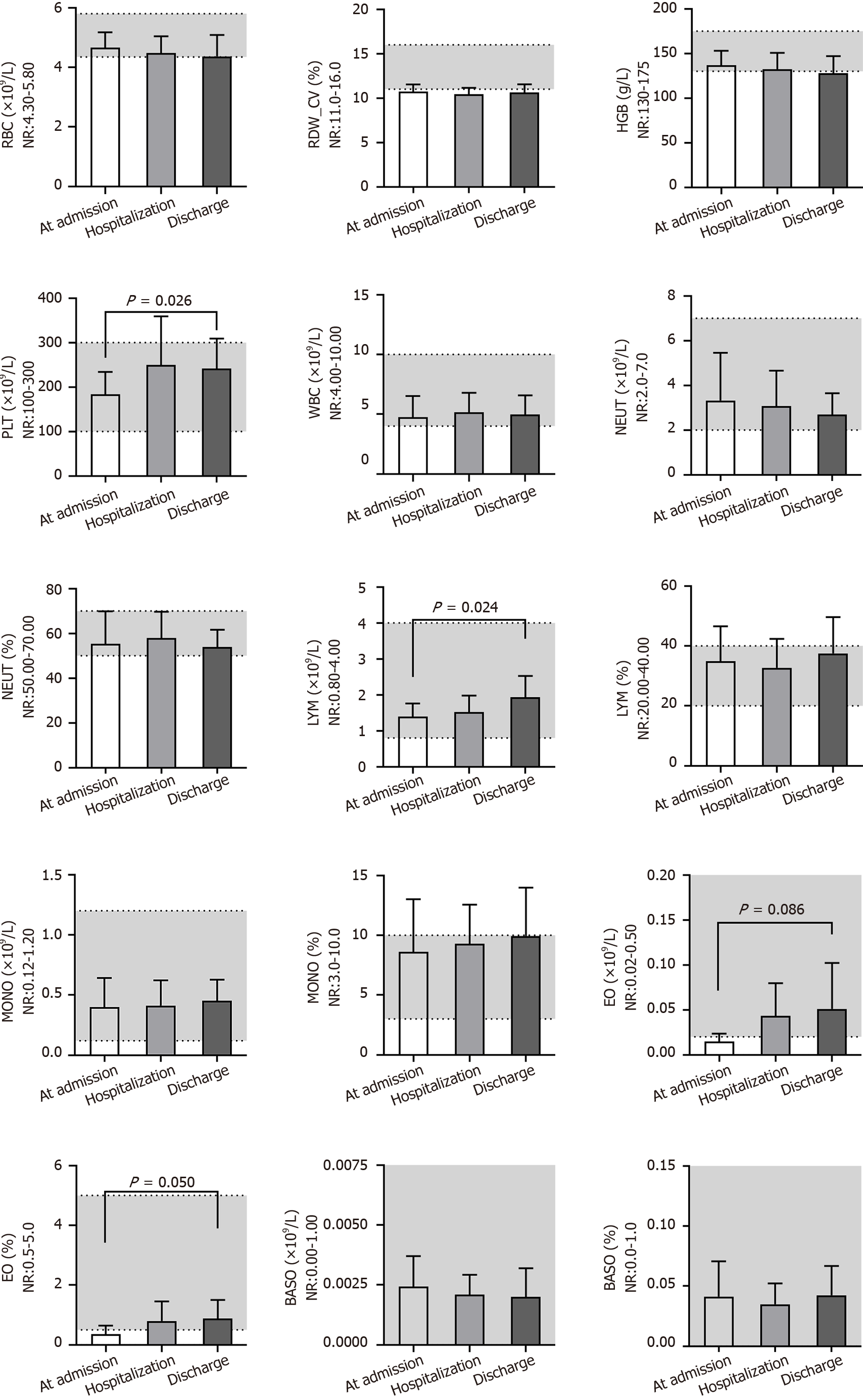
By comparing the results of routine blood examination at different times (Figure 1), we found that the red blood cell (RBC) count [(4.66 ± 0.51) × 109/L], RBC distribution width_CV (RDW_CV) (10.7% ± 0.8%), hemoglobin content (127 ± 34 g/L), white blood cell (WBC) count [(4.75 ± 1.77) × 109/L], neutrophil count [(3.3 ± 2.1) × 109/L] and percent of neutrophils (55.28% ± 14.65%) were close to the lower end of the normal range (NR) or below at admission and did not change significantly during the three time periods. The platelet count was normal at admission but increased [(241 ± 68) × 109/L; P = 0.026] significantly after treatment. The lymphocyte count [(1.39 ± 0.37) × 109/L] was lower at admission but increased [(1.93 ± 0.60) × 109/L, P = 0.024] significantly after treatment. The eosinophil (EO) count [(0.0145 ± 0.009) × 109/L] and percent of EO% (0.36% ± 0.29%) were below the NR at admission but increased significantly after treatment, although they were still at the lower end of the NR. Additionally, the percentage of lymphocytes, monocyte count, percentage of monocytes, basophil count and percentage of basophils were nearly in the NR and did not change at different times.
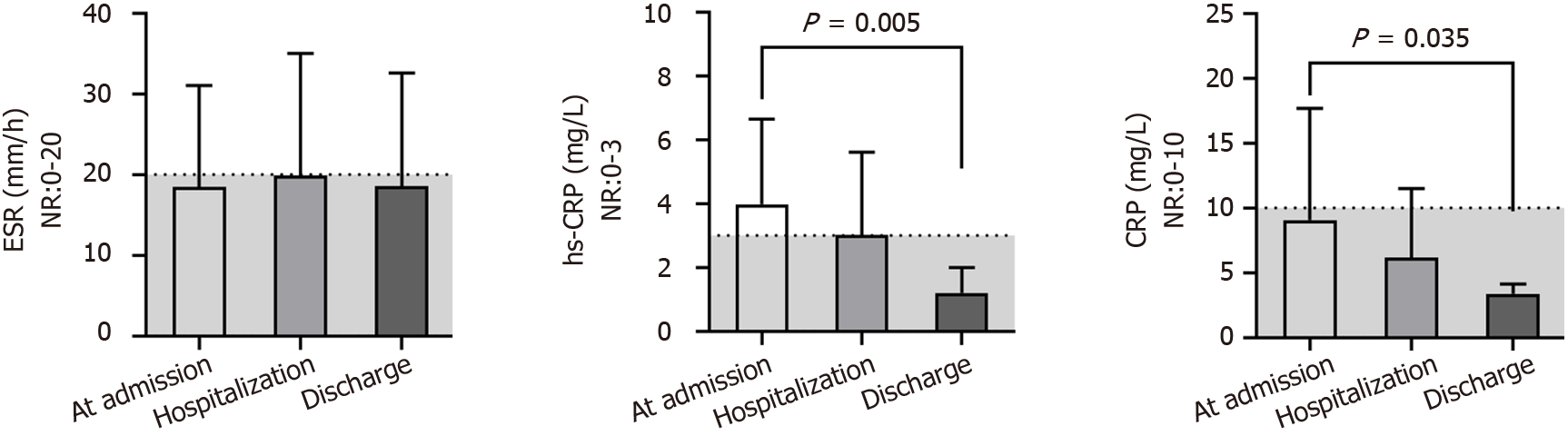
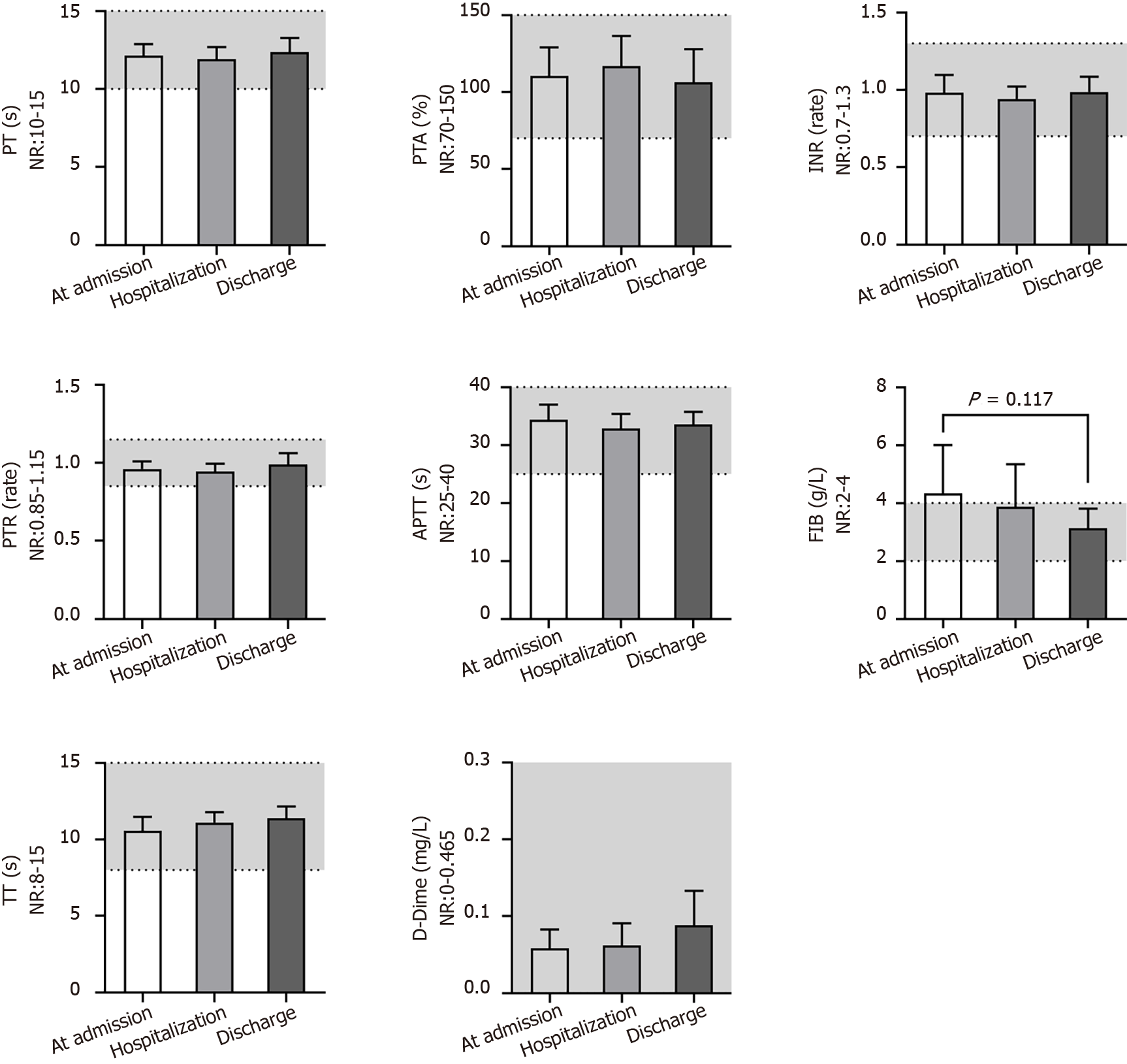
