Published online Mar 6, 2022. doi: 10.12998/wjcc.v10.i7.2138
Peer-review started: September 30, 2021
First decision: December 4, 2021
Revised: December 17, 2021
Accepted: January 20, 2022
Article in press: January 20, 2022
Published online: March 6, 2022
Processing time: 152 Days and 19.5 Hours
Osteonecrosis of the femoral head (ONFH) is a frequent and refractory disease whose pathogenesis has not yet been elucidated. Infection and other factors that reduce the local blood supply can lead to bone necrosis.
To aim of this study was to assess the relationship of ONFH with bone infection by use of metagenomic sequencing.
Twelve patients with idiopathic ONFH and 12 comparable controls who were undergoing hip arthroplasty were followed up in parallel. Necrotic femoral head specimens were collected for bacterial and fungal cultures using standard methods. Bone specimens were subjected to preliminary processing, and metagenomics sequencing of microorganisms was performed. A one-way analysis of variance was used to compare bacterial species in the two groups.
Bacterial and fungal cultures exhibited no evidence of microbial growth in all isolated necrotic femoral head tissues. We thus performed metagenomic sequencing and classified the species as suspected pathogens or suspected background microorganisms based on known bacterial pathogenicity. There was no evidence of viruses, fungi, parasites, M. tuberculosis complex, or mycoplasma/chlamydia. There were also no significant differences in suspected pathogens or suspected background microorganisms (P > 0.05).
Although we found no pathogens specific for ONFH in necrotic femoral head tissue, our research provides a foundation for future research on the metagenomics of bone pathogens.
Core Tip: Osteonecrosis of the femoral head (ONFH) is a frequent and refractory disease whose pathogenesis has not been elucidated. Infection and other factors that reduce the local blood supply can lead to osteonecrosis. We performed culture and metagenomic sequencing to examine the presence of pathogens in necrotic femoral head tissue. The results confirmed the absence of viruses, fungi, parasites, or mycoplasma/chlamydia in the specimens, and no significant differences in bacterial distribution and suspected background microorganisms. Although we did not identify an ONFH-specific pathogen, our study lays the groundwork for future research on metagenomics of bone pathogens.
- Citation: Liu C, Li W, Zhang C, Pang F, Wang DW. Previously unexplored etiology for femoral head necrosis: Metagenomics detects no pathogens in necrotic femoral head tissue. World J Clin Cases 2022; 10(7): 2138-2146
- URL: https://www.wjgnet.com/2307-8960/full/v10/i7/2138.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i7.2138
Osteonecrosis of the femoral head (ONFH) is a common and refractory disease characterized by the death of osteocytes and bone marrow cells, and is generally caused by an inadequate blood supply. Direct and indirect factors that reduce the local blood supply, such as femoral neck fracture, hip dislocation, long-term corticosteroid use, and excessive alcohol consumption, can lead to ONFH[1-3]. However, many patients develop idiopathic ONFH, in which there are no obvious causes or risk factors[4]. Infection is one of the various factors that can disrupt the local blood supply to the femoral head and lead to necrosis. Several specific pathogens can cause microvascular disease and lead to secondary local necrosis, such as intra-proliferative proliferative nephritis, which is often associated with type A hemolytic streptococcal infection[5,6]. Our purpose was to identify ONFH-related pathogens in the necrotic femoral head, and to examine the relationship between ONFH and infection. To our knowledge, this is the first study to examine whether pathogenic microbial infection is responsible for idiopathic ONFH in patients who do not have obvious risk factors. Our findings may have important consequences for clinical decision-making for the early intervention and follow-up treatment of ONFH.
All patient data were extracted from the hospital's medical records. All included patients had diagnoses of ONFH based on clinical symptoms and imaging features, and had no known risk factors. All patients had abnormal gaits; reduced Harris hip scores; positive results in the Thomas experiment and Patrick experiment; and X-ray features showing femoral head collapse, a crescent sign, and Ficat stage Ⅲ or Ⅳ. The time from the onset of pain to diagnosis ranged from 2 mo to 5 years. All patients underwent total hip arthroplasty, and all of their Harris hip scores improved after surgery. The control group, consisting of 12 patients with acute femoral neck fractures who were undergoing hip arthroplasty, were followed up in parallel.
