Published online Mar 6, 2022. doi: 10.12998/wjcc.v10.i7.2206
Peer-review started: October 10, 2021
First decision: November 11, 2021
Revised: November 21, 2021
Accepted: January 17, 2022
Article in press: January 17, 2022
Published online: March 6, 2022
Processing time: 142 Days and 16 Hours
Aortoesophageal fistula (AEF) induced by esophageal fishbones is a rare complication of esophageal foreign bodies and is very difficult to treat. Although the current view suggests that endovascular stent-graft treatment is useful for AEF, whether a subsequent thoracic operation is necessary remains controversial. The purpose of this report is to describe our experience using endovascular stent-graft treatment without combined thoracic operations for the treatment of AEF in two specific cases.
We presented two cases of patients complaining of retrosternal discomfort treated in our department for an aortoesophageal fistula caused by the accidental ingestion of a fishbone. The two patients were effectively managed with combined means of endoscopic, medical (broad-spectrum antibiotic therapy, fasting, gastrointestinal decompression, etc.) and endovascular stent-graft treatment. The main difference in treatment was that the first patient presented with hematemesis after endoscopic removal of the fishbone. Subsequently, the patient underwent endovascular stent-graft treatment. The second case was managed with endoscopic removal of the fishbone with simultaneous endovascular stent-graft treatment, without any signs of hematemesis or melena. Both patients had successful postoperative management and were discharged home. Long-term follow-up is ongoing.
The treatment decision-making process should depend on the patients’ specific situations. Our practice indicates that endovascular stent-graft treatment without combined thoracic operations could be a valuable alternative in selected patients.
Core Tip: Esophageal foreign body is the most common cause of aortoesophageal fistula. Aortoesophageal fistula is easily confused with other upper gastrointestinal bleeding, and it is very dangerous and has serious consequences. In this work, we presented two cases of perforation of esophagus due to foreign body and analyzed the 8 similar cases recorded in PubMed, MEDLINE from January 2000 to October 2021. This pooled analysis of involving endovascular stent-graft treatment of aortoesophageal fistula or other aortic injury secondary to foreign body, in view of clinical course, imaging, treatment and prognosis may provide a better understanding of aortoesophageal fistula.
- Citation: Gong H, Wei W, Huang Z, Hu Y, Liu XL, Hu Z. Endovascular stent-graft treatment for aortoesophageal fistula induced by an esophageal fishbone: Two cases report. World J Clin Cases 2022; 10(7): 2206-2215
- URL: https://www.wjgnet.com/2307-8960/full/v10/i7/2206.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i7.2206
Esophageal foreign bodies are one of the most common reasons for emergency treatment, and fishbones are one of the most common esophageal foreign bodies. Regions of esophageal stenosis are the most common sites of esophageal obstruction and perforation[1]. The main causes of aortoesophageal fistula (AEF) are aneurysms, ingestion of foreign bodies, and advanced esophageal cancer. AEF is a rare disease with high mortality, with an annual incidence of 0.007 per million[2]. AEF is easily confused with other causes of upper gastrointestinal bleeding, is very dangerous and has serious consequences[3]. Currently, considerable controversy remains regarding the appropriate management strategies for this disease. Here, we present our successful experience in managing two consecutive patients with AEF using endovascular stent-graft treatment with multidisciplinary integrated management without combined thoracic operations.
Case 1: A 71-year-old Chinese woman presented to our department complaining of ingestion of a fish bone foreign body, with worsening retrosternal chest pain for 4 d.
Case 2: A 48-year-old man presented to the emergency room with a history of intense retrosternal discomfort lasting for 2 d after swallowing a fish bone.
Case 1: There were no complaints of fever, haematemesis, or any other symptoms.
Case 2: There were no other symptoms.
No special circumstances.
No special circumstances.
No obvious abnormal physical examinations.
Case 1: No obvious abnormal laboratory markers were observed.
Case 2: A routine blood analysis showed a white blood cell (WBC) counting of 11.34 × 109/L.