The ESR was always close to the middle upper end of the NR or above at different times (Figure 2). However, most patients who had undergone examination of the high sensitivity CRP (hs-CRP) test at admission had obviously increased levels (4.0 ± 2.7 mg/L), and all patients returned to the NR before discharge (1.2 ± 0.8 mg/L; P = 0.005). A similar change trend was found for the CRP levels (P = 0.035). The indices associated with the function of coagulation (Figure 3) - i.e., the prothrombin time, prothrombin activity, international normalized ratio, prothrombin ratio, activated partial thromboplastin time, thrombin time and D-dimer level-were always in the NR for all patients, except for the fibrinogen (FIB) level, which was above the NR (4.3 ± 1.4 g/L) and returned to the NR (P = 0.117) after anticoagulant treatment.
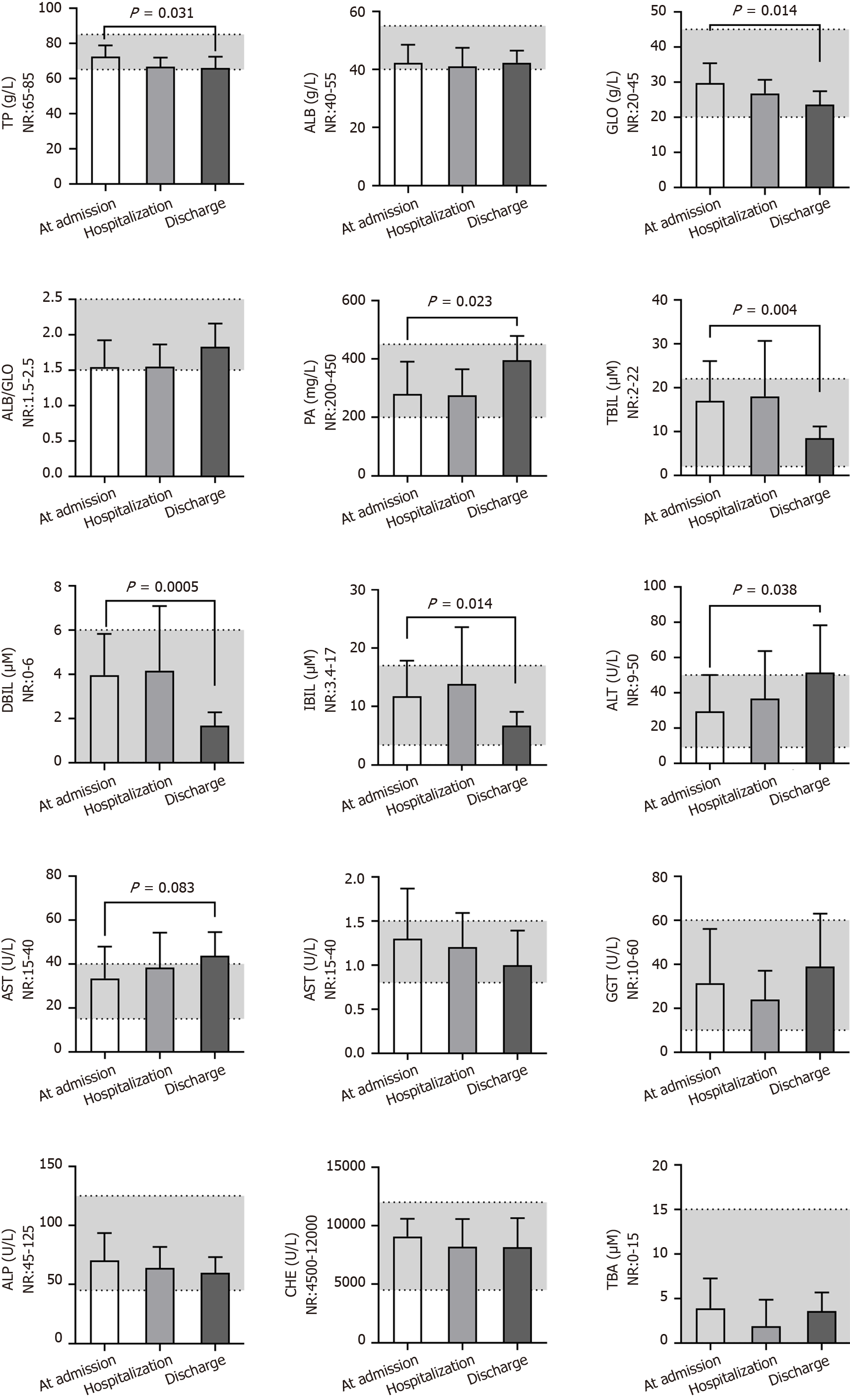
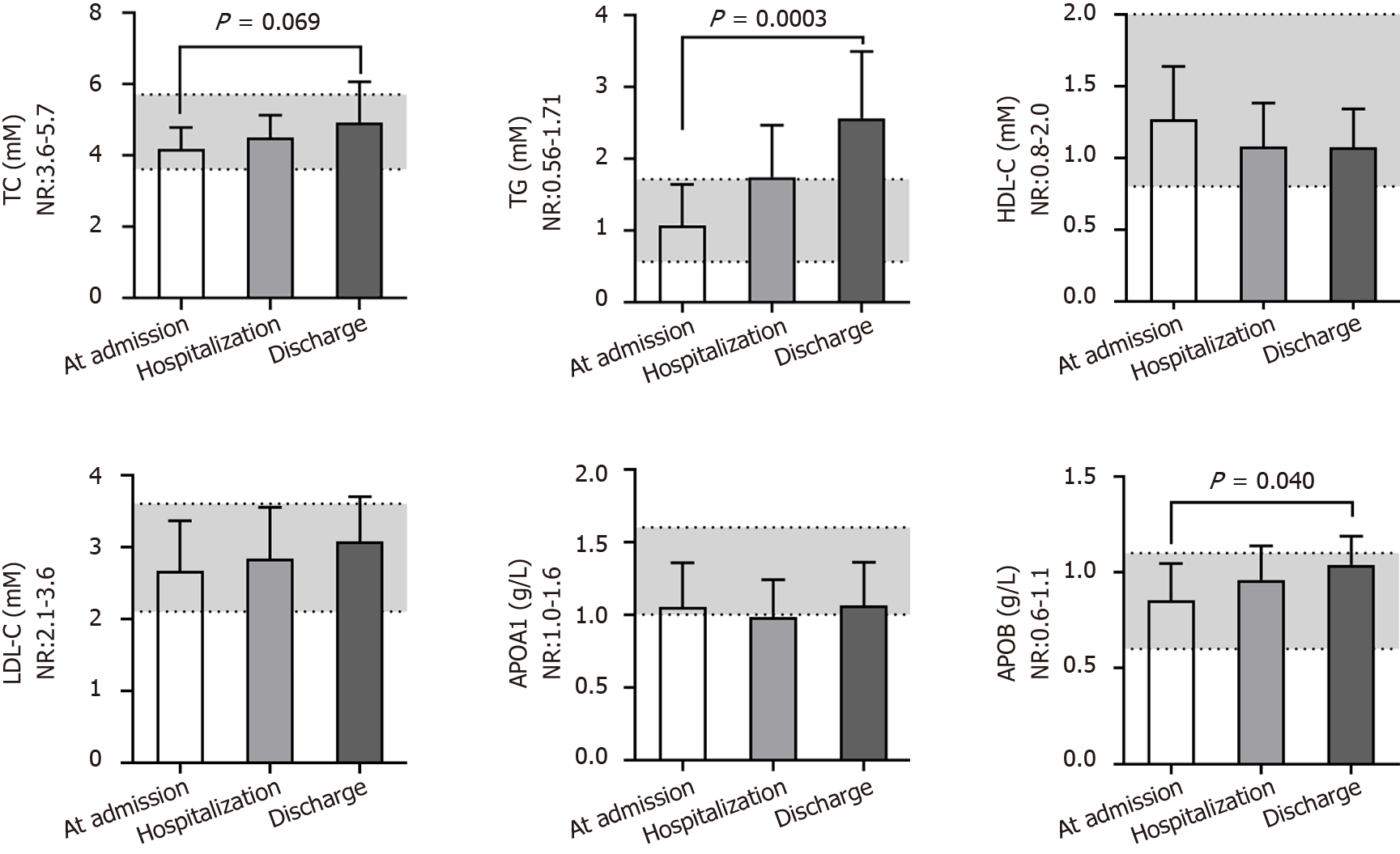
Among the fifteen blood biochemistry indices reflecting the liver function of patients (Figure 4), glutamyl transpeptidase (GGT), alkaline phosphatase, cholinesterase and total bile acid were nearly in the NR at different times. The total protein (TP) level (72 ± 6 g/L), albumin (ALB) level (42 ± 6 g/L), ratio of ALB and globulin (GLO) (1.5 ± 0.4), and prealbumin (PA) level (280 ± 110 