All experimental protocols were approved by the Institutional Ethics Committee of the authors’ affiliated institutions, and informed consent was obtained from all patients.
All specimens were collected in a thousand-level laminar flow operating room that had constant temperature and humidity to minimize contamination. In addition, bedside sampling was performed following aseptic procedures. Thus, sampling personnel wore hats, masks, and gloves, and avoided speaking to prevent possible contamination by dental bacteria. A fresh necrotic femoral head tissue sample that was about the size of a soybean (about 200 mg) was ground into pieces as small as possible using a rongeur, and equal amounts were then placed into three clean Eppendorf tubes. The first tube contained RNase-free pure water prepared for RNA sequencing, the second tube was used for DNA sequencing, and the third tube was used for bacterial and fungal cultures. The first tube was gently shaken to submerge the specimen, sealed, and immediately placed in liquid nitrogen to ensure cryopreservation of RNA. The sample in the third tube was cultured for identification of bacteria, fungi, and Mycobacterium, using standard methods.
The first and second tubes were stored vertically in dry ice (-80°C) and sent to Huada Gene Company Tianjin Branch within 4 h after collection. These specimens were received within 48 h, and subjected to further homogenization using previously described ortexing/sonication methods[7]. The eluted specimens were initially tested for nucleic acid content to assure they met standard requirements, and were then subjected to sequencing.
Each tissue sample, with 0.5 mm glass beads, was added to a microcentrifuge tube with a lysis buffer and centrifuged at 3000 rpm for 30 min. Then a 0.3 mL aliquot was transferred into a new microcentrifuge tube, and DNA was extracted using the TIANamp Micro DNA Kit (Tiangen Biotech) according to the manufacturer's recommendations. The same procedures were used for RNA extraction using the QIAAmp Viral RNA Mini Kit (52904#, Qiagen), and then cDNA was generated from an RNA template by reverse transcription. The DNA was then fragmented, end-repaired, and a ligated adapter and PCR amplification were used to construct DNA libraries. The Agilent 2100 Bioanalyzer was used for quality control, and eligible libraries were sequenced as described below[8].
Metagenomic next-generation sequencing (mNGS) was performed using the BGISEQ-50 gene sequencer (Huada Gene Company). Data quality was achieved by removing low quality and short (< 35 bp) reads. Then, the Burrows-Wheeler transformation was used to perform alignment to the reference genome (hg19). By aligning four microbial genome databases (for bacteria, viruses, fungi, and parasites), the rest of the data were analyzed, except for low-complexity reads. The classification reference databases were from NCBI (ftp://ftp.ncbi.nlm.nih.gov/genomes/). RefSeq contains 1424 genomic sequences of DNA viruses, 2637 genomic sequence of RNA viruses, 2406 genomic sequences of bacteria, 83 genomes or scaffolds of Mycobacterium, and sequences of 199 fungi, 135 parasites, and 41 mycoplasma/chlamydia that are related to human infections. The detection limit was 100 to 1000 copies/mL for microbial nucleic acids; the detection specificity for microorganisms with copy numbers greater than the detection limit was greater than 99%; and the repeatability was greater than 99%.
All data analyses were performed using SPSS version 20 (IBM Corp., Armonk, NY, United States). A one-way analysis of variance was used to determine statistically significant differences. A P value below 0.05 was considered significant (P < 0.05).
Twelve patients with idiopathic ONFH were enrolled (Table 1). The median patient age was 61 years (range: 48-69, mean: 58, standard deviation: 7). There were 7 patients with left femoral necrosis, 3 with bilateral femoral necrosis, and 2 with right femoral necrosis. Three patients had comorbidities that were unrelated to ONFH (lumbar disc herniation, lumbar disc herniation and lumbar spinal stenosis, prostatic hyperplasia and bronchitis).