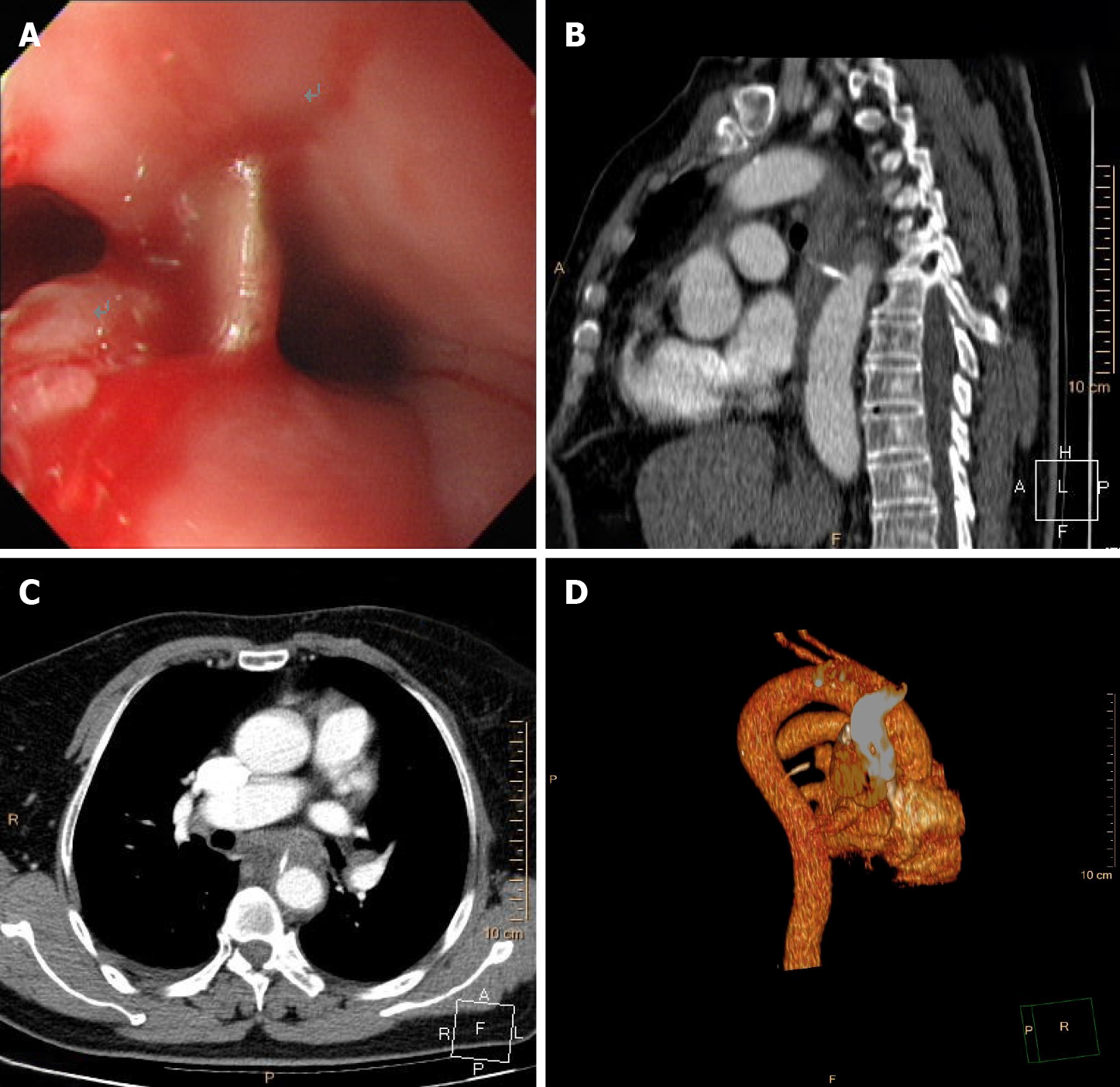
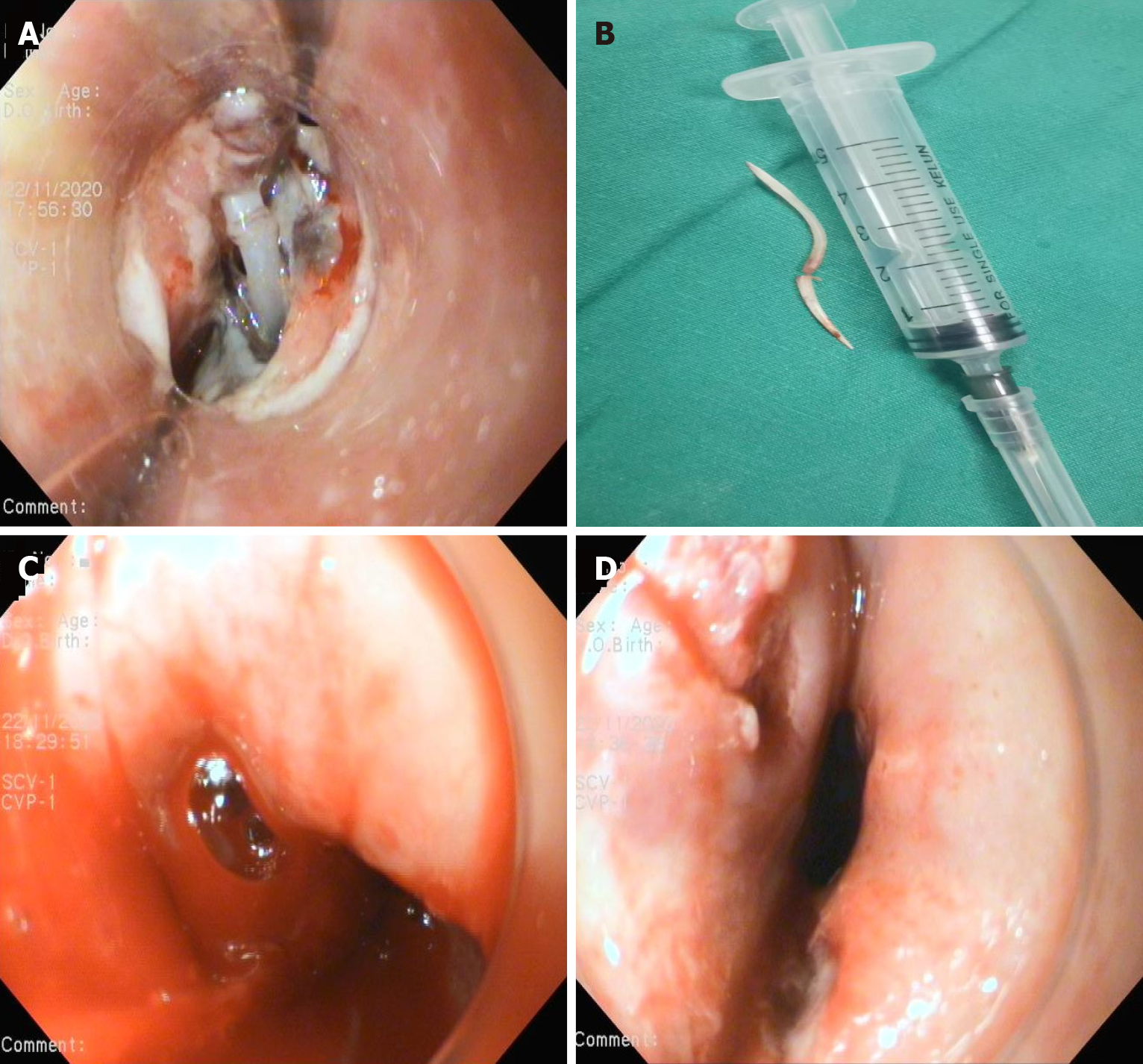
Case 1: Esophagogastroduodenoscopy (EGD) (Figure 1A) and chest computed tomography angiography (CTA) (Figure 1B-D) showed that a fish bone (length 2.0 cm) penetrated the wall of the esophagus and entered the thoracic aorta.
