Published online Feb 26, 2022. doi: 10.12998/wjcc.v10.i6.2007
Peer-review started: September 1, 2021
First decision: October 18, 2021
Revised: October 27, 2021
Accepted: January 11, 2022
Article in press: January 11, 2022
Published online: February 26, 2022
Processing time: 175 Days and 7.2 Hours
Dedifferentiated liposarcoma (DDLPS) is an extremely rare neoplasm that exhibits various morphologies. The tumor is characterized by immunoreactivity to MDM2 and CDK4 and can be confirmed by detecting MDM2 amplification via fluorescence in situ hybridization (FISH). Herein, we report an unusual case of DDLPS arising from the duodenum.
A 64-year-old man presented with repeated abdominal pain and weight loss. Radiologic studies revealed a mass of the duodenum involving the pancreas. The patient was treated with pylorus-preserving pancreaticoduodenectomy. Histologically, the tumor showed a high-grade sarcoma. Immunohistochemistry demonstrated that the tumor cells were positive for MDM2 and CDK4 expression. MDM2 amplification was detected via FISH, leading to the final diagnosis of DDLPS. Following surgery, the patient was treated in the intensive care unit due to peritonitis, and died 60 d after surgery.
To the best of the authors’ knowledge, this is the first case of primary duodenal DDLPS in Korea and the third case in the English-language literature. Care must be taken not to misdiagnose DDLPS as another high-grade tumor. Liposarcoma should be in the differential diagnosis list.
Core Tip: Primary dedifferentiated liposarcoma (DDLPS) originating from the duodenum are rare and can be diagnosed based on histology, immunohistochemistry and MDM2 amplification via fluorescence in situ hybridization. Differential diagnoses are required along with consideration of DDLPS.
- Citation: Kim NI, Lee JS, Choi C, Nam JH, Choi YD, Kim HJ, Kim SS. Primary duodenal dedifferentiated liposarcoma: A case report and literature review. World J Clin Cases 2022; 10(6): 2007-2014
- URL: https://www.wjgnet.com/2307-8960/full/v10/i6/2007.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i6.2007
Liposarcoma is the most common form of malignant soft tissue tumor and tends to occur in the retroperitoneum, deep soft tissues of the trunk, and extremities[1]. Primary liposarcoma of the gastrointestinal tract is extremely rare, with only a few cases reported in the literature[2-10]. In this report, we describe a unique case of dedifferentiated liposarcoma (DDLPS) originating from the duodenum and review previously reported cases. Pathologists should keep liposarcoma in the differential diagnosis list.
A 64-year-old male presented with a 7-d duration of repeated abdominal pain.
The patient also complained of 8 kg weight loss in 3 mo.
The patient had been on antihypertensive medication for 20 years.
He had no other significant personal or family medical history.
Physical examination revealed diffuse abdominal tenderness, but a palpable mass was not detected.
Laboratory findings showed slight elevation of aspartate aminotransferase (55 U/L). Tumor markers including carcinoembryonic antigen (2.13 mg/mL), were within the normal range.
