Published online Mar 6, 2022. doi: 10.12998/wjcc.v10.i7.2330
Peer-review started: October 5, 2021
First decision: November 11, 2021
Revised: November 21, 2021
Accepted: January 19, 2022
Article in press: January 19, 2022
Published online: March 6, 2022
Processing time: 147 Days and 19.1 Hours
Alström syndrome (AS) is a rare autosomal recessive disease that is generally induced by mutations of the Alström syndrome 1 (ALMS1) gene. We report a case of AS, extend the spectrum of ALMS1 mutations and highlight the biological role of ALMS1 to explore the relationship between dilated cardiomyopathy (DCM) and mutations in ALMS1.
We present the case of an infant with AS mainly manifesting with DCM that was caused by a novel mutation of the ALMS1 gene. Whole-exome sequencing revealed a simultaneous large deletion and point mutation in ALMS1, leading to frameshift and missense mutations, respectively, rather than nonsense or frameshift mutations, which have been reported previously. Upon optimized anti-remodeling therapy, biohumoral exams and arrhythmic burden of the infant were alleviated at follow-up after 6 mo.
We identified novel mutations of ALMS1 and extended the spectrum of ALMS1 mutations in an infant with AS.
Core Tip: We present the case of an infant with dilated cardiomyopathy (DCM) who was diagnosed with Alström syndrome at the early stage of the disease. Whole-exome sequencing revealed that a large deletion and point mutation simultaneously occurred in the Alström syndrome 1 (ALMS1) gene, leading to frameshift and missense mutations, respectively, rather than nonsense or frameshift mutations, which have been reported previously. Likewise, to date, few interpretations have been made of the related mechanism of the novel ALMS1 gene mutation to induce DCM in infants.
- Citation: Jiang P, Xiao L, Guo Y, Hu R, Zhang BY, He Y. Novel mutations of the Alström syndrome 1 gene in an infant with dilated cardiomyopathy: A case report. World J Clin Cases 2022; 10(7): 2330-2335
- URL: https://www.wjgnet.com/2307-8960/full/v10/i7/2330.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i7.2330
Alström syndrome (AS; MIM# 203800) is an unusual autosomal recessive genetic disorder that involves multiple systems and progressive dysfunction and is characterized by visual disturbance, hearing impairment, cardiomyopathy, hypertriglyceridemia, accelerated nonalcoholic fatty liver disease, and recurrent respiratory disease[1]. It is caused by mutations of the Alström syndrome 1 (ALMS1) gene, which is located on chromosome 2p13. The ALMS1 gene contains 23 exons and encodes a 461.2-kDa protein of 4169 amino acids[2]. To date, over 268 variants in ALMS1 have been identified[2]. The ALMS1 protein localizes to centrosomes and the base of cilia[3]; however, the function of the protein is not clear, and the explicit molecular pathological mechanisms of dilated cardiomyopathy (DCM) have not been fully demonstrated. Here, we present the case of a 1-month-old girl who was initially diagnosed with DCM induced by a novel mutation of the ALMS1 gene and describe the likely pathogenesis of DCM as a result of variants in ALMS1.
A 1-month-old girl was brought to the hospital because of cyanosis and dyspnea.
She had a persistent cough with recurrent choking for 4 d, and the symptoms deteriorated in the last 12 h, manifesting with cyanosis and dyspnea.
She had a history of recurrent respiratory infections and had nystagmus at birth.
Her parents denied a family history of cardiomyopathy and genetic disease.
Her body weight was 4.5 kg, and her body length was 50 cm. Her heart border was enlarged to the left midaxillary line, and she had a few rales in both lower lungs.
Clinical laboratory tests indicated a plasma triglyceride level of 3.17 mmol/L (normal < 1.7 mmol/L), high-density lipoprotein (HDL) cholesterol level of 0.99 mmol/L (normal 1.15–2.25 mmol/L), serum cardiac troponin T (cTnT) level of 0.05 µg/L (normal< 0.024 µg/L) and N-terminal pro-brain natriuretic peptide level of 23 681 pg/mL (normal < 125 pg/mL).