mg/L) were close to the middle lower end of the NR or below at admission, and the total bilirubin (TBIL) (17 ± 9 μM), direct bilirubin (DBIL) (4.0 ± 1.8 μM), and indirect bilirubin (IBIL) (11.7 ± 6.1 μM) levels and aspartate transferase (AST) activity (33 ± 14 U/L) were close to the middle upper end of the NR or above at admission; these findings predicted the impaired liver function of patients after developing COVID-19. Even worse, alanine transaminase (ALT) activity (51 ± 27 U/L; P = 0.038) and AST activity (44 ± 11 U/L; P = 0.083) increased, whereas the GLO level (23.6 ± 3.8 g/L; P = 0.014) decreased after hospitalization, observations that predicted aggravated liver dysfunction after treatment. These data are consistent with the obviously increased levels of total cholesterol (TC) (4.9 ± 1.1 mmol/L; P = 0.069), triglyceride (TG) (2.56 ± 0.93 mmol/L; P = 0.0003), and apolipoprotein B (APOB) (1.0 ± 0.1 g/L; P = 0.040), slightly decreased level of high-density lipoprotein cholesterol (HDL-C) (P = 0.217) and slightly increased level of low-density lipoprotein cholesterol (LDL-C) (P = 0.200) after treatment (Figure 5). We speculated that the most substantial reason was the secondary liver injury likely induced by virus infection as well as overdosed medicines used during the treatment.