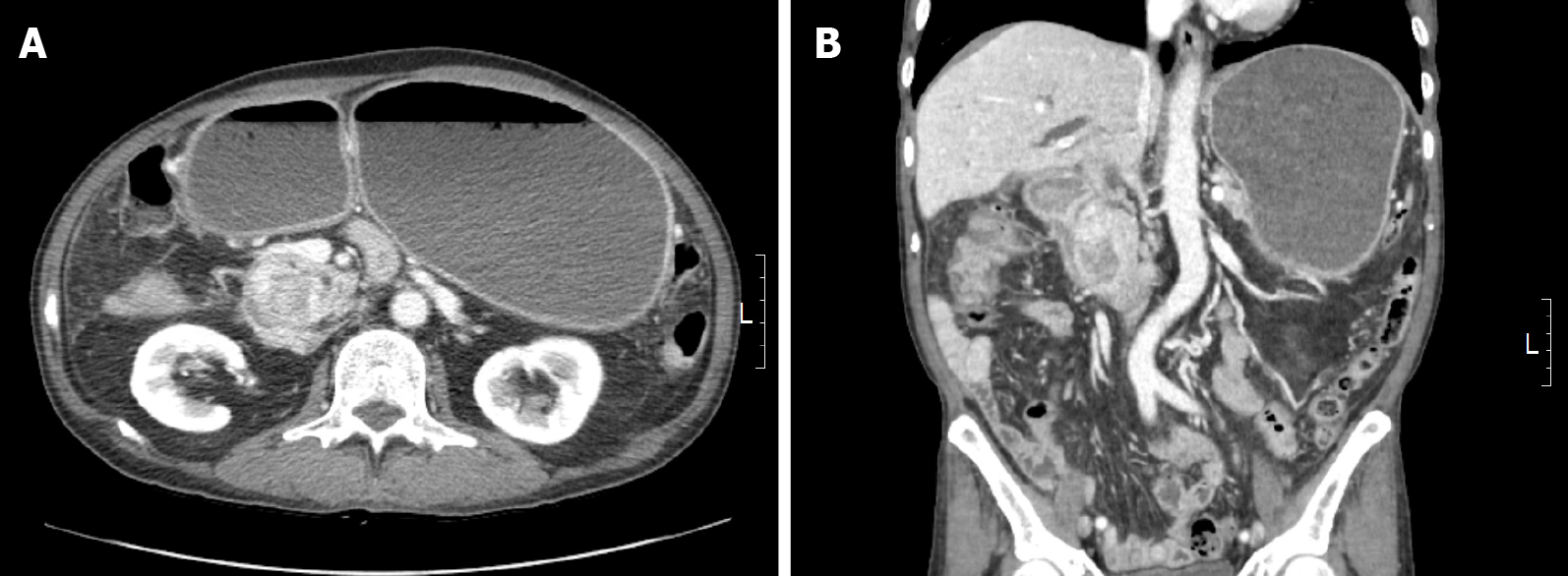
Abdominal computed tomography (CT) revealed a 3 cm-sized heterogeneously enhancing mass in the pancreaticoduodenal groove, causing the obstruction of the second portion of the duodenum.
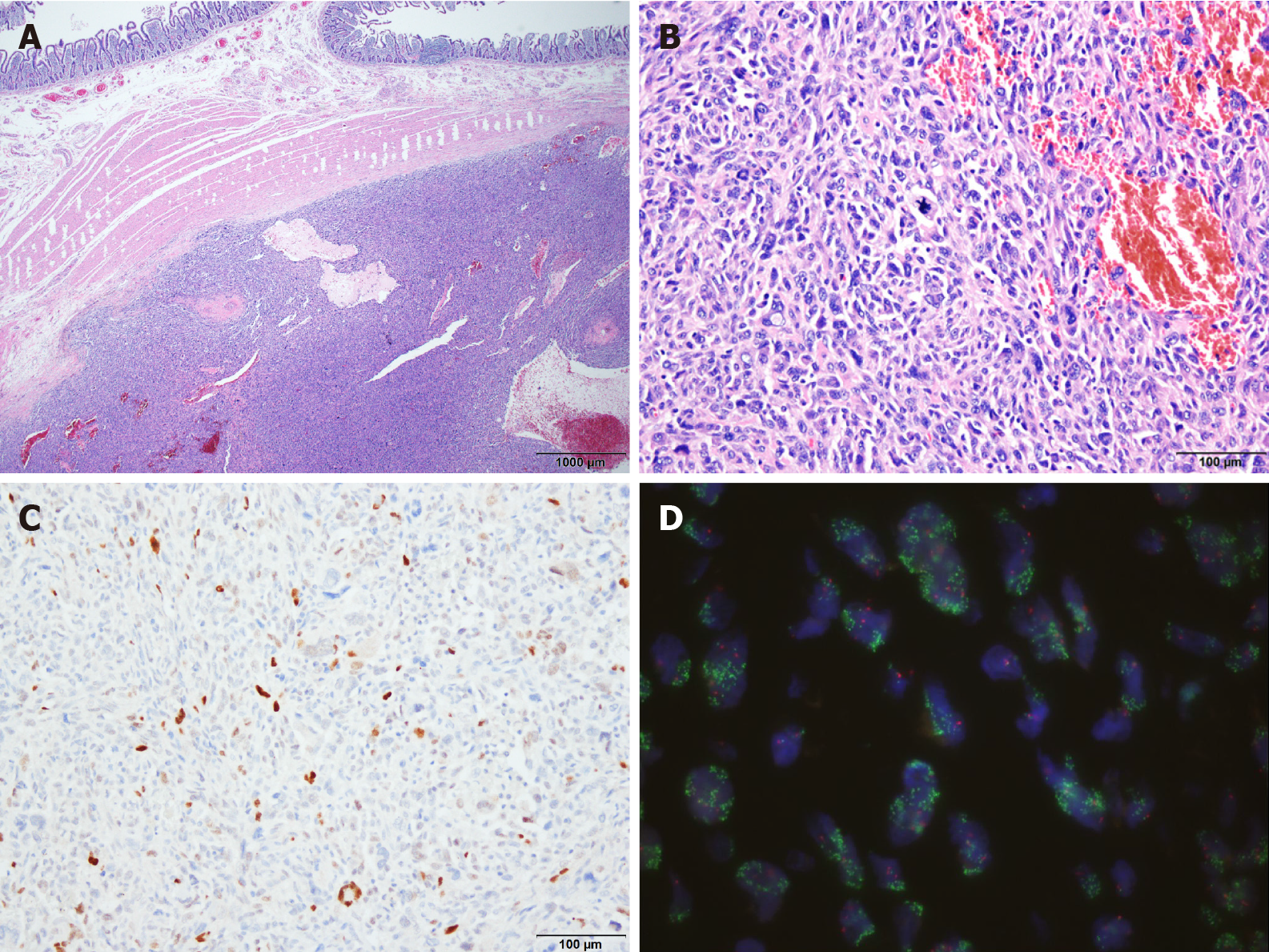
The preoperative differential diagnosis was duodenal adenocarcinoma, gastro-intestinal stromal tumor (GIST), or leiomyosarcoma (LMS). An accurate diagnosis via preoperative upper endoscopy was not possible because only the mucosal surface was collected due to duodenal stenosis. The duodenal tumor was diagnosed as DDLPS by combining all clinical, radiologic, and intraoperative findings and histologic data (Figure 1 and Figure 2).
The patient underwent pylorus-preserving pancreaticoduodenectomy with en bloc right hemicolectomy, superior mesenteric vein segmental resection, and inferior vena cava wedge resection. Intraoperatively, the duodenal mass invaded the pancreas and hepatic flexure of the colon, resulting in gastric and duodenal distension. The tumor also appeared to invade adjacent large blood vessels. Although the duodenal mass was surgically removed, the entire tumor could not be completely removed as the tumor had already spread throughout the body.