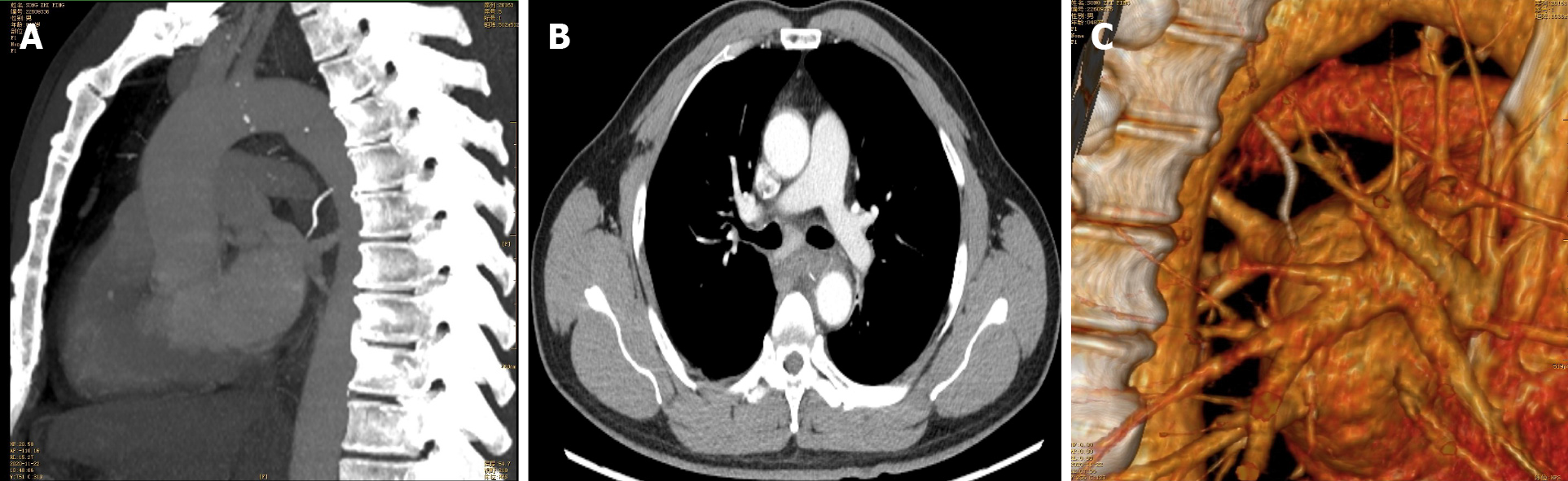
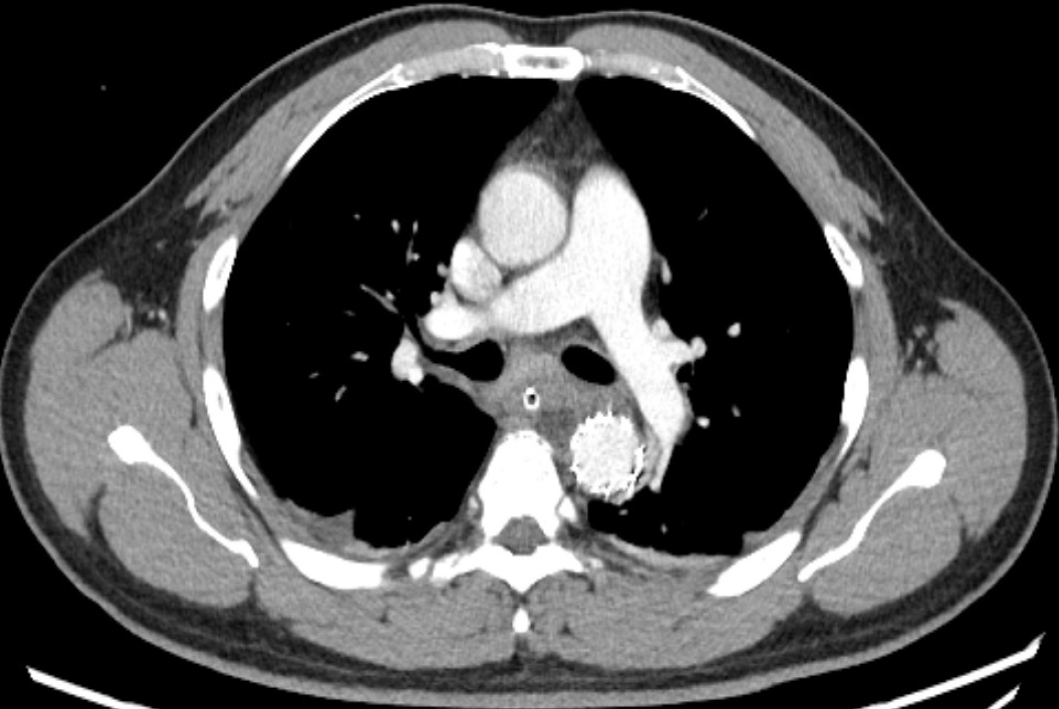
Case 2: Chest CTA (Figure 2) confirmed that the fishbone puncturing through the esophageal wall was tightly appressed to the descending aortic wall, with mediastinal hematoma.