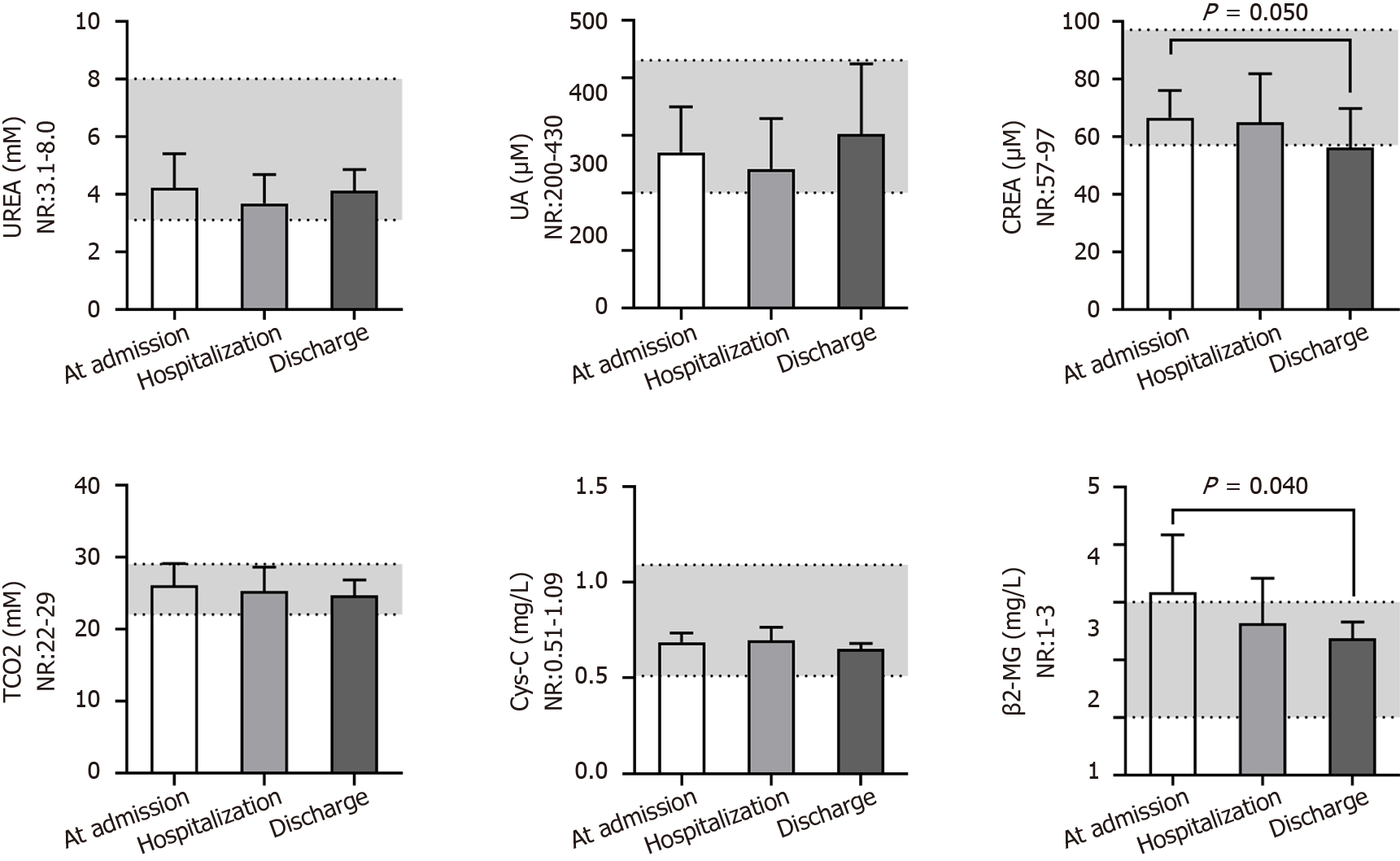
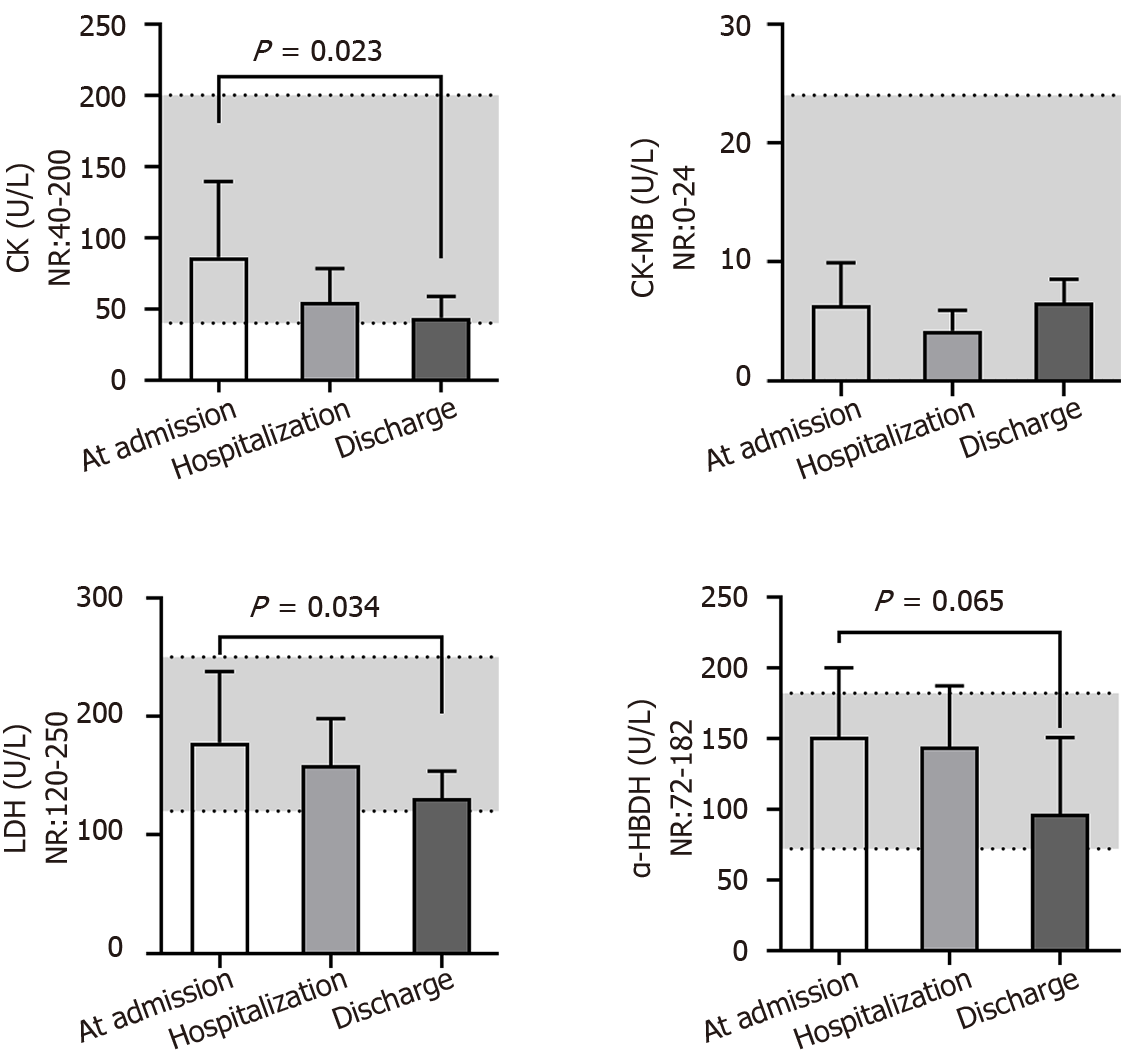
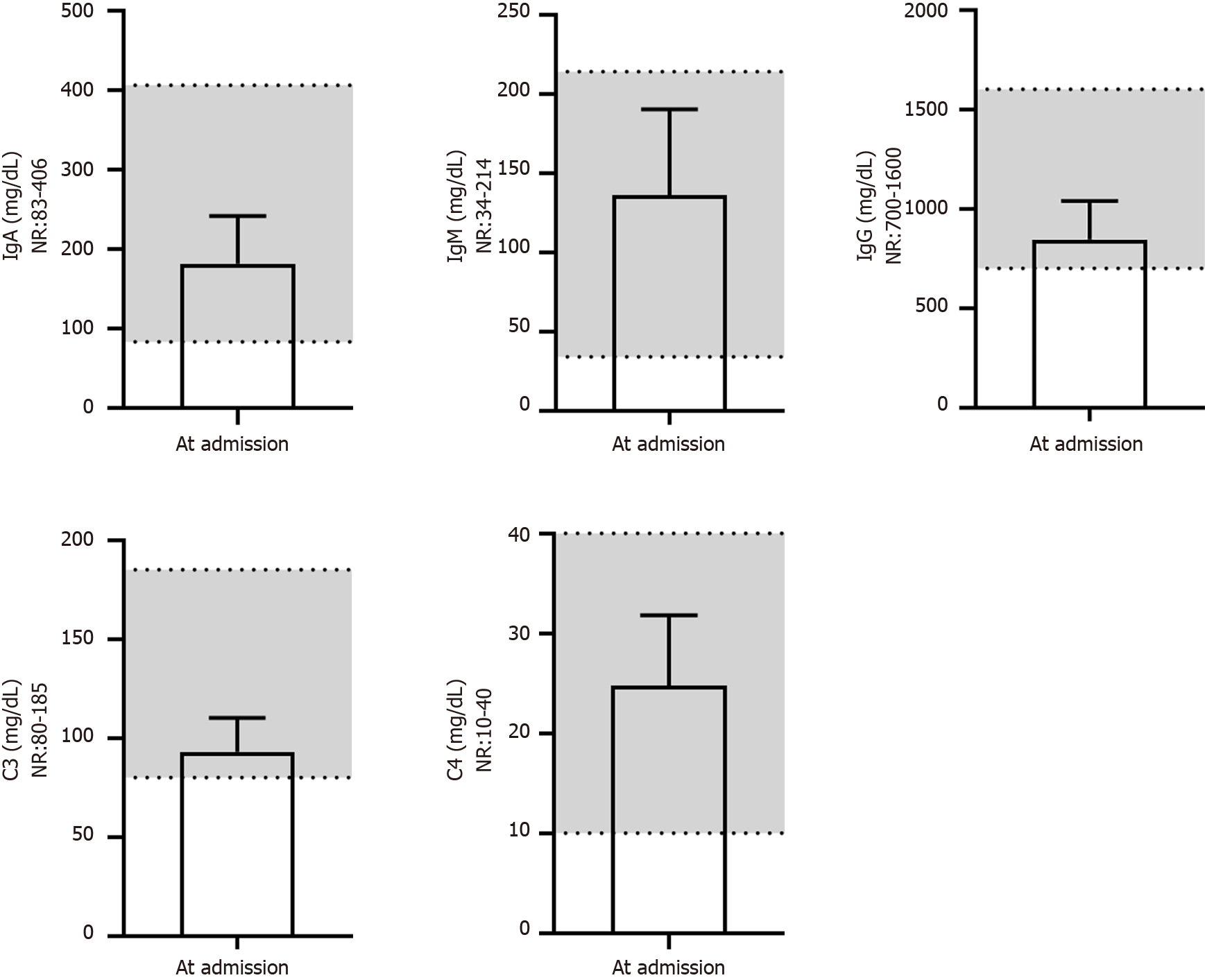
We next evaluated the renal function of patients at different times (Figure 6). Serum urea, uric acid, total CO2 and cystatin C levels were nearly in the NR at different times. The level of serum creatinine (66 ± 9 μM) was close to the lower end of the NR at admission and further reduced (56 ± 13 μM, P = 0.050) after treatment, which may indirectly reflect liver injury during virus infection. Additionally, the serum β2-MG (3.2 ± 1.0 mg/L) level in most patients was above the NR at admission and gradually returned to the normal level after treatment (P = 0.040), predicting kidney injury in patients after developing COVID-19. The serum biochemical indices associated with cardiac function (Figure 7) - i.e., creatine phosphokinase isoenzyme activity - were always in the NR at different times. However, the activities of creatine kinase (CK), lactic dehydrogenase (LDH) and α-hydroxybutyric dehydrogenase (α-HBDH) gradually decreased after treatment, indicating potentially slight heart damage in patients after developing COVID-19. Among the five blood biochemical markers associated with immunity (Figure 8), immunoglobulin M (IgM) and complement C4 were in the NR at admission, but IgA (181 ± 60 mg/dL), IgG (845 ± 193 mg/dL) and complement C3 (93 ± 17 mg/dL) were close to the middle lower end of the NR at admission, indicating low immunity for most patients after developing COVID-19.