Upon macroscopic examination, the surgical resection specimen appeared to have originated from the submucosal layer of the duodenum, measuring 3x3 cm at its greatest dimension. The nodular mass showed a white-to-tan colored cut surface with a focal area of hemorrhage. Pathologic evaluation revealed a tumor arising from the duodenum and extending to the pancreas, colon, and omentum. Histology showed the epicenter of the tumor was the submucosal layer with normal-looking mucosa. The tumor was relatively well circumscribed and composed of high-grade pleomorphic cells. The tumor was arranged in a haphazard, fascicular growth pattern with telangiectatic-like feature. The majority of the tumor cells exhibited marked nuclear atypia with brisk mitotic activity. Undifferentiated tumor cells displayed large vesicular or hyperchromatic nuclei and prominent macronucleoli. Discohesive polygonal giant cells with abundant eosinophilic cytoplasm were also observed. Immunohistochemistry was positive for MDM2 and CDK4 but was negative for CK, Actin, Desmin, CD117, CD34, ERG, S100, TFE3, Melan A, and HMB45. The tumor was also found to harbor MDM2 amplification via fluorescence in situ hybridization (FISH).
Subsequently, chest CT and whole-body positron emission tomography/CT scans were performed to check for preexisting and unidentified intraabdominal liposarcoma outside the gastrointestinal tract and secondary duodenal involvement. No specific abnormalities or metastases were found.