Perforation of the esophagus due to a foreign body with AEF.
Perforation of the esophagus due to a foreign body without exclusion of perforating wounds to the descending aorta.
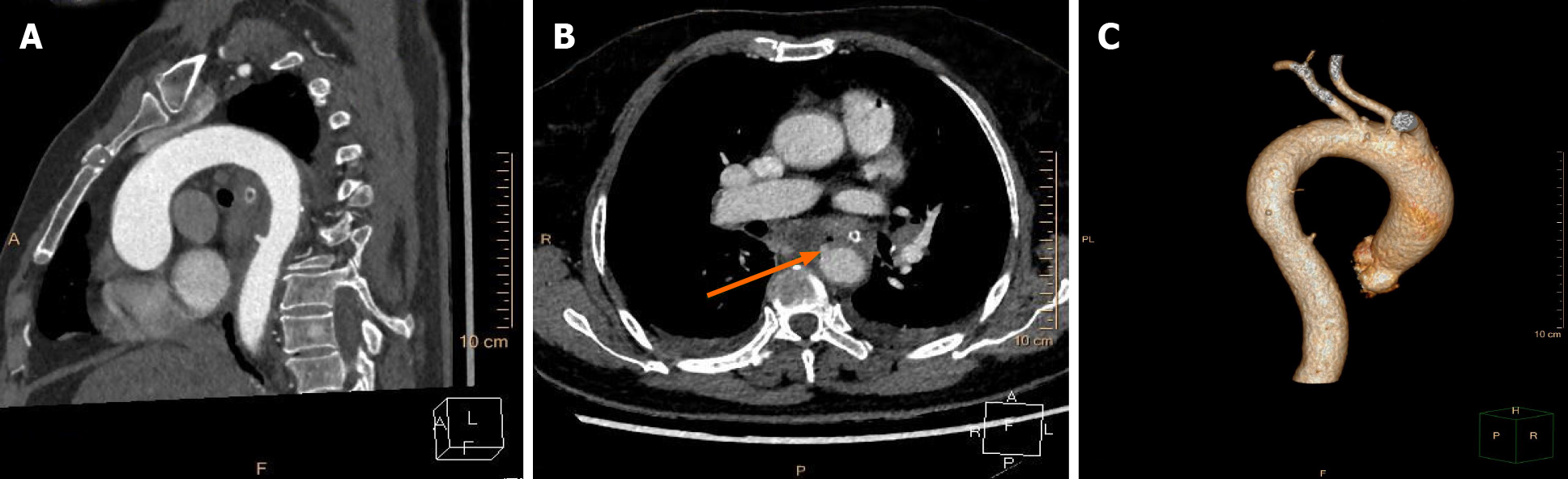
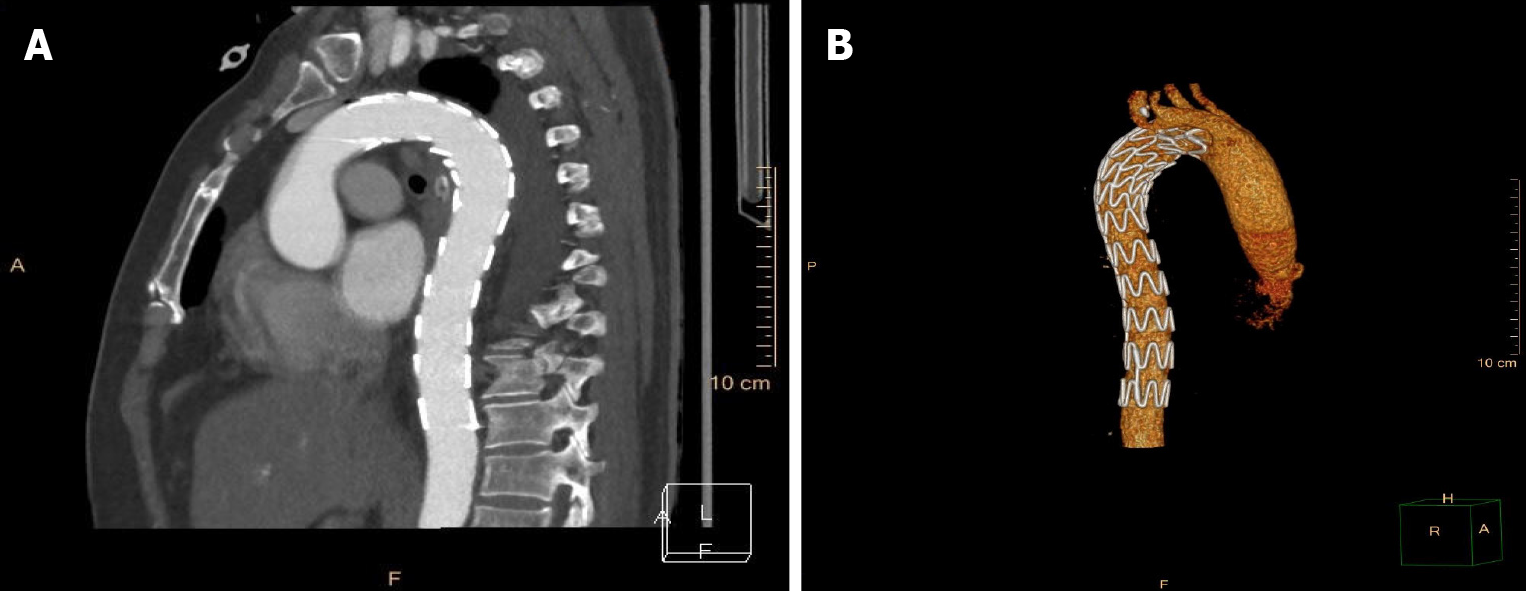
According to the wishes of the patient and her family, the patient was transferred to an affiliated hospital of a provincial medical college for further treatment. The foreign body was removed from the mouth using biopsy forceps by a local endoscopist on the first day after transfer to the hospital. However, the patient coughed up blood several times the next morning. The management of the patient is unclear due to a lack of medical records. She requested discharge from the affiliated hospital after fluid resuscitation, with relatively steady vital signs. Eventually, the patient was referred to the intensive care unit (ICU) of our hospital for evaluation and remediation. A reexamination with chest CTA (Figure 3) demonstrated thoracic aorta extravasation of contrast medium and swollen and thickened walls of the middle esophagus. We collaborated closely with a multidisciplinary team comprising gastroenterology, cardiovascular medicine, vascular surgery, and cardiothoracic surgery specialists. The patient underwent closure of the perforated aortic cavity via a minimally invasive interventional technique performed by vascular surgeons, with cardiothoracic teams on standby in case conversion to open surgery was necessary. An endovascular stent graft (30 mm × 200 mm, Ankura™, Shenzhen, China) was placed inside the descending aorta, and intraoperative high-pressure angiography confirmed successful results, with no extravasation of contrast medium. The patient underwent continuous broad-spectrum antibiotic therapy (intravenous vancomycin for 1 wk, piperacillin-tazobactam and metronidazole for 4 wk), nasogastric tube decompression and total parenteral nutrition for 3 wk after the intervention, during which she was constantly observed for indications of secondary or delayed bleeding or fever. The final chest CTA (Figure 4) demonstrated that the thoracic aortic stent graft was in the thoracic aorta and unobstructed without contrast agent extravasation. In addition, the swollen and thickened wall of the middle esophagus was significantly improved.
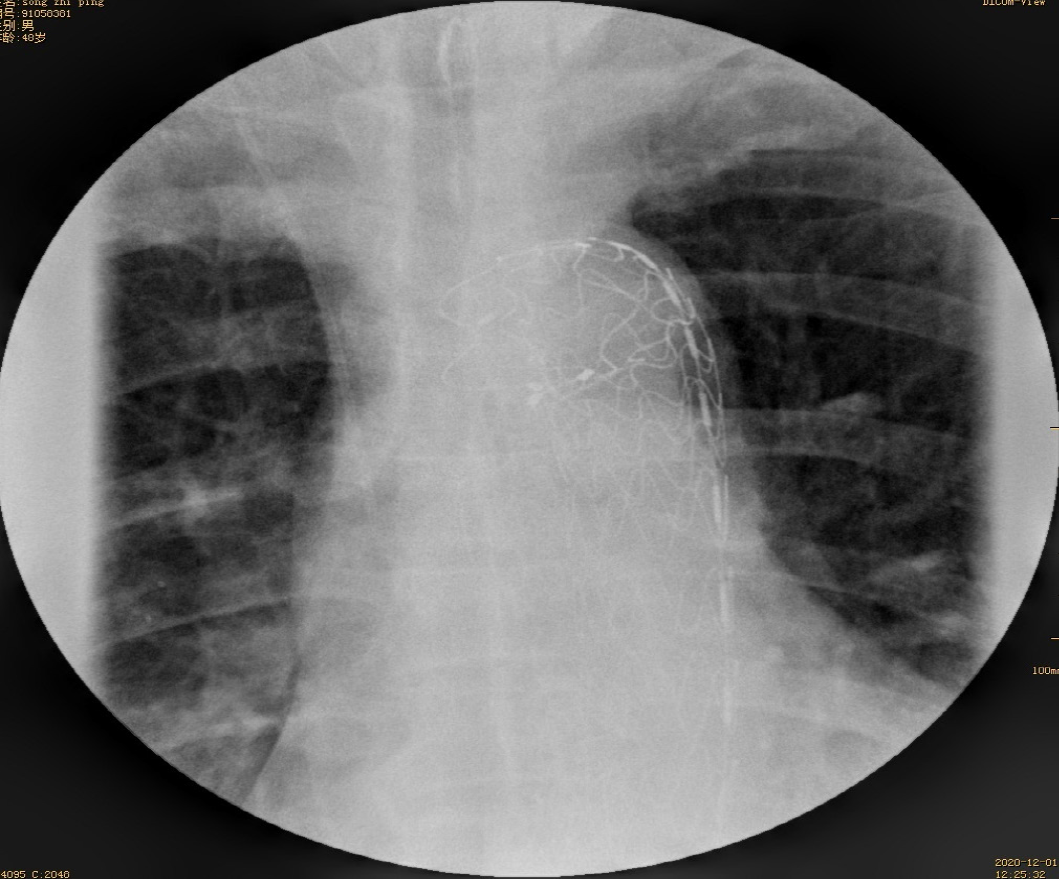
After a multidisciplinary consultation, including gastroenterology, cardiovascular medicine, vascular surgery, and cardiothoracic surgery specialists, an aorta impaled by a foreign body was suspected. The angiographic catheter was first guided into the thoracic aorta, and arteriography revealed a 1 cm vascular niche in the descending aorta. Then, an endovascular stent graft that had not yet been released was delivered to the selected location by a vascular surgeon. EGD showed that both ends of the fish bones inserted into the esophageal wall 28 cm from the incisors (Figure 