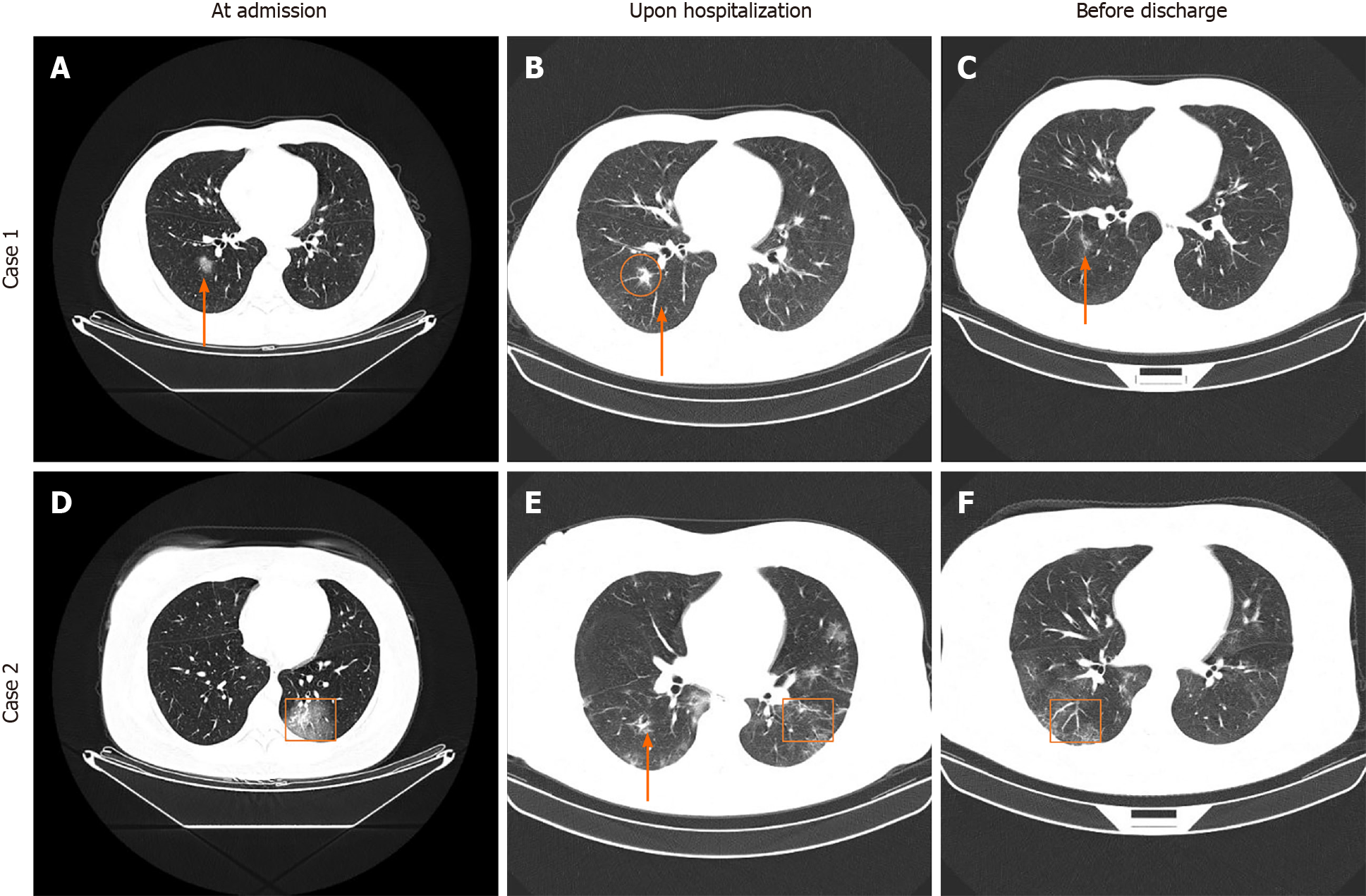
According to chest CT image analysis of all patients at the three time periods, we found dynamic changes in CT characteristics for most patients (Figure 9). During the initial stage, ground-glass opacity was observed in the lungs. During the progressive stage, the lesions became larger, more consolidated or increased in number, more pulmonary lobes were affected, and fibrous stripes and the paving stone sign were observed. During the severe stage, consolidated lesions diffusely distributed in double lungs, enlarged fibrous stripes, signs of white lung and air bronchogram were observed. During the recovery stage, partial absorption of lesions was observed in the pulmonary lobes, and patients were in recovery. Representative CT images (2 cases) with various lesions are shown in Figure 9.
In the present study, we reported the epidemiological and clinical characteristics of 16 cases of laboratory-confirmed COVID-19 infections admitted to the People’s Hospital of Hebi (Hebi, China), a representative agricultural city in North China with one-fifth of the population working in other places year around. The first 6 confirmed cases returned from Wuhan after the Chinese Lunar New Year holiday began. Because the latency of COVID-19 has been reported to be as short as 2 d[15], a rapid increase in confirmed cases emerged in Hebi in the following week, followed by zero cases in the subsequent four days. These cases were likely imported and linked to the cases that appeared in Wuhan, China. From February 5, another 10 cases were confirmed, and 9 cases were identified in four family clusters; one case was of unknown origin. The latency of patients infected in Hebi ranged from 6 to 10 d, which was longer than that of the Wuhan exposure or Shenzhen exposure[15,16]. Notably, one patient who had returned from Wuhan had a latency as long as 22 d, and the onset of this patient was even later than that of his family member. No new cases were identified after February 11, 2020. Overall, COVID-19 is an efficient person-to-person transmission disease with different latencies and can be spread through asymptomatic patients. Therefore, the most effective precautionary measures for general areas were the lockdown and screening of people from epidemic areas and avoiding crowded areas, and more stringent public health measures should be implemented in epidemic areas before universal vaccination can be reached or effective medicines are developed.
Fever and the resulting fatigue were the typical symptoms for most patients at admission in Hebi, a finding similar to that reported for patients in Wuhan[17]. Therefore, the simplest approach to screen potential infections is the monitoring of body temperature. Additionally, respiratory infection symptoms such as dry cough, cough and expectoration and digestive system symptoms such as anorexia were the common symptoms in our study. The median time of hospital stay was 17 (14, 20) d in this study, and all the patients were cured to discharge. We predicted that the high cure rate might be associated with the relatively young age of the patients. These findings agree with most of the study findings showing that patients aged older than 60 years are more likely to become critical cases[18,19].
According to the laboratory test results, the WBC count, neutrophil count, percent of neutrophils, and lymphocyte count were close to the lower end of the NR or below at admission. Among these, leukopenia is a significant laboratory index for COVID-19 infection and its increase often predicts an improvement of this disease[20]. In our study, the lymphocyte count of most patients increased after adequate treatment and nearly recovered to the NR before discharge. Notably, the EO count and percent of EO% in most patients were below the NR at admission and increased to varying degrees after hospitalization. A similar phenomenon is observed in patients with COVID-19 and other severe infections[21], indicating that eosinopenia is associated with lower immune function and is an independent indicator of COVID-19 infection. Based on the available evidence, anemia is associated with severe infection of COVID-19 due to the interaction of SARS-CoV-2 and hemoglobin and resultant hemolysis[22,23]. Consistently, we found that the RBC count, RDW_CV and levels of hemoglobin were always close to the lower end of the NR or below during the entire treatment. Additionally, a previous study revealed that pro-inflammatory cytokines, including interleukin-2 receptor (IL-2R) and IL-6 in serum, increased significantly in severe patients with COVID-19[24], suggesting injury to cytokine storms caused by COVID-19 infection. CRP is a typical inflammatory marker in plasma produced during the acute phase of inflammation[25]. Similarly, our study also found that most patients showed increased levels of CRP, particularly hs-CRP, which recovered to the NR before discharge. ESR, another marker of systemic inflammation and a less expensive alternative to CRP, was also found to be increased in some patients at admission. The hypercoagulable state and secondary hyperfibrinolysis marked by an increased level of D-dimer are important pathological features of severe to critical types of COVID-19 infection[26]. Although increased D-dimer levels were not detected in our study, we still found upregulated plasma FIB levels in most patients at admission and recovery after anticoagulant treatment with heparin. Our findings are in accordance with a previous study showing that inflammatory and hematologic markers such as CRP, were elevated in patients with COVID-19 and suggested to be predictors of severe outcomes[27].
Liver damage is common in patients with COVID-19, and severe cases have a higher risk of liver dysfunction[28]. A recent study reported that the expression level of the ACE2 receptor in cholangiocytes is similar to that of alveolar type 2 cells, suggesting that SARS-CoV-2 directly binds to cholangiocytes and leads to liver damage[29]. However, a pathological study on liver tissue from a deceased COVID-19 patient showed that the viral titer was relatively low because no viral inclusion body was observed in the liver, but moderate microvascular steatosis and mild active inflammation of the hepatic lobular portal area were observed[30]. We found that GGT, an accurate diagnostic biomarker for cholangiocyte injury, was not elevated in most patients. However, relatively low levels of TP, ALB and PA, a decreased ratio of ALB/GLO, relatively high levels of TBIL, DBIL, and IBIL, and high AST activity were detected in most patients at admission, indicating that SARS-CoV-2 might directly damage liver function. Furthermore, the ALT and AST activities further increased, and the GLO level decreased after hospitalization. Additionally, increased levels of TC, TG, and APOB, slightly decreased levels of HDL-C and increased levels of LDL-C were detected in most patients after treatment. We predicted that the aggravated liver impairment and elevated blood lipids may be due to hepatotoxicity induced by both viruses and overdosed medicines used during hospitalization. Therefore, patients with severe liver dysfunction at admission may develop liver failure and must be given personalized medication to protect the liver, and patients should undergo regular evaluation of liver function even after discharge.