| Case | Sex/age (yr) | Site | Comorbidities | Physical examination | X-ray features | Ficat stage | Time from pain onset to admission | Surgical approach | Outcome |
| 1 | M/50 | Bilateral | Lumbar disc herniation | Limb gait, Harris score: 25 (left), 19 (right), left hip joint mobility: flexion 90°, extension 0°, abduction 15°, adduction 10°, external rotation 0°, internal rotation 10°, right hip joint mobility: flexion 90°, extension 0°, abduction 10°, adduction 0°, external rotation 0°, internal rotation 0°, Thomas experiment (+), Patrick experiment (+) | Bilateral femoral head collapse, crescent sign, joint space narrowing, with hip osteoarthritis | IV (left); IV (right) | 2 yr | Bilateral artificial total hip arthroplasty | Harris score: 48 (left), 47 (right) |
| 2 | F/62 | Left | None | Limb gait, Harris score: 35, hip joint mobility: flexion 90°, extension 5°, abduction 20°, adduction 15°, external rotation 15°, internal rotation 5°, Thomas experiment (+), Patrick experiment (+) | Femoral head collapse occurs, crescent sign | III | 7 mo | Left artificial total hip arthroplasty | Harris score: 52 |
| 3 | F/68 | Left | None | Limb gait, Harris score: 35, hip joint mobility: flexion 70°, extension 0°, abduction 10°, adduction 10°, external rotation 10°, internal rotation 10°, Thomas experiment (+), Patrick experiment (+) | Femoral head collapse occurs, crescent sign | III | 2 mo | Left artificial total hip arthroplasty | Harris score: 54 |
| 4 | F/69 | Right | None | Limb gait, Harris score: 17, hip joint mobility: flexion 60°, extension 0°, abduction 0°, adduction 0°, external rotation 0°, internal rotation 5°, Thomas experiment (+), Patrick experiment (+) | Femoral head collapse occurs, crescent sign | IV | 15 mo | Right artificial total hip arthroplasty | Harris score: 48 |
| 5 | M/48 | Bilateral | None | Limb gait, Harris score: 24 (left), 37 (right), left hip joint mobility: flexion 90°, extension 0°, abduction 40°, adduction 10°, external rotation 15°, internal rotation 10°, right hip joint mobility: flexion 110°, extension 0°, abduction 60°, adduction 15°, external rotation 15°, internal rotation 15°, Thomas experiment (+), Patrick experiment (+) | Femoral head collapse occurs, crescent sign (left); No femoral head collapse, appear cystic (right) | III (left); II (right) | 15 mo | Left artificial total hip arthroplasty | Harris score: 56 (left), 37 (right) |
| 6 | F/63 | Left | Lumbar disc herniation and lumbar spinal stenosis | Limb gait, Harris score: 32, hip joint mobility: flexion 70°, extension 0°, abduction 25°, adduction 10°, external rotation 10°, internal rotation 10°, Thomas experiment (+), Patrick experiment (+) | Femoral head collapse, joint space narrowing | IV | 3 yr and 6 mo | Left artificial total hip arthroplasty | Harris score: 49 |