5A); The fish bone was gently removed endoscopically (Figure 5B), with subsequent active blood spurting noted in the esophageal defect (Figure 5C). At the critical moment, the aortal stent graft (28 mm × 200 mm, Ankura™, Shenzhen, China) was immediately released by a vascular surgeon, and angiography showed that the aortal defect was closed well. After endoscopic irrigation with normal saline, two longitudinal ulcers were observed (Figure 5D). A nasogastric tube was inserted to drain the digestive juice. After intervention, the patient fasted and received broad-spectrum antibiotic therapy (intravenous vancomycin for 1 wk, cefoperazone-sulbactam and metronidazole for 3 wk). After spending one day in the ICU because of a fever, the patient was transferred back to our department. No active bleeding was observed. He had no fever and no hematemesis or melena. CTA showed a well-positioned aortic stent graft and no contrast extravasation from the aorta (Figure 6). On the 7th day after the operation, esophageal imaging with meglumine diatrizoate demonstrated no leakage (Figure 7), and the gastrointestinal decompression tube was removed.
The patient started oral intake on the 21st postoperative day and was discharged on day 28; later, her treatment was changed to oral antibiotics for 4 wk. The patient’s symptoms improved without fever and hematemesis. The patient was alive at the 19-mo follow-up.
The patient started liquid intake on the 7th postoperative day and was discharged on day 21, with a home therapy antibiotic course for 4 wk. The patient was symptom-free and alive at the 5-mo follow-up.
The treatments of AEF mainly include the following: (1) Enteral feeding with broad-spectrum antibiotics and proton pump inhibitors, as well as percutaneous endoscopic gastrostomy or esophageal fistula bypass, but these approaches are often ineffective due to recurrent bleeding and severe infection[4]; (2) Endovascular stent-graft treatment is a rapid, minimally invasive and effective alternative to emergent surgical treatment of AEF and can quickly identify the bleeding site and achieve hemostasis through stent implantation[5]; and (3) Surgical repair is the typical treatment approach and was considered the only definitive treatment for foreign body-related AEF before the emergence of endovascular treatment, with in-hospital mortality and reintervention rate of nearly 40% and 50%, respectively[6,7]. A left thoracotomy followed by aortic replacement with a prosthetic/cryopreserved homograft is the typical approach for open AEF repair[7]. The popularization of endovascular therapy has provided more treatment options for AEF. A study[8] have attempted to close aortic ruptures during the acute phase using endovascular therapy, especially for patients with significant upper gastrointestinal bleeding in poor general condition who are not suitable for open surgery. In a systematic review, endovascular treatment for AEF offered