Renal dysfunction or even acute kidney injury occurs in approximately half of patients with COVID-19, the main features of which are proteinuria, hematuria, increased levels of blood urea nitrogen and serum creatinine[31]. This finding implies the possibility that cells in the kidney may be directly infected by SARS-CoV-2. Based on the online analysis of ACE2 expression in different human organs and immunohistochemistry tests, Fan et al[32] proved that ACE2 is highly expressed in renal tubular cells. Similarly, our study also demonstrated that more than half of patients showed a sustainable increase in β2-MG levels at admission and a decrease after treatment. Considering this evidence, renal function evaluation of confirmed cases should be performed at admission, and more intensive surveillance and potential interventions should be given for severe patients to prevent fatality. Additionally, SARS-CoV-2 can cause fatal consequences for patients who have underlying cardiovascular diseases or cardiac risk factors and cardiac injury for patients without potential cardiovascular disease[33]. For more detailed information on the relationship between COVID-19 and the cardiovascular system, please refer to the review elaborately summarized by Kwenandar et al[34]. In our study, the myocardial enzyme activities associated with cardiac function were nearly in the NR at admission, but the activities of CK, LDH and α-HBDH were reduced to varying degrees after the treatment. To our knowledge, the human population has no immunity to SARS-CoV-2, and older people are susceptible to COVID-19 and may develop more severe symptoms than younger people[19], indirectly indicating that immunity is the most effective way to block and fight against COVID-19 infection. According to the results of immunity-associated tests, the levels of IgA, IgG and complement C3 were relatively low for most patients at admission, demonstrating the low immunity of these cases.
This retrospective study still has significant limitations. First, we had a relatively small number of confirmed cases, and not all patients were examined for the above laboratory tests during the three time periods (at admission, upon hospitalization and before discharge) because of condition limitation; hence, the number of cases in some figures was less than 16. Second, the specific Ct values of RT-PCR to identify the genes of SARS-CoV-2 were largely lacking, and no method exists to analyze the correlation of virus titers with the severity of symptoms and data concerning laboratory tests. Third, follow-up studies on most patients at discharge regarding abnormal phenomena such as eosinopenia and aggravated liver function were also lacking. Thus, a stricter experimental design and complete data collection are required in future studies.
Collectively, our study indicated that COVID-19 is a highly contagious disease; hence, stringent public health measures are the most effective strategy to cut off its transmission before universal vaccination is reached or effective antivirals are developed. Leukopenia, eosinopenia, decreased EO%, elevated hs-CRP and FIB levels, and abnormal liver and renal function are valuable biochemical indices for most patients with COVID-19 infection, and aggravated liver dysfunction will be detected for most patients during treatment. Therefore, adequate laboratory tests, CT scanning at admission and dynamic monitoring of blood biochemical indices will be beneficial to identify and predict the response of patients to various treatment modalities. We hope our study on the 16 cases in Hebi will provide useful information to understand the prevention, diagnosis and treatment of COVID-19.
There are no studies on the dynamic changes of biochemical indices of patients with coronavirus disease 2019 (COVID-19) at admission, upon hospitalization and before discharge.
The epidemiological features and dynamic changes in blood biochemical indices for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)-infected patients in Hebi, a representative city with a large floating population in North China, have not been well characterized.
This retrospective study aimed to analyze the data of patients with COVID-19 at admission, upon hospitalization and before discharge in order to clarify the epidemiological features and dynamic changes in blood biochemical indices of this disease.
All available medical information, especially the laboratory data, was collected from 16 cases of laboratory-confirmed COVID-19 infection at admission, upon hospitalization and before discharge.
Fever and fatigue were the common symptoms of COVID-19 infection. Leukopenia, eosinopenia, decreased percentage of eosinophils, elevated high sensitivity C-reactive protein and fibrinogen levels, abnormal liver and renal function were valuable biochemical indices for patients with COVID-19. Aggravated liver dysfunction could be detected in most patients during treatment.
Patients with COVID-19 have certain common symptoms at admission and dynamic changes in blood biochemical indices at admission, upon hospitalization and before discharge. Dynamic monitoring of blood biochemical indices can be beneficial to identify and predict the response of patients to various treatment modalities.
Further investigations on stricter experimental design, larger samples and complete data collection are required to confirm whether the findings of this study could be applied on a broader scale.
| 1. | Schiffrin EL, Flack JM, Ito S, Muntner P, Webb RC. Hypertension and COVID-19. Am J Hypertens. 2020;33:373-374. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 212] [Cited by in RCA: 208] [Article Influence: 34.7] [Reference Citation Analysis (0)] |
| 2. | Haitao T, Vermunt JV, Abeykoon J, Ghamrawi R, Gunaratne M, Jayachandran M, Narang K, Parashuram S, Suvakov S, Garovic VD. COVID-19 and Sex Differences: Mechanisms and Biomarkers. Mayo Clin Proc. 2020;95:2189-2203. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 212] [Cited by in RCA: 204] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 3. | Saha S, Al-Rifai RH, Saha S. Diabetes prevalence and mortality in COVID-19 patients: a systematic review, meta-analysis, and meta-regression. J Diabetes Metab Disord. 2021;1-12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 4. | Chan JF, Kok KH, Zhu Z, Chu H, To KK, Yuan S, Yuen KY. Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerg Microbes Infect. 2020;9:221-236. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1813] [Cited by in RCA: 1974] [Article Influence: 329.0] [Reference Citation Analysis (0)] |
| 5. | Kaur N, Singh R, Dar Z, Bijarnia RK, Dhingra N, Kaur T. Genetic comparison among various coronavirus strains for the identification of potential vaccine targets of SARS-CoV2. Infect Genet Evol. 2021;89:104490. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 68] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 6. | Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497-506. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35178] [Cited by in RCA: 30484] [Article Influence: 5080.7] [Reference Citation Analysis (13)] |
| 7. | Das G, Mukherjee N, Ghosh S. Neurological Insights of COVID-19 Pandemic. ACS Chem Neurosci. 2020;11:1206-1209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 105] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 8. | Alqahtani SA, Schattenberg JM. Liver injury in COVID-19: The current evidence. United European Gastroenterol J. 2020;8:509-519. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 176] [Cited by in RCA: 166] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 9. | Cheng Y, Luo R, Wang K, Zhang M, Wang Z, Dong L, Li J, Yao Y, Ge S, Xu G. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. 2020;97:829-838. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1757] [Cited by in RCA: 1838] [Article Influence: 306.3] [Reference Citation Analysis (0)] |