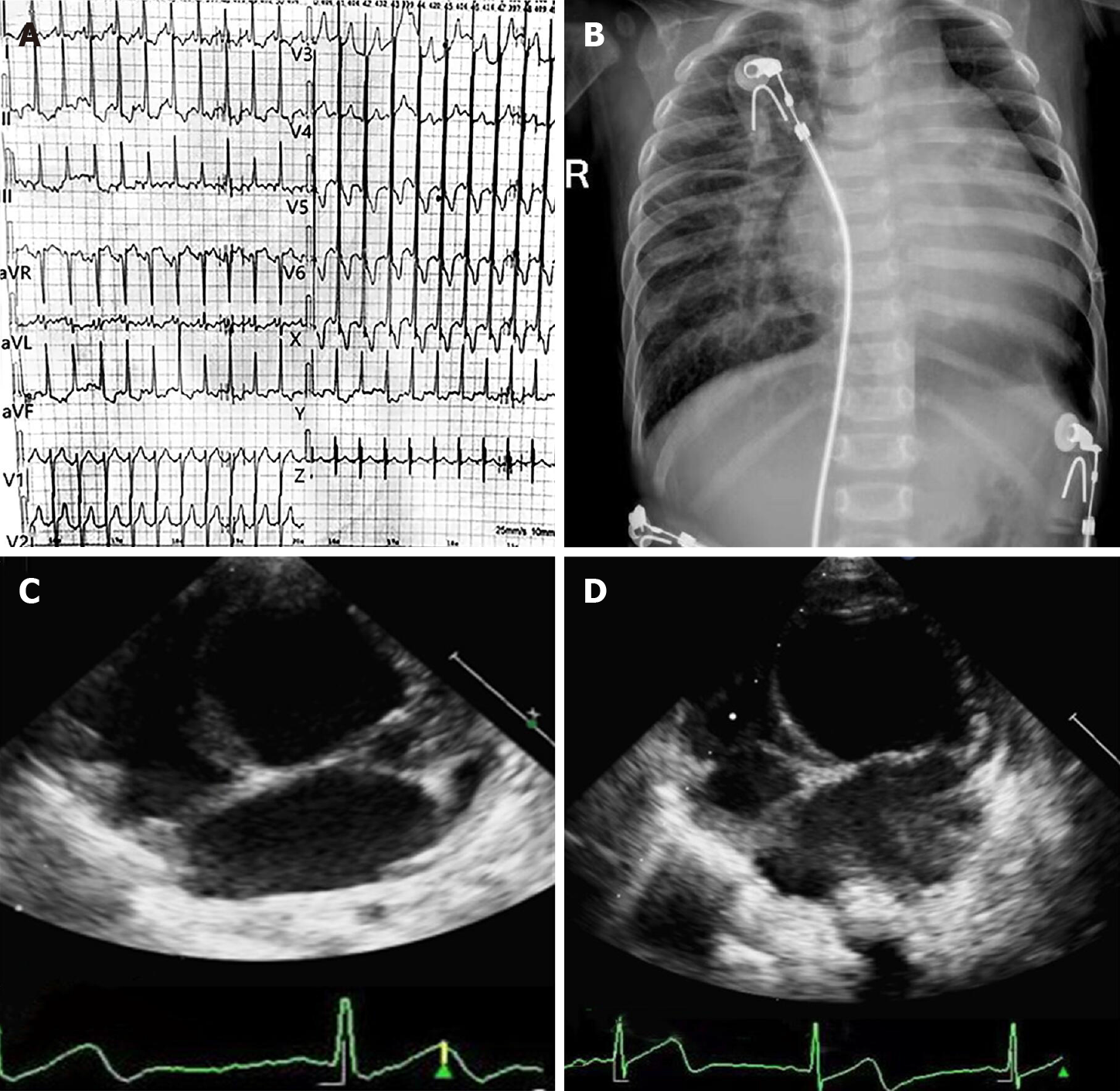
Twelve-lead ECG documented high voltages in the left precordial leads and diffuse T wave inversion (Figure 1A). There were two episodes of paroxysmal atrial tachycardia in 24-h Holter ECG monitoring, and the maximum heart rate was 180 beats/min, whereas ventricular arrhythmia was not recorded. Chest radiography demonstrated cardiac enlargement and pulmonary congestion (Figure 1B). Transthoracic echocardiography (TTE) indicated severe left ventricular dilatation and heart failure with reduced ejection fraction (Figure 1C).