However, due to the vessel invasion of the tumor, cancer seeding contributed to anastomotic dehiscence. Seven days after initial surgery, a 5 mm-sized leakage of the hepaticojejunal anastomosis occurred, leading to generalized peritonitis. The patient underwent a second operation. Large amounts of necrotic fluid had filled the abdominal cavity, and severe adhesions were observed. Hepaticojejunal anastomosis repair with superior mesenteric vein thrombectomy was performed. Fourteen days later, the patient developed sepsis due to the perforation of the gastrojejunal-anastomosis. A life saving emergency operation was performed. Intraoperatively, massive hematoma and bowel adhesion were observed in the upper abdomen. Overall, the tissue was very friable and edematous due to severe inflammation. An external stent was inserted and repaired using a T-tube in the gastrojejunal perforation site. Since the possibility of perforation was very high in the case of primary repair of the jejunal limb, an external stent was inserted using a hemovac drain. After massive irrigation, the operation was terminated.
Unfortunately, the patient did not recover from disseminated intravascular coagulation and peritonitis, and he died 60 d after surgery.
Liposarcomas are subclassified as atypical lipomatous tumor/well-differentiated liposarcoma (ALT/WDLPS), DDLPS, myxoid liposarcoma, pleomorphic liposarcoma, or myxoid pleomorphic liposarcoma according to the World Health Organization classification[11]. WDLPS is a typically indolent histologic subtype that presents as slowly growing masses but can be locally aggressive with minimal to no distant metastatic potential, while DDLPS is a higher grade histology with the potential for rapid growth and distant metastatic potential[12,13].
The term DDLPS was first introduced by Evans in 1979[14] and is defined as a combination of ALT/WDLPS and a high-grade non-lipogenic sarcoma-like component of variable histologic grade. DDLPS can occur de novo (90%), with about 10% occurring from a pre-existing WDLPS[15]. The histologic hallmark of DDLPS is the transition from ALT/WDLPS to non-lipogenic sarcoma, although a well-differentiated component may not be identifiable.
The tumor usually presents as a large painless mass in late adult life with an equal distribution between males and females. The retroperitoneum is the most common location and occurrence in the extremities and subcutaneous tissue is very rare[16]. Since DDLPS primarily involving the intestine is extremely unusual, less than 10 cases have been reported to date[1-3,5-10,17]. Of those tumors arising in the small bowel, four originated in the jejunum, five in the ileum, and two in the duodenum (Table 1). Clinical information on the precise locations of the other three small bowel tumors is not available. The literature reveals that occurrences of DDLPS in the small intestine can cause various symptoms, such as intussusception, bleeding, obstruction, and abdominal discomfort. Since Okabayashi et al[6] reported the first case in 2013, there has only been one additional report of DDLPS in the duodenum[10].
| No. | Ref. | Age (yr) | Sex | Clinical presentation | Location | Histology | Treatment |
| 1 | Atik et al[2] | 58 | F | Intussusception | Jejunum | LPS | Not stated |
| 2 | Papadopoulos et al[7] | 52 | M | Abdominal discomfort, vomiting | Jejunum | WDLPS | Small bowel resection |
| 3 | Rivkind et al[9] | Unknown | Unknown | Mimicking appendicitis | Small intestine | Myxoid LPS | Unknown |
| 4 | Benaragama et al[3] | 76 | M | Small bowel perforation | Iliac fossa | WDLPS | Segmental resection |
| 5 | Patel et al[8] | 59 | M | Palpable lump | Iliac fossa | DDLPS with WD component | Resection |
| 6 | Jeong et al[30] | 45 | M | Abdominal discomfort | Jejunum | DDLPS with WD component | Excision |
| 7 | Okabayashi et al[6] | 55 | M | Melena | Duodenum | DDLPS with WD component | Pancreaticoduodenectomy with partial colon resection |
| 8 | Nennstiel et al[17] | 60 | M | Gastrointestinal bleeding | Ileocecal valve | Pleomorphic LPS | Ileum segmental resection |
| 9 | Matsuo et al[5] | 84 | M | Intussusception | Ileum | DDLPS | Ileocecal resection |
| 10 | Gajzer et al[1] | 51 | M | Not provided | Small intestine | DDLPS with myofibiorblastic differentiation | Excision |
| 11 | Gajzer et al[1] | 75 | M | Intestinal obstruction | Ileum | DDLPS with WD component | Segmental ileectomy |
| 12 | Gajzer et al[1] | 53 | M | Intestinal obstruction | Jejunum | DDLPS | Segmental jejunectomy |
| 13 | Gajzer et al[1] | 68 | M | Not provided | Small intestine | DDLPS with WD component | Excision |
| 14 | Whitham et al[10] | 59 | F | Fatigue, palpitation, shortness of breath | Duodenum | DDLPS | Segmental duodenal resection and distal gastrectomy |
| 15 | Present case | 64 | M | Abdominal pain,weight loss | Duodenum | DDLPS | Pylorus-preserving pancreaticoduodenectomy |
Microscopically, the tumor exhibits variable histologic features but mostly undifferentiated pleomorphic cells with striking nuclear atypia. Such tumors with unusual locations and histopathological features may pose a diagnostic challenge. Thus, sarcomatoid carcinoma, GIST, LMS, malignant melanoma and other high-grade sarcomas should be among the list of differential diagnoses.