| 7 | M/61 | Left | Prostatic hyperplasia, bronchitis | Limb gait, Harris score: 45, hip joint mobility: flexion 100°, extension 0°, abduction 20°, adduction 10°, external rotation 15°, internal rotation 10°, Thomas experiment (+), Patrick experiment (+) | Femoral head collapse occurs, crescent sign | III | 5 yr | Left artificial total hip arthroplasty | Harris score: 55 |
| 8 | M/56 | Right | None | Limb gait, Harris score: 30, hip joint mobility: flexion 80°, extension 10°, abduction 15°, adduction 5°, external rotation 0°, internal rotation 5°, Thomas experiment (+), Patrick experiment (+) | Femoral head collapse occurs, crescent sign | IV | 16 mo | Right artificial total hip arthroplasty | Harris score: 51 |
| 9 | F/62 | Left | None | Limb gait, Harris score: 40, hip joint mobility: flexion 90°, extension 10°, abduction 15°, adduction 5°, external rotation 10°, internal rotation 15°, Thomas experiment (+), Patrick experiment (+) | Femoral head collapse occurs, crescent sign | III | 5 mo | Left artificial total hip arthroplasty | Harris score: 57 |
| 10 | M/46 | Bilateral | None | Limb gait, Harris score: 26 (left), 28 (right), left hip joint mobility: flexion 85°, extension 10°, abduction 35°, adduction 15°, external rotation 10°, internal rotation 10°, right hip joint mobility: flexion 90°, extension 10°, abduction 25°, adduction 10°, external rotation 10°, internal rotation 15°, Thomas experiment (+), Patrick experiment (+) | Bilateral femoral head collapse, crescent sign | III (left); III (right) | 3 yr | Bilateral artificial total hip arthroplasty | Harris score: 46 (left), 48 (right) |
| 11 | F/55 | Left | None | Limb gait, Harris score: 40, hip joint mobility: flexion 95°, extension 15°, abduction 15°, adduction 20°, external rotation 10°, internal rotation 10°, Thomas experiment (+), Patrick experiment (+) | Femoral head collapse occurs, crescent sign | III | 9 mo | Left artificial total hip arthroplasty | Harris score: 58 |
| 12 | M/61 | Left | None | Limb gait, Harris score: 22, hip joint mobility: flexion 65°, extension 0°, abduction 5°, adduction 0°, external rotation 5°, internal rotation 0°, Thomas experiment (+), Patrick experiment (+) | Femoral head collapse occurs, crescent sign | IV | 4 yr | Left artificial total hip arthroplasty | Harris score: 46 |
Aerobic bacteria and anaerobic bacteria cultures were negative on the third day, fungal cultures were negative for 56 d, and tuberculosis liquid cultures were negative for 42 d. These results indicate the need to perform culture-independent analysis, such as mNGS.