encouraging perioperative outcomes, with an acceptable 30-d mortality rate (19.4%)[9]. However, when fistulas are present in the aorta and esophagus and are markedly contaminated, the use of endovascular stent grafts to close the contaminated fistula can lead to long-term artificial stent infection and a remarkable increase in the area of the aorta that needs to be resected. Currently, there are two main areas of controversy. First, it is questionable whether thoracic operations can be completely replaced by endovascular therapy. Second, if endovascular therapy cannot be fully substituted for thoracic surgery, it is unclear when further thoracic surgery should be performed.
In this paper, we compiled the reports of esophageal foreign bodies complicated with AEF or other aortic injuries in recent years, all of which involve endovascular stent treatment[10-17] (Table 1). These experiences indicate that endovascular stent treatment seems to be gaining popularity as a preferred method for AEF, and the severity of infection throughout the course of the disease is directly related to the prognosis of the patient. With regard to the management of esophageal fistula, we consider that the healing of the esophageal fistula could be assessed through esophageal imaging with meglumine diatrizoate and EGD a few days after removal of the foreign body and that the esophagus could be repaired by a viable pedicle flap of the omentum or a pedicled intercostal muscle flap or even through reconstitution of the gastrointestinal tract if a fistula exists[11,12,15]. More notably, Mezzetto et al[14] reported that the presence of a low-flow esophageal fistula could be endoscopically closed by long clips after removal of the foreign body. With regard to surgical management, thoracic surgery may not be necessary if the mediastinal infection gradually improves or if no obvious signs of infection are observed for several days after the operation, as reported by Mezzetto et al[14] and Zeng et al[17]. In the presence of obvious signs of infection and chest imaging suggestive of aortic pseudoaneurysm, most experts decided to perform immediate thoracic surgery after endovascular treatment for adequate mediastinal debridement, irrigation and drainage[10,11,13,15]. However, Kelly et al[12] reported successful treatment of a patient who had evidence of sepsis, high fever and hypotension with a stent that became infected 51 d after a single insertion of an endovascular stent graft; the patient later underwent definitive open surgical repair in which the stent was removed, the aorta was replaced with a homograft and covered with a left trapezius flap under deep hypothermic arrest, and the gastrointestinal tract was reconstituted. Conversely, in the case of an infected endovascular aorta stent for which no further surgery was performed due to the presence of comorbid conditions, the patient ultimately died, as reported by Rawala et al[16].