Sarcomatoid carcinomas should be considered as the primary differential diagnosis. These tumors are predominantly composed of poorly differentiated spindle cells and/or undifferentiated bizarre anaplastic cells resembling fibrosarcoma or LMS. Sarcomatoid carcinomas can be diagnosed by immunohistochemistry using cytokeratin to demonstrate epithelial derivation.
GIST may occur anywhere in the gastrointestinal tract with 30% arising in the small bowel, including the duodenum. The tumor also exhibits a broad morphologic spectrum. While most instances of GIST are spindle or epithelioid cell tumors, progression to high-grade sarcomatous morphology can be seen rarely. The majority of GISTs show expression of CD117, DOG1 and CD34, which may be helpful for diagnosis.
Gastrointestinal LMS is also very rare, with fewer than 100 cases reported in the English-language literature since 2000[18-21]. Typical LMS shows spindle cells with blunt-ended nuclei and eosinophilic fibrillary cytoplasm. The tumor cells are arranged in intersecting fascicles with varying degree of nuclear atypia, necrosis, and brisk mitotic activity. LMS can exhibit a poorly differentiated, pleomorphic appearance in addition to typical areas; this is known as pleomorphic LMS or dedifferentiated LMS[22,23]. For this diagnosis to be established, morphological features characteristic of classic LMS must be present, and are usually positive for at least one myogenic marker, although staining is often weaker and more focal than in typical leiomyosarcomatous areas[22,23]. The diagnosis of LMS should be made on the basis of immunohistochemical stains along with the appropriate morphological features.
Most melanomas of the gastrointestinal tract are metastases from the skin, and primary small bowel melanoma of duodenal origin is extremely rare[24,25]. Because malignant melanomas display various histologic appearance, strong clinical suspicion and precise evaluation are needed to diagnose primary duodenal melanoma.
Based on the morphology of tumor cells in DDLPS, other types of high-grade tumors such as undifferentiated pleomorphic sarcoma, malignant peripheral nerve sheath tumor, and angiosarcoma should be included among the differential diagnoses. The tumor in the present case showed positive immunoreactivity for MDM2 and CDK4. DDLPS was diagnosed based on the histological and immunohistological findings combined with MDM2 amplification via FISH.
Dual staining with MDM2 and CDK4 has been shown to be both sensitive and specific to DDLPS[26]. This is a result of the overexpression of the protein product from chromosomal amplification in the 12q13–15 region of the MDM2 and CDK4 oncogenes. Amplification of these genes can then be confirmed with FISH if diagnostic uncertainty remains[27,28].
However, MDM2 positivity by immunohistochemistry is not a specific indicator of MDM2 amplification because MDM2 positivity is observed in many other sarcomas, including WDLPS, DDLPS, intimal sarcoma, LMS, angiosarcoma, and myxofibro-sarcoma[29]. Validation of MDM2 amplification by FISH, which is currently a gold standard, is mandatory to confirm the diagnosis of DDLPS.
While the biologic behavior of DDLPS appears to be unfavorable, the most effective treatment modality is surgical resection. There have been no published studies of adjuvant therapy due to the paucity of intestinal DDLPS. Because there are only a limited number of cases, we cannot predict the outcomes for DDLPS arising in the small bowel, but we expect similar prognoses compared with soft tissue DDLPS.