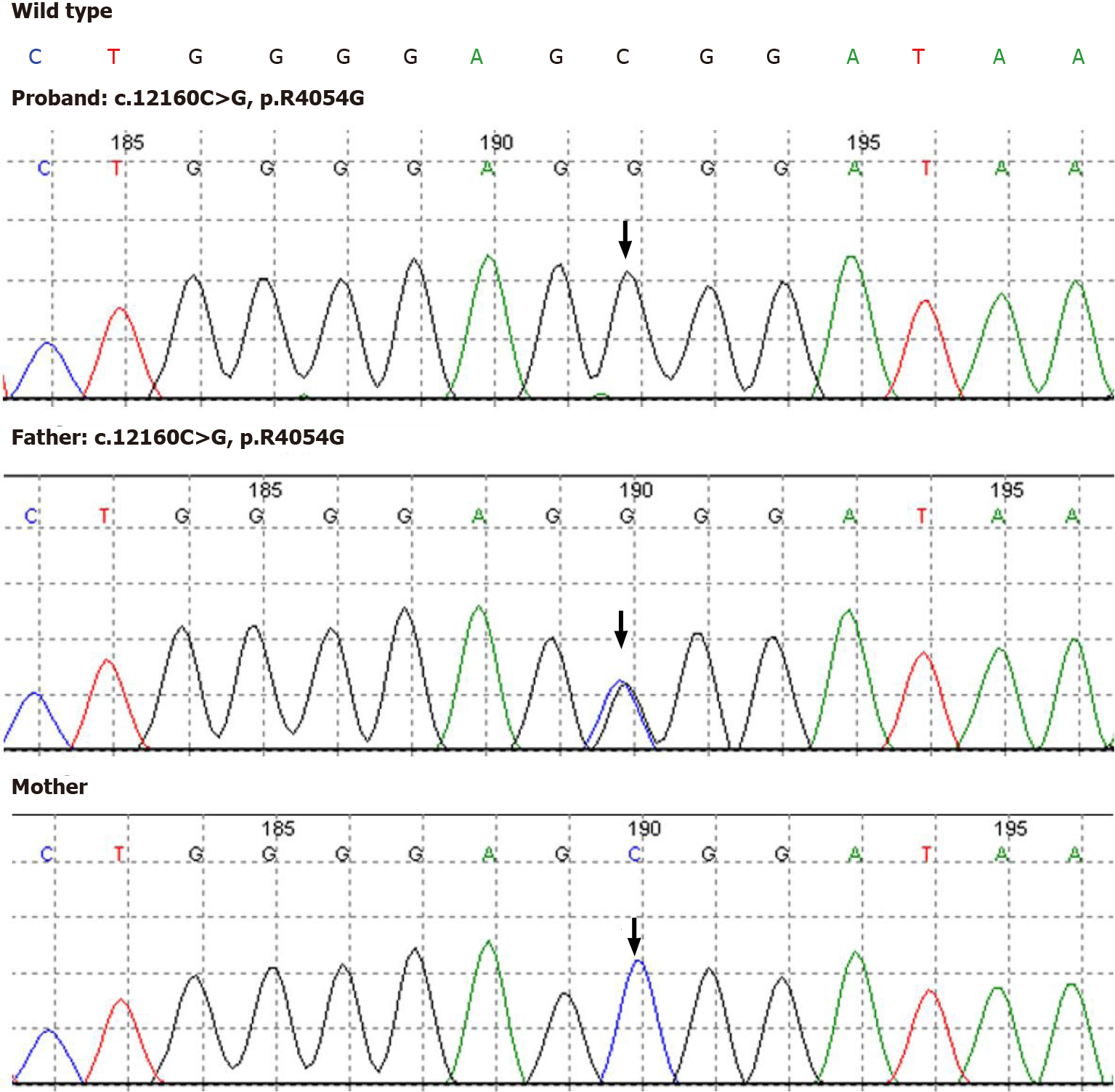
Nuclear genomic DNA was extracted from peripheral blood samples of the infant and her parents for amplification with targeted capture of the coding regions of the genome. Then, amplicons were subjected to whole-exome sequencing by a NextSeq500 sequencer (Illumina, San Diego, CA, United States). Novel genetic mutations in ALMS1 were identified, and genetic analysis showed that the ALMS1 gene (NM_015120) had two mutations on chr2: 73829360 (c.12160C>G, p.R4054G) in exon 20 and chr2: 73827805-73830431 deletion in exons 18-21 (Figure 2). The mutations were confirmed by the Sanger sequencing method, which revealed that c.12160C>G (p.R4054G) and a deletion removing the entire exons 18-21 were acquired by paternal and maternal inheritance, respectively.