| No. | Sex | Age | Foreign body | Signs of infection | Aortal injury | Esophagoscopy | Surgical Management | The time interval between endovascular treatment and thoracic operations | Prognosis |
| Case 1 | Male | 22 yr | Chicken bone | WBC count of 19.96 × 109/L | CTA demonstrated a mediastinal esophageal fistula with an aortic pseudoaneurysm at the descending aorta | Esophageal stent-graft was implanted to isolate the esophageal perforation followed by the placement of a three-lumen gastrojejunal tube | Two F16 silicone tubes were placed in the pleural cavity and the mediastinum to facilitate postoperative drainage after mediastinal debridement and irrigation using the thoracoscope. | Immediately | Succeseeful salvage |
| Case 2 | Male | 32 yr | Fishbone | No abnormalities | CTA demonstrated an AEF | Emergency gastroscopy could not identify a bleeding focus due to massive amounts of clot and fresh blood | The esophagus was primarily repaired and reinforced by a pedicled intercostal muscle flap to cover the exposed stent-graft through a right thoracotomy | Immediately | Succeseeful salvage |
| Case 3 | Male | 59 yr | Fishbone | Evidence of sepsis with a high fever and hypotension | CTA demonstrated an AEF | There was some active bleeding after the fishbone was removed by gastroscopy | The stent became infected and definitive open surgical repair involved removing the stent, replacing the aorta with a homograft and coverage with a left trapezius flap while under deep hypothermic arrest, and reconstitution of the gastrointestinal tract | 53 d | Succeseeful salvage |
| Case 4 | Male | 25 yr | Fishbone | WBC count of 15.7 × 109/L | CTA demonstrated a large aortic pseudoaneurysm was seen on the descending aorta but not a punctured aorta | Not done | Two drainage tubes were placed in the left thoracic cavity and the mediastinum after the pseudoaneurysm was opened and mediastinal debridement and irrigation were performed by exploratory thoracotomy | Immediately | Succeseeful salvage |
| Case 5 | Male | 79 yr | Goat bone | WBC count of 12.4 × 109/L with a high fever | CTA demonstrated the presence of a focal irregularity of the aortic medial profile at the level of the thoracic aorta, suspected for an adventitial tear | The bone fragment was endoscopically removed and an endoscopic closure of the esophageal laceration by means of 2 long clips was performed. | Not done | Not done | Succeseeful salvage |
| Case 6 | Male | 40 yr | Chicken bone | WBC count of 10.9 × 109/L | CTA demonstrated an AEF and a large saccular pseudoaneurysm at the aortic isthmus, accompanied by mediastinal hematoma and bilateral pleural effusion | Esophagoscopy showed fresh and clotted blood coming from two irregular mural ulcers in the upper and middle thirds of the esophagus | After careful debridement, the dead space between the aorta and esophagus was filled with a viable pedicle flap of the omentum by an exploratory left thoracotomy. Two chest tubes were placed for mediastinal irrigation and drainage | Immediately | Succeseeful salvage |
| Case 7 | Female | 80 yr | Not mentioned | Elevated WBC count and MRSA-positive blood cultures | CT demonstrated a thoracic aorta aneurysm and an AEF | A mass was compressing the distal third of the esophagus, and an ulcer was present. An esophageal stent was placed to cover the whole esophagus | Not done | Not done | Died 3 months after the thoracic aorta stent was placed |
| Case 8 | Male | 58 yr | Duck bone | Not mentioned | CTA confirmed a descending intramural hematoma | The duck bone was removed gently using an endoscope | Not done | Not done | Succeseeful salvage |
Our two cases were unique in that they involved the process of improving clinical competence. AEF is a life-threatening complication, and discharge to a peripheral hospital should be avoided. Based on the presence of such clinical complications, angio-computed tomography should be performed in all cases of foreign body removal, even if the patient is clinically asymptomatic. In the first case, 1 d after removal of the esophageal foreign body, the patient suddenly suffered from hematemesis and other conditions, which were suggested to be caused by aortoclasia. The patient was in critical condition and could have died at any time. We performed timely and effective thoracic aorta stent-graft placement and closed the aortic fistula to achieve hemostasis. Based on our experience with the first case, a combined plan was drafted for endoscopic removal of the fishbone with simultaneous closure of the aortic defect using minimally invasive endovascular treatment in the second case. The patient’s hemoglobin was within normal limits, without hematemesis or melena. The order of events involving endoscopic fishbone removal and endovascular stent placement is another concern; a prematurely placed stent could be damaged by the foreign body impaling the aortic wall from the esophagus. We ensured persistent jejunal nutrient and an anti-infectious state (especially against gram-positive bacteria) after conducting sufficient multidisciplinary discussions. Because there were no clinical signs of infection in either patient, we did not perform thoracic surgery at a later stage. It is unprecedented that 2 continuous patients did not develop mediastinal infection and gradually resumed a normal diet. Because the esophageal fistulas were small, the esophagus was allowed to heal naturally. Thus, we did not place sutures through thoracic surgery. Conversely, patients with AEF and clinical signs of infection who are in good physical condition and considered low risk for open surgery should be considered for thoracic surgery.