We presented a unique case of DDLPS originating from the duodenum, one of the rarest locations for gastrointestinal sarcomas. DDLPS should be thoroughly distinguished from its morphological mimickers, as the tumor may be more highly aggressive and extensive than clinically and radiologically expected. While histopathologic features and immunohistochemistry offer evidence of DDLPS, MDM2 FISH is essential to confirm the diagnosis. Pathologists should keep DDLPS among the initial histologic differential diagnoses for high-grade tumors of the gastrointestinal tract.
We are grateful to the patient for allowing us to use his medical records in our case report.
| 1. | Gajzer DC, Fletcher CD, Agaimy A, Brcic I, Khanlari M, Rosenberg AE. Primary gastrointestinal liposarcoma-a clinicopathological study of 8 cases of a rare entity. Hum Pathol. 2020;97:80-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 2. | Atik M, Whittlesey RH. Liposarcoma of jejunum. Ann Surg. 1957;146:837-842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 79] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 3. | Benaragama KS, Neequaye SK, Maudgil D, Gordon AG. Small bowel liposarcoma--a rare cause of small bowel perforation. BMJ Case Rep. 2010;2010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 4. | De Monti M, Mangoni I, Gobatti D, Ghilardi G, Scorza R. [Primary jejunal liposarcoma]. Minerva Gastroenterol Dietol. 2000;46:119-122. [PubMed] |
| 5. | Matsuo K, Inoue M, Shirai Y, Kataoka T, Kagota S, Taniguchi K, Lee SW, Uchiyama K. A rare case of primary small bowel de-differentiated liposarcoma causing intussusception: A case report. Medicine (Baltimore). 2018;97:e11069. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 6. | Okabayashi T, Shima Y, Iwata J, Sumiyoshi T, Kozuki A, Tokumaru T, Hata Y, Noda Y, Inagaki T, Morishita S, Morita M. Primary liposarcoma of the duodenum: a first case presentation. Intern Med. 2013;52:2753-2757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 7. | Papadopoulos T, Kirchner T, Bergmann M, Müller-Hermelink HK. Primary liposarcoma of the jejunum. Pathol Res Pract. 1990;186:803-6; discussion 807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 8. | Patel J, Deb R, Speake W, Macculloch TA. Primary small bowel liposarcoma (atypical lipomatous tumour) with myogenic differentiation. Sarcoma. 2010;2010. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 9. | Rivkind AI, Admon D, Yarom R, Schreiber L. Myxoid liposarcoma of the small intestine mimicking acute appendicitis. Eur J Surg. 1994;160:251-252. [PubMed] |
| 10. | Whitham Z, Blackham A, Loven V. A Case of Primary Duodenal Liposarcoma. Case Rep Oncol. 2020;13:649-654. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 11. | Dei Tos AP, Marino-Enriquez A, Pedeutour F. WHO classification of tumours of soft tissue and bone. 5th ed. Lyon: IARC, 2020. |
| 12. | Crago AM, Dickson MA. Liposarcoma: Multimodality Management and Future Targeted Therapies. Surg Oncol Clin N Am. 2016;25:761-773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 119] [Article Influence: 11.9] [Reference Citation Analysis (5)] |
| 13. | Crago AM, Singer S. Clinical and molecular approaches to well differentiated and dedifferentiated liposarcoma. Curr Opin Oncol. 2011;23:373-378. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 189] [Cited by in RCA: 201] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 14. | Evans HL. Liposarcoma: a study of 55 cases with a reassessment of its classification. Am J Surg Pathol. 1979;3:507-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 402] [Cited by in RCA: 378] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 15. | Henricks WH, Chu YC, Goldblum JR, Weiss SW. Dedifferentiated liposarcoma: a clinicopathological analysis of 155 cases with a proposal for an expanded definition of dedifferentiation. Am J Surg Pathol. 1997;21:271-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 450] [Cited by in RCA: 441] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 16. | Dei Tos AP. Liposarcomas: diagnostic pitfalls and new insights. Histopathology. 2014;64:38-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 143] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 17. | Nennstiel S, Mollenhauer M, Schlag C, Becker V, Neu B, Hüser N, Gertler R, Schmid RM, von Delius S. Small bowel pleomorphic liposarcoma: a rare cause of gastrointestinal bleeding. Case Rep Gastrointest Med. 2014;2014:391871. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 18. | Yamamoto H, Handa M, Tobo T, Setsu N, Fujita K, Oshiro Y, Mihara Y, Yoshikawa Y, Oda Y. Clinicopathological features of primary leiomyosarcoma of the gastrointestinal tract following recognition of gastrointestinal stromal tumours. Histopathology. 2013;63:194-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 68] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 19. | Miettinen M, Furlong M, Sarlomo-Rikala M, Burke A, Sobin LH, Lasota J. Gastrointestinal stromal tumors, intramural leiomyomas, and leiomyosarcomas in the rectum and anus: a clinicopathologic, immunohistochemical, and molecular genetic study of 144 cases. Am J Surg Pathol. 2001;25:1121-1133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 387] [Cited by in RCA: 325] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 20. | Miettinen M, Sarlomo-Rikala M, Sobin LH, Lasota J. Gastrointestinal stromal tumors and leiomyosarcomas in the colon: a clinicopathologic, immunohistochemical, and molecular genetic study of 44 cases. Am J Surg Pathol. 2000;24:1339-1352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 175] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 21. | Miettinen M, Sobin LH, Lasota J. True smooth muscle tumors of the small intestine: a clinicopathologic, immunhistochemical, and molecular genetic study of 25 cases. Am J Surg Pathol. 2009;33:430-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 22. | Nicolas MM, Tamboli P, Gomez JA, Czerniak BA. Pleomorphic and dedifferentiated leiomyosarcoma: clinicopathologic and immunohistochemical study of 41 cases. Hum Pathol. 2010;41:663-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 46] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 23. | Oda Y, Miyajima K, Kawaguchi K, Tamiya S, Oshiro Y, Hachitanda Y, Oya M, Iwamoto Y, Tsuneyoshi M. Pleomorphic leiomyosarcoma: clinicopathologic and immunohistochemical study with special emphasis on its distinction from ordinary leiomyosarcoma and malignant fibrous histiocytoma. Am J Surg Pathol. 2001;25:1030-1038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 89] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 24. | Anvari K, Gharib M, Jafarian AH, Saburi A, Javadinia SA. Primary duodenal malignant melanoma: A case report. Caspian J Intern Med. 2018;9:312-315. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 25. | Espinoza-Ríos J, Salas Y, Leiva Reyes N, Prochazka Zárate R, García Encinas C, Cok Garcia J, Pinto Valdivia J, Bravo Paredes E, Bussalleu Rivera A. [Duodenal melanoma: a case report and review of the literature]. Rev Gastroenterol Peru. 2017;37:267-270. [PubMed] |
| 26. | Chung L, Lau SK, Jiang Z, Loera S, Bedel V, Ji J, Weiss LM, Chu PG. Overlapping features between dedifferentiated liposarcoma and undifferentiated high-grade pleomorphic sarcoma. Am J Surg Pathol. 2009;33:1594-1600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 27. | Hostein I, Pelmus M, Aurias A, Pedeutour F, Mathoulin-Pélissier S, Coindre JM. Evaluation of MDM2 and CDK4 amplification by real-time PCR on paraffin wax-embedded material: a potential tool for the diagnosis of atypical lipomatous tumours/well-differentiated liposarcomas. J Pathol. 2004;202:95-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 81] [Article Influence: 3.5] [Reference Citation Analysis (1)] |
| 28. | Sirvent N, Coindre JM, Maire G, Hostein I, Keslair F, Guillou L, Ranchere-Vince D, Terrier P, Pedeutour F. Detection of MDM2-CDK4 amplification by fluorescence in situ hybridization in 200 paraffin-embedded tumor samples: utility in diagnosing adipocytic lesions and comparison with immunohistochemistry and real-time PCR. Am J Surg Pathol. 2007;31:1476-1489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 261] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 29. | Sciot R. MDM2 Amplified Sarcomas: A Literature Review. Diagnostics (Basel). 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 61] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 30. | Jeong D, Kim SW. Dedifferentiated subserosal liposarcoma of the jejunum: sonographic and computed tomographic findings with pathologic correlation. Clin Imaging. 2012;36:390-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Esch M, Sperti C, Wang XJ S-Editor: Wang JL L-Editor: A P-Editor: Wang JL