According to the results of the mNGS, we cannot make final conclusion regarding the causative pathogens without further clinical assessments. Nonetheless, we used a method to rank the microbes detected from the samples. First, we compared microbes detected with background microbes in the database, and labeled them as “background” microbes. Second, we compared the microbes detected using negative controls; if the number of reads was less than 50, the difference was considered to be “more than 5-fold” and if the number of reads was more than 50, the difference was considered to be “more than 3-fold”. For bacteria, we reported the top 5 genera and the 2 major species in each genus; for fungi and parasites, we reported the top 5 genera and the 1 major species in each genus; for viruses, we reported all detected species; each species has two types and each type has two subtypes.
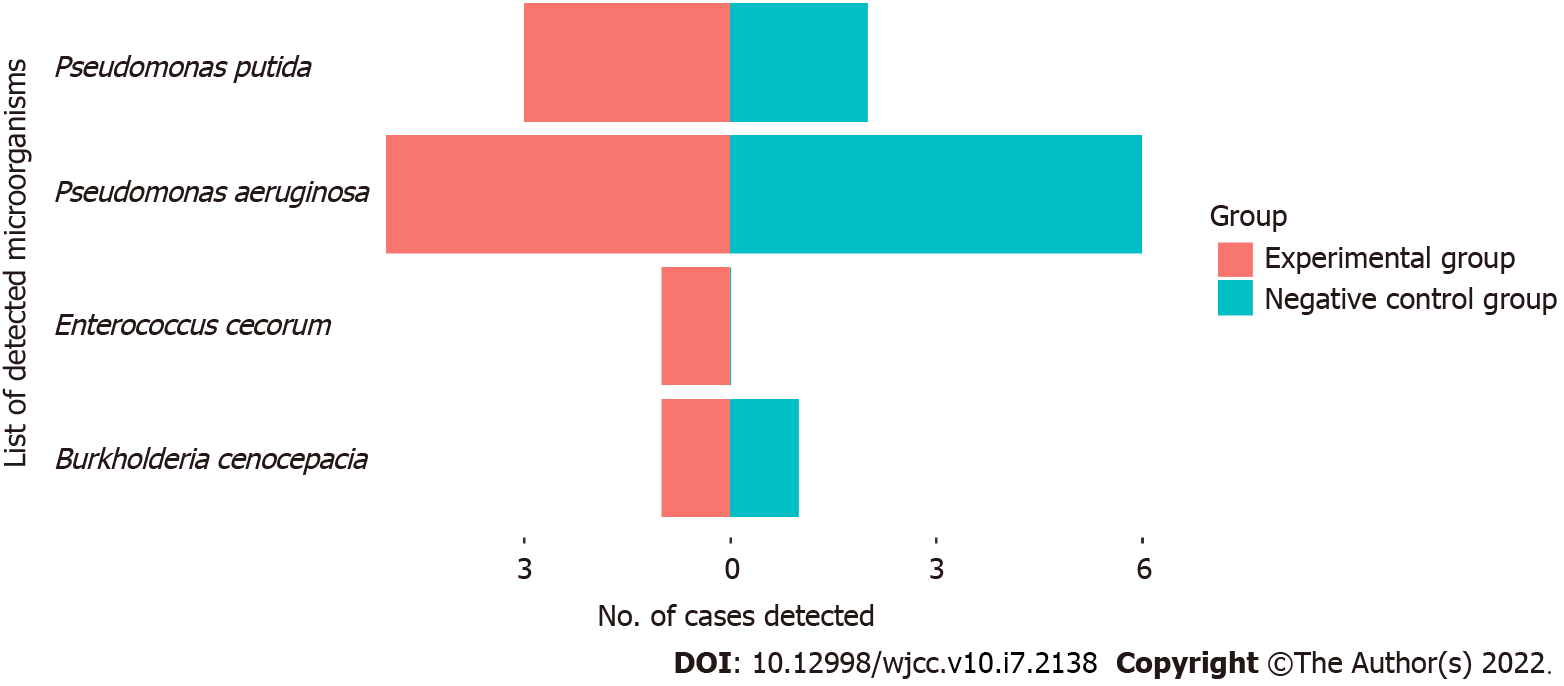
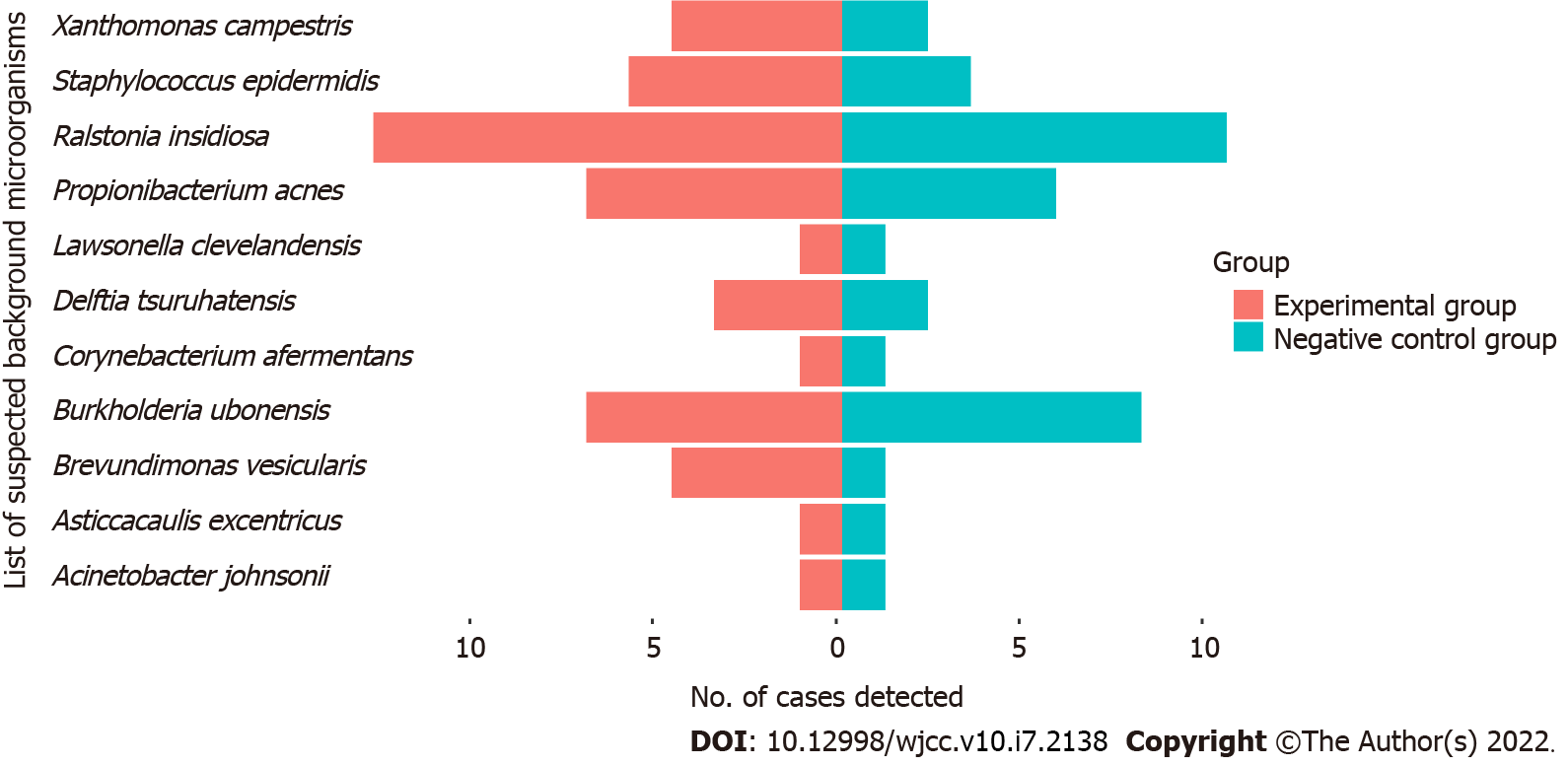
We sorted the microbial sequencing results according to the number of sequences, and then classified detected bacterial sequences as suspected pathogens or suspected background microorganisms, based on known common clinical pathogens. The suspected pathogens in the patients and controls were Pseudomonas aeruginosa, Burkholderia cenocepacia, Enterococcus cecorum, and Pseudomonas putida (Figure 1 and Supplementary Table 1). The major suspected background microorganisms in the patients and controls were Ralstonia insidiosa, Propionibacterium acnes, and Burkholderia ubonensis, and several other species (Figure 2 and Supplementary Table 2). There was no evidence of viruses, fungi, parasites, M. tuberculosis complex, or mycoplasma/chlamydia. Statistical analysis indicated there were no significant differences in suspected pathogens or suspected background microorganisms (both P > 0.05, Supplementary Table 3).
Idiopathic osteonecrosis is a painful disorder that mainly affects individuals who are 30 to 40 years-old[9]. The basic features of ONFH pathogenesis are impaired blood circulation that ultimately leads to necrosis. Some studies attributed the disease to a combination of metabolic factors, genetic susceptibility, and insufficient local blood supply. ONFH can progress to symptomatic osteoarthritis of the hip joint and collapse, or even destruction of the femoral head[10,11]. Microscopic or macroscopic disruption of the blood supply to the femoral head are considered the hallmarks of this condition, because they lead to necrosis of bone forming cells[6,12]. Many pathogenic bacteria and viruses can release damaging toxins or cause inflammation of small blood vessels, thereby resulting in ischemia, infarction, and tissue necrosis. For example, Helicobacter pylori can cause gastritis, hepatitis B virus can cause chronic hepatitis, and streptococcal infection can lead to acute glomerulonephritis[13]. Furthermore, infectious pathogens can cause a local immune response, leading to microemboli in the arteries. However, our sequencing results provided no evidence that idiopathic ONFH is associated with infections by any particular microorganisms. Traditional culturing methods also have not