Based on our limited experience with these two cases, we suggest that the management options for AEF patients should depend on the severity of the mediastinal infection, recovery from the esophageal fistula, extent of aortic injury, and the patients’ general condition. Our cases indicate that endovascular stent-graft treatment without combined thoracic operations could be a valuable alternative in selected patients.
| 1. | Tao K, Cheng H, Hu Z, Kong M. An aorto-oesophageal fistula treated with endovascular aortic repair: the fate of untreated oesophageal lesion on endoscopic follow-up. Interact Cardiovasc Thorac Surg. 2017;25:990-992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 2. | Beuran M, Negoi I, Negoi RI, Hostiuc S, Paun S. Primary Aortoduodenal Fistula: First you Should Suspect it. Braz J Cardiovasc Surg. 2016;31:261-263. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 3. | Dołęga-Kozierowski B, Sokratous K, Dyś K, Lis M, Ferenc S, Drelichowski S, Witkiewicz W. Aortoesophageal fistula as a complication of thoracic aorta aneurism stent grafting - a case report and literature review. Pol J Radiol. 2012;77:77-80. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 4. | Tao M, Shlomovitz E, Darling G, Roche-Nagle G. Secondary aorto-esophageal fistula after thoracic aortic aneurysm endovascular repair treated by covered esophageal stenting. World J Clin Cases. 2016;4:233-237. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 17] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 5. | Okita Y, Yamanaka K, Okada K, Matsumori M, Inoue T, Fukase K, Sakamoto T, Miyahara S, Shirasaka T, Izawa N, Ohara T, Nomura Y, Nakai H, Gotake Y, Kano H. Strategies for the treatment of aorto-oesophageal fistula. Eur J Cardiothorac Surg. 2014;46:894-900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 6. | Sia KJ, Ashok GD, Ahmad FM, Kong CK. Aorto-oesophageal fistula and aortic pseudoaneurysm caused by a swallowed fish bone. Hong Kong Med J. 2013;19:542-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 7. | Kieffer E, Chiche L, Gomes D. Aortoesophageal fistula: value of in situ aortic allograft replacement. Ann Surg. 2003;238:283-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 76] [Article Influence: 3.3] [Reference Citation Analysis (1)] |
| 8. | Chiesa R, Melissano G, Marone EM, Kahlberg A, Marrocco-Trischitta MM, Tshomba Y. Endovascular treatment of aortoesophageal and aortobronchial fistulae. J Vasc Surg. 2010;51:1195-1202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 55] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 9. | Canaud L, Ozdemir BA, Bee WW, Bahia S, Holt P, Thompson M. Thoracic endovascular aortic repair in management of aortoesophageal fistulas. J Vasc Surg. 2014;59:248-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 88] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 10. | Chen X, Li J, Chen J, Zhou Y, Zhang Y, Ding H, Huang S, Zhang Z. A combined minimally invasive approach for the treatment of aortoesophageal fistula caused by the ingestion of a chicken bone: case report and literature review. Clinics (Sao Paulo). 2012;67:195-197. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 11. | Assink J, Vierhout BP, Snellen JP, Benner PM, Paul MA, Cuesta MA, Wisselink W. Emergency endovascular repair of an aortoesophageal fistula caused by a foreign body. J Endovasc Ther. 2005;12:129-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 12. | Kelly SL, Peters P, Ogg MJ, Li A, Smithers BM. Successful management of an aortoesophageal fistula caused by a fish bone--case report and review of literature. J Cardiothorac Surg. 2009;4:21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 43] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 13. | Xi EP, Zhu J, Zhu SB, Liu Y, Yin GL, Zhang Y, Zhang XM, Dong YQ. Surgical treatment of aortoesophageal fistula induced by a foreign body in the esophagus: 40 years of experience at a single hospital. Surg Endosc. 2013;27:3412-3416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 14. | Mezzetto L, Treppiedi E, Scorsone L, Giacopuzzi S, Perandini S, Macrì M, Veraldi GF. Thoracic Aortic Pseudoaneurysm after Esophageal Perforation and Mediastinitis Caused by Accidental Ingestion of a Mutton Bone: A Case Report on Staged Endoscopic and Endovascular Treatments. Ann Vasc Surg. 2016;30:307.e15-307.e19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 15. | Shen JY, Zhang HW, Fan KJ, Liao H, Zhang EY, Hu J. Aortoesophageal fistula and arch pseudoaneurysm after removing of a swallowed chicken bone: a case report of one-stage hybrid treatment. BMC Surg. 2018;18:3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 16. | Rawala MS, Badami V, Rizvi SB, Nanjundappa A. Aortoesophageal Fistula: A Fatal Complication of Thoracic Endovascular Aortic Stent-Graft Placement. Am J Case Rep. 2018;19:1258-1261. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 17. | Zeng L, Shu W, Ma H, Hu J. Aortic injury caused by esophageal foreign body-case reports of 3 patients and literature review. Medicine (Baltimore). 2020;99:e20849. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cardiac and cardiovascular systems
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Costache RS, Mezzetto L S-Editor: Liu JH L-Editor: A P-Editor: Liu JH