According to diagnostic criteria for AS[4], the infant met two major criteria and one minor criterion. The mutation sites associated with clinical features were in favor of the diagnosis of AS.
Both sacubitril/valsartan and dapagliflozin are strongly recommended for adult patients with heart failure with reduced ejection fraction, according to the 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure[5], but not in infants, because the safety and efficacy of both have not been confirmed in infants, and further study is needed for evaluation[6,7]. Therefore, drug therapies such as angiotensin-converting enzyme inhibitors, beta blockers, spironolactone, digoxin and diuretics were administered according to consensus clinical management guidelines for AS[1].
In follow-up at 6 mo, clinical laboratory tests indicated that the N-terminal pro-brain natriuretic peptide level decreased to 1879 pg/mL, the cTnT concentration declined to normal, and there was no arrhythmic burden in repeated 24-h Holter ECG monitoring. Further, TTE revealed that cardiac function of the infant had not deteriorated with the current medication (Figure 1D).
AS is an extremely rare autosomal recessive disease induced by a mutation of the ALMS1 gene, with an estimated incidence of 1 case per 1000000 live births[1]. In the present case, the patient had mutations in ALMS1 and visual symptoms, DCM, repeated respiratory infection, and hypertriglyceridemia with low HDL levels, which conformed to the diagnostic standard for AS[4]. Mutations in ALMS1 are associated with AS in the individual, and both DCM and visual symptoms are cardinal manifestations of AS[1]. Consequently, the classic phenotype in infants with AS is closely related to the genotype. Mutations in exons 18-21 of ALMS1 were not identified in the mutational hotspots located in exons 8, 10 and 16. Variants in non-hotspot exons could result in classical phenotype deficiency or atypical phenotypes, such as the delayed age of obesity and diabetes onset. In contrast, most of the variants in ALMS1 in previous reports were nonsense and frameshift mutations[8], but a large deletion and point mutation simultaneously occurring in the infant caused frameshift and missense mutations, respectively, both of which are reported for the first time. Casey and colleagues[9] also identified two infant siblings with DCM who were finally diagnosed with AS as a result of mutant alleles in exons 20 and 5 rather than in the mutational hotspots. Thus, an increasing number of diseases are caused by variants in the ALMS1 gene outside the recognized mutational hotspots.
To date, little is known about the mechanism by which ALMS1 gene mutation can lead to DCM in infants. In our case, the mutations that affected ALMS1 protein expression were missense and frameshift mutations in exons 20 and 18-21, respectively, which can lead to abnormal structure of the ALMS1 protein and subsequent loss of function. A previous study showed that the ALMS1 protein plays an important role in postnatal cardiomyocyte mitosis by affecting centrosomes and regulating cell cycle arrest, and ALMS1 protein deficiency can impair the terminal differentiation of cardiomyocytes[10], leading to cardiac dysfunction or progressive functional deterioration. Additionally, deficiency of the ALMS1 protein can activate β-catenin-dependent WNT signaling[10], which has been demonstrated to contribute to the inflammatory response and fibrosis in tissues and cells in animal experiments[11]. The local cardiac inflammatory response and cardiac fibrosis may be important mechanisms in the process of DCM.
We identified novel mutations of the ALMS1 gene and extended the spectrum of known ALMS1 mutations. It is essential to perform ALMS1 gene sequencing in infants with DCM.