identified the causative pathogens. mNGS provides enhanced capabilities by offering culture-independent, comprehensive diagnostic assessment of the microbial composition of clinical samples[14,15]. This motivated our use of mNGS to investigate the presence of microorganisms in the necrotic femoral head of patients with ONFH. One of the limitations of the present study is that we only examined a relatively small number of cases. Our sequencing results indicated that the femoral head tissues of the ONFH and control groups had no significant differences in pathogenic microorganisms or background microorganisms. Thus, we have no evidence that idiopathic ONFH is associated with any specific femoral head pathogens, indicating there is still a need to identify the cause of ONFH in these patients. Another limitation is that mNGS can lead to false-positive and false-negative results. In particular, the presence of background bacteria can interfere with the results. In fact, we identified several background bacteria in both groups (R. insidiosa, P. acnes, and B. ubonensis). These background bacteria often appear because they are common in the laboratory and other environments, and are occasionally associated with patient infections. Moreover, our culturing of necrotic femoral head tissue indicated no bacterial growth. Some studies have reported that HIV infection is a risk factor for ONFH[16,17], although there is no reliable evidence of a causative relationship. Patients infected with HIV often receive radiotherapy, chemotherapy, and corticosteroid treatments, and these may favor the development of ONFH. Thus, it is difficult to identify the specific causes of ONFH in patients infected with HIV[18]. Some studies support the hypothesis that the protease inhibitors used by HIV-infected patients promotes the development of osteonecrosis because they cause hyperlipidemia[19]. Although our results were negative, our proof-of-concept study indicated there are still many other possible routes for examining the metagenomics of pathogens in ONFH. The present study was apparently limited by the relatively small sample size and by our detection of a small number of suspected pathogens (Pseudomonas aeruginosa, Burkholderia cenocepacia, Enterococcus cecorum, and Pseudomonas putida). In addition, our mNGS results detected no microbial growth in isolated necrotic femoral head tissues of some patients, a finding that complicates interpretation of differential gene expression profiling. Therefore, future multicenter randomized and prospective studies with larger sample sizes are ongoing to validate the specificity and sensitivity of mNGS for the diagnosis of idiopathic ONFH. We also plan to use robust genetic classifiers in this population to distinguish specific pathogens from non-infectious microvascular diseases in idiopathic ONFH.