| 10. | Akhmerov A, Marbán E. COVID-19 and the Heart. Circ Res. 2020;126:1443-1455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 434] [Cited by in RCA: 472] [Article Influence: 78.7] [Reference Citation Analysis (0)] |
| 11. | Israfil SMH, Sarker MMR, Rashid PT, Talukder AA, Kawsar KA, Khan F, Akhter S, Poh CL, Mohamed IN, Ming LC. Clinical Characteristics and Diagnostic Challenges of COVID-19: An Update From the Global Perspective. Front Public Health. 2020;8:567395. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 12. | Liu Y, Yang Y, Zhang C, Huang F, Wang F, Yuan J, Wang Z, Li J, Feng C, Zhang Z, Wang L, Peng L, Chen L, Qin Y, Zhao D, Tan S, Yin L, Xu J, Zhou C, Jiang C, Liu L. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci China Life Sci. 2020;63:364-374. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1522] [Cited by in RCA: 1373] [Article Influence: 228.8] [Reference Citation Analysis (0)] |
| 13. | Hu B, Guo H, Zhou P, Shi ZL. Characteristics of SARS-CoV-2 and COVID-19. Nat Rev Microbiol. 2021;19:141-154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2083] [Cited by in RCA: 3312] [Article Influence: 662.4] [Reference Citation Analysis (1)] |
| 14. | Lin L, Li TS. [Interpretation of "Guidelines for the Diagnosis and Treatment of Novel Coronavirus (2019-nCoV) Infection by the National Health Commission (Trial Version 5)"]. Zhonghua Yi Xue Za Zhi. 2020;100:805-807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 24] [Reference Citation Analysis (0)] |
| 15. | Backer JA, Klinkenberg D, Wallinga J. Incubation period of 2019 novel coronavirus (2019-nCoV) infections among travellers from Wuhan, China, 20-28 January 2020. Euro Surveill. 2020;25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1082] [Cited by in RCA: 920] [Article Influence: 153.3] [Reference Citation Analysis (0)] |
| 16. | Bi Q, Wu Y, Mei S, Ye C, Zou X, Zhang Z, Liu X, Wei L, Truelove SA, Zhang T, Gao W, Cheng C, Tang X, Wu X, Sun B, Huang S, Sun Y, Zhang J, Ma T, Lessler J, Feng T. Epidemiology and transmission of COVID-19 in 391 cases and 1286 of their close contacts in Shenzhen, China: a retrospective cohort study. Lancet Infect Dis. 2020;20:911-919. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1284] [Cited by in RCA: 1251] [Article Influence: 208.5] [Reference Citation Analysis (0)] |
| 17. | Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, Du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY, Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Zhong NS; China Medical Treatment Expert Group for Covid-19. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382:1708-1720. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19202] [Cited by in RCA: 19025] [Article Influence: 3170.8] [Reference Citation Analysis (9)] |
| 18. | Zhang G, Zhang J, Wang B, Zhu X, Wang Q, Qiu S. Analysis of clinical characteristics and laboratory findings of 95 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a retrospective analysis. Respir Res. 2020;21:74. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 209] [Cited by in RCA: 194] [Article Influence: 32.3] [Reference Citation Analysis (1)] |
| 19. | Applegate WB, Ouslander JG. COVID-19 Presents High Risk to Older Persons. J Am Geriatr Soc. 2020;68:681. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 106] [Cited by in RCA: 117] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 20. | Khaled SA, Hafez AA. Aplastic anemia and COVID-19: how to break the vicious circuit? Am J Blood Res. 2020;10:60-67. [PubMed] |
| 21. | Zhang JJ, Dong X, Cao YY, Yuan YD, Yang YB, Yan YQ, Akdis CA, Gao YD. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy. 2020;75:1730-1741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2139] [Cited by in RCA: 2350] [Article Influence: 391.7] [Reference Citation Analysis (0)] |
| 22. | Cavezzi A, Troiani E, Corrao S. COVID-19: hemoglobin, iron, and hypoxia beyond inflammation. A narrative review. Clin Pract. 2020;10:1271. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 283] [Cited by in RCA: 285] [Article Influence: 47.5] [Reference Citation Analysis (0)] |
| 23. | Hariyanto TI, Kurniawan A. Anemia is associated with severe coronavirus disease 2019 (COVID-19) infection. Transfus Apher Sci. 2020;59:102926. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 66] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 24. | Chen L, Liu HG, Liu W, Liu J, Liu K, Shang J, Deng Y, Wei S. [Analysis of clinical features of 29 patients with 2019 novel coronavirus pneumonia]. Zhonghua Jie He He Hu Xi Za Zhi. 2020;43:203-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 108] [Reference Citation Analysis (0)] |
| 25. | Powell LJ. C-reactive protein--a review. Am J Med Technol. 1979;45:138-142. [PubMed] |
| 26. | Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844-847. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3992] [Cited by in RCA: 4077] [Article Influence: 679.5] [Reference Citation Analysis (0)] |
| 27. | Hariyanto TI, Japar KV, Kwenandar F, Damay V, Siregar JI, Lugito NPH, Tjiang MM, Kurniawan A. Inflammatory and hematologic markers as predictors of severe outcomes in COVID-19 infection: A systematic review and meta-analysis. Am J Emerg Med. 2021;41:110-119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 154] [Cited by in RCA: 146] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 28. | Feng G, Zheng KI, Yan QQ, Rios RS, Targher G, Byrne CD, Poucke SV, Liu WY, Zheng MH. COVID-19 and Liver Dysfunction: Current Insights and Emergent Therapeutic Strategies. J Clin Transl Hepatol. 2020;8:18-24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 301] [Cited by in RCA: 280] [Article Influence: 46.7] [Reference Citation Analysis (0)] |
| 29. | Zippi M, Fiorino S, Occhigrossi G, Hong W. Hypertransaminasemia in the course of infection with SARS-CoV-2: Incidence and pathogenetic hypothesis. World J Clin Cases. 2020;8:1385-1390. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 23] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 30. | Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, Liu S, Zhao P, Liu H, Zhu L, Tai Y, Bai C, Gao T, Song J, Xia P, Dong J, Zhao J, Wang FS. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420-422. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5228] [Cited by in RCA: 5832] [Article Influence: 972.0] [Reference Citation Analysis (3)] |
| 31. | Hassanein M, Radhakrishnan Y, Sedor J, Vachharajani T, Vachharajani VT, Augustine J, Demirjian S, Thomas G. COVID-19 and the kidney. Cleve Clin J Med. 2020;87:619-631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 44] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 32. | Fan C, Lu W, Li K, Ding Y, Wang J. ACE2 Expression in Kidney and Testis May Cause Kidney and Testis Infection in COVID-19 Patients. Front Med (Lausanne). 2020;7:563893. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 139] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 33. | Madjid M, Safavi-Naeini P, Solomon SD, Vardeny O. Potential Effects of Coronaviruses on the Cardiovascular System: A Review. JAMA Cardiol. 2020;5:831-840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1095] [Cited by in RCA: 1267] [Article Influence: 211.2] [Reference Citation Analysis (2)] |
| 34. | Kwenandar F, Japar KV, Damay V, Hariyanto TI, Tanaka M, Lugito NPH, Kurniawan A. Coronavirus disease 2019 and cardiovascular system: A narrative review. Int J Cardiol Heart Vasc. 2020;29:100557. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 80] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kurniawan A S-Editor: Wang JJ L-Editor: A P-Editor: Li X