The authors are grateful to the parents of the infant for their agreement to the publication of this report and accompanying images.
| 1. | Tahani N, Maffei P, Dollfus H, Paisey R, Valverde D, Milan G, Han JC, Favaretto F, Madathil SC, Dawson C, Armstrong MJ, Warfield AT, Düzenli S, Francomano CA, Gunay-Aygun M, Dassie F, Marion V, Valenti M, Leeson-Beevers K, Chivers A, Steeds R, Barrett T, Geberhiwot T. Consensus clinical management guidelines for Alström syndrome. Orphanet J Rare Dis. 2020;15:253. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 70] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 2. | Dassie F, Favaretto F, Bettini S, Parolin M, Valenti M, Reschke F, Danne T, Vettor R, Milan G, Maffei P. Alström syndrome: an ultra-rare monogenic disorder as a model for insulin resistance, type 2 diabetes mellitus and obesity. Endocrine. 2021;71:618-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 3. | Sylla BS, Huberdeau D, Bourgaux-Ramoisy D, Bourgaux P. Site-specific excision of integrated polyoma DNA. Cell. 1984;37:661-667. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 80] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 4. | Marshall JD, Beck S, Maffei P, Naggert JK. Alström syndrome. Eur J Hum Genet. 2007;15:1193-1202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 187] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 5. | McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, Burri H, Butler J, Čelutkienė J, Chioncel O, Cleland JGF, Coats AJS, Crespo-Leiro MG, Farmakis D, Gilard M, Heymans S, Hoes AW, Jaarsma T, Jankowska EA, Lainscak M, Lam CSP, Lyon AR, McMurray JJV, Mebazaa A, Mindham R, Muneretto C, Francesco Piepoli M, Price S, Rosano GMC, Ruschitzka F, Kathrine Skibelund A; ESC Scientific Document Group. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42:3599-3726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8225] [Cited by in RCA: 8584] [Article Influence: 1716.8] [Reference Citation Analysis (0)] |
| 6. | Das BB, Scholl F, Vandale B, Chrisant M. Sacubitril/Valsartan: potential treatment for paediatric heart failure. Cardiol Young. 2018;28:1077-1081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 7. | Tirucherai GS, LaCreta F, Ismat FA, Tang W, Boulton DW. Pharmacokinetics and pharmacodynamics of dapagliflozin in children and adolescents with type 2 diabetes mellitus. Diabetes Obes Metab. 2016;18:678-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 8. | Ozantürk A, Marshall JD, Collin GB, Düzenli S, Marshall RP, Candan Ş, Tos T, Esen İ, Taşkesen M, Çayır A, Öztürk Ş, Üstün İ, Ataman E, Karaca E, Özdemir TR, Erol İ, Eroğlu FK, Torun D, Parıltay E, Yılmaz-Güleç E, Atabek ME, Elçioğlu N, Satman İ, Möller C, Muller J, Naggert JK, Özgül RK. The phenotypic and molecular genetic spectrum of Alström syndrome in 44 Turkish kindreds and a literature review of Alström syndrome in Turkey. J Hum Genet. 2015;60:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 9. | Casey J, McGettigan P, Brosnahan D, Curtis E, Treacy E, Ennis S, Lynch SA. Atypical Alstrom syndrome with novel ALMS1 mutations precluded by current diagnostic criteria. Eur J Med Genet. 2014;57:55-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 10. | Shenje LT, Andersen P, Halushka MK, Lui C, Fernandez L, Collin GB, Amat-Alarcon N, Meschino W, Cutz E, Chang K, Yonescu R, Batista DA, Chen Y, Chelko S, Crosson JE, Scheel J, Vricella L, Craig BD, Marosy BA, Mohr DW, Hetrick KN, Romm JM, Scott AF, Valle D, Naggert JK, Kwon C, Doheny KF, Judge DP. Mutations in Alström protein impair terminal differentiation of cardiomyocytes. Nat Commun. 2014;5:3416. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 67] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 11. | Burgy O, Königshoff M. The WNT signaling pathways in wound healing and fibrosis. Matrix Biol. 2018;68-69:67-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 145] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; externally peer reviewed.
Peer-review model: Single blind
Specialty type: Genetics and heredity
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Barison A, Bronson SC S-Editor: Liu JH L-Editor: A P-Editor: Liu JH