In summary, this is the first study to use mNGS to identify specific pathogens in the femoral heads of patients who have idiopathic ONFH with no obvious risk factors. Although we found no differences in the ONFH and control groups, we demonstrated that mNGS can detect microbes in femoral head tissues and has potential for use in association with the analysis of transcription factors related to the host immune response and the microbiome.
The immediate cause of femoral head necrosis is localized damage to the blood flow to the femoral head and disruption of the intraosseous circulation. Inflammatory as well as infectious factors are thought to lead to microangiopathy and depletion of the intraosseous circulation, which in turn leads to femoral head necrosis.
The motivation for this study was to assess the relationship between infection and osteonecrosis of the femoral head (ONFH) using metagenomic next-generation sequencing (mNGS) sequencing.
Our objectives were to identify the pathogens associated with ONFH in necrotic femoral heads and to investigate the relationship between ONFH and infection.
Two groups of subjects, the ONFH group and the control group, were recruited and femoral head specimens were collected using standard methods. Differences in the microbial distribution of the femoral head specimens between the two groups were assessed by culture and mNGS sequencing methods.
No evidence of microbial growth could be detected by bacterial or fungal culture methods in any of the femoral head specimens isolated from the subjects. Metagenomic sequencing results also did not support the presence of viruses, fungi, parasites, Mycobacterium tuberculosis complex or mycoplasma/chlamydia. There were no significant differences in the suspected background microorganisms between the two subject groups (P > 0.05).
No specific pathogens associated with the pathogenesis of ONFH were identified, this study lays the foundation for mNGS studies of ONFH disease.
So far as we are aware, this is the first study to examine whether pathogenic microbial infections are the cause of idiopathic ONFH in patients with no apparent risk factors.
| 1. | Moya-Angeler J, Gianakos AL, Villa JC, Ni A, Lane JM. Current concepts on osteonecrosis of the femoral head. World J Orthop. 2015;6:590-601. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 266] [Cited by in RCA: 341] [Article Influence: 31.0] [Reference Citation Analysis (11)] |
| 2. | Shah KN, Racine J, Jones LC, Aaron RK. Pathophysiology and risk factors for osteonecrosis. Curr Rev Musculoskelet Med. 2015;8:201-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 193] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 3. | Mont MA, Cherian JJ, Sierra RJ, Jones LC, Lieberman JR. Nontraumatic Osteonecrosis of the Femoral Head: Where Do We Stand Today? J Bone Joint Surg Am. 2015;97:1604-1627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 355] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 4. | Guerado E, Caso E. The physiopathology of avascular necrosis of the femoral head: an update. Injury. 2016;47 Suppl 6:S16-S26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 90] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 5. | Mahony T, Sidell D, Gans H, Cooperstock M, Brown K, Cheung JM, Farhadian B, Gustafson M, Thienemann M, Frankovich J. Palatal Petechiae in the Absence of Group A Streptococcus in Pediatric Patients with Acute-Onset Neuropsychiatric Deterioration: A Cohort Study. J Child Adolesc Psychopharmacol. 2017;27:660-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 6. | Aoyama T, Goto K, Kakinoki R, Ikeguchi R, Ueda M, Kasai Y, Maekawa T, Tada H, Teramukai S, Nakamura T, Toguchida J. An exploratory clinical trial for idiopathic osteonecrosis of femoral head by cultured autologous multipotent mesenchymal stromal cells augmented with vascularized bone grafts. Tissue Eng Part B Rev. 2014;20:233-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 61] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 7. | Thoendel MJ, Jeraldo PR, Greenwood-Quaintance KE, Yao JZ, Chia N, Hanssen AD, Abdel MP, Patel R. Identification of Prosthetic Joint Infection Pathogens Using a Shotgun Metagenomics Approach. Clin Infect Dis. 2018;67:1333-1338. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 227] [Cited by in RCA: 193] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 8. | Wang C, Yang Y, Xu M, Mao F, Yang P, Yuan S, Gao R, Gan S. Deep Sequencing of the Rat MCAO Cortexes Reveals Crucial circRNAs Involved in Early Stroke Events and Their Regulatory Networks. Neural Plast. 2021;2021:9942537. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 9. | Kaushik AP, Das A, Cui Q. Osteonecrosis of the femoral head: An update in year 2012. World J Orthop. 2012;3:49-57. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 102] [Cited by in RCA: 107] [Article Influence: 7.6] [Reference Citation Analysis (5)] |
| 10. | Malizos KN, Karantanas AH, Varitimidis SE, Dailiana ZH, Bargiotas K, Maris T. Osteonecrosis of the femoral head: etiology, imaging and treatment. Eur J Radiol. 2007;63:16-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 186] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 11. | Tanino H, Ito H. Agent for preventing onset of idiopathic osteonecrosis of femoral head and/or suppressing progress of same. US Patent Application, 2018. |
| 12. | Inoue S, Igarashi M, Karube S, Oda H. Vitamin D3 metabolism in idiopathic osteonecrosis of femoral head. Nihon Seikeigeka Gakkai Zasshi. 1987;61:659-666. [PubMed] |
| 13. | Weis S, Sonnberger M, Dunzinger A. Infections: Bacteria. Imaging Brain Diseases. Springer, Vienna, 2019; 653-692. |
| 14. | Wilson MR, Naccache SN, Samayoa E, Biagtan M, Bashir H, Yu G, Salamat SM, Somasekar S, Federman S, Miller S, Sokolic R, Garabedian E, Candotti F, Buckley RH, Reed KD, Meyer TL, Seroogy CM, Galloway R, Henderson SL, Gern JE, DeRisi JL, Chiu CY. Actionable diagnosis of neuroleptospirosis by next-generation sequencing. N Engl J Med. 2014;370:2408-2417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 609] [Cited by in RCA: 700] [Article Influence: 58.3] [Reference Citation Analysis (0)] |
| 15. | Doan T, Wilson MR, Crawford ED, Chow ED, Khan LM, Knopp KA, O'Donovan BD, Xia D, Hacker JK, Stewart JM, Gonzales JA, Acharya NR, DeRisi JL. Illuminating uveitis: metagenomic deep sequencing identifies common and rare pathogens. Genome Med. 2016;8:90. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 149] [Cited by in RCA: 148] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 16. | Ries MD, Barcohana B, Davidson A, Jergesen HE, Paiement GD. Association between human immunodeficiency virus and osteonecrosis of the femoral head. J Arthroplasty. 2002;17:135-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Morse CG, Mican JM, Jones EC, Joe GO, Rick ME, Formentini E, Kovacs JA. The incidence and natural history of osteonecrosis in HIV-infected adults. Clin Infect Dis. 2007;44:739-748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 83] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 18. | Yombi JC, Vandercam B, Wilmes D, Dubuc JE, Vincent A, Docquier PL. Osteonecrosis of the femoral head in patients with type 1 human immunodeficiency virus infection: clinical analysis and review. Clin Rheumatol. 2009;28:815-823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 19. | Matos MA, Alencar RW, Matos SS. Avascular necrosis of the femoral head in HIV infected patients. Braz J Infect Dis. 2007;11:31-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; externally peer reviewed.
Peer-review model: Single blind
Specialty type: Orthopedics
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Arumugam VA, Dauyey K S-Editor: Liu JH L-Editor: A P-Editor: Liu JH