Published online Feb 26, 2022. doi: 10.12998/wjcc.v10.i6.1929
Peer-review started: August 3, 2021
First decision: October 20, 2021
Revised: November 1, 2021
Accepted: January 14, 2022
Article in press: January 14, 2022
Published online: February 26, 2022
Processing time: 204 Days and 7.9 Hours
Ankylosing spondylitis (AS) is an autoimmune disease characterized by sacroiliitis and spondylitis, with a few hematological abnormalities. Myelodysplastic syndromes (MDS) are a heterogeneous group of hematopoietic stem cell disorders with frequent autoimmune phenomena. The relationship between AS and MDS remains unknown.
We describe a rare case of concurrent AS and MDS. An 18-year-old man with low back pain and anemia was diagnosed with AS; however, the cause of anemia could not be determined by the first bone marrow examination. He recovered from anemia and the symptoms of AS resolved after treatment with etanercept, glucocorticoid, and blood transfusion, but he developed pancytopenia with an increased myeloblast count (from 2.5% to 9%). Chromosome analysis revealed del(7q) and trisomy 8. Refractory anemia with excess of blasts-1 (RAEB-1)/MDS was confirmed by repeating the bone marrow examination. He became blood transfusion-dependent and received decitabine-based chemotherapy but eventually died.
We suspect that AS may be an early autoimmune phenomenon related to MDS. However, a condition of coexistence cannot be excluded.
Core Tip: We report a case of simultaneous presentation of ankylosing spondylitis (AS) and myelodysplastic syndrome (MDS). Patients with MDS may have autoimmune manifestations. AS may be an early autoimmune phenomenon associated with MDS; however, the possibility of a coincidence cannot be excluded. Most importantly, AS may cause anemia, but it is usually mild. If a patient with AS presents with severe anemia, it must be diagnosed as a hematopoietic system pathology. Chemotherapy or bone marrow transplantation should be considered for acute leukemia.
- Citation: Xu GH, Lin J, Chen WQ. Concurrent ankylosing spondylitis and myelodysplastic syndrome: A case report. World J Clin Cases 2022; 10(6): 1929-1936
- URL: https://www.wjgnet.com/2307-8960/full/v10/i6/1929.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i6.1929
Myelodysplastic syndromes (MDS) are a heterogeneous group of hematopoietic stem cell disorders characterized by abnormal hematopoiesis and peripheral cytopenia. In some patients, MDS may ultimately transform into acute myeloid leukemia. Bone marrow failure results in transfusion dependence and infection. Autoimmune syndromes often develop in patients with MDS, and various autoimmune phenomena, including acute systemic vasculitis, chronic autoimmune disorders, connective tissue disorders, and asymptomatic immunologic abnormalities, have been reported[1,2]. Evidence shows immune dysregulation in patients with MDS, which may cause autoimmune myelosuppression and immune-mediated cytopenias[3,4].
Ankylosing spondylitis (AS), an autoimmune disease of unknown etiology, is characterized by sacroiliitis and spondylitis, along with few systemic complications and hematological or biochemical abnormalities. Mild anemia has been reported in some patients with AS. However, the concurrence of AS and MDS in the same patient has been rarely described in the literature. The relationship between the two diseases remains unknown, and the therapeutic strategy and prognosis are unclear. This case report of a simultaneous presentation of AS and MDS aims to describe and clarify the relationship between them.
An 18-year-old man complained of intermittent low back pain and fatigue.
In 2008, an 18-year-old man complained of intermittent low back pain with frequent relapses, relieved by non-steroidal anti-inflammatory drugs (NSAIDs). In July 2009, he was admitted to a local hospital due to severe low back pain and fatigue. Initial laboratory evaluation revealed severe anemia (Hb concentration, 40 g/L), increased mean corpuscular volume (MCV; 122.7 fl); mild leucopenia (WBC 3.8 × 109/L); and normal platelet count. His blood folate and vitamin B12 concentrations were slightly decreased. However, he refused to undergo a bone marrow test. He was treated with red blood cell transfusion plus folic acid and vitamin B12; however, his symptoms were not relieved. Subsequently, in August 2009, he was referred to our hematology department for evaluation and further management of the severe anemia. He denied having traveled or being in contact with patients with tuberculosis or other infectious diseases recently. No sexual history was reported. His mother and father were healthy. There was no positive family history.
The patient had no previous disease history.
No personal and family disease history.
Examination revealed pronounced skin pallor, a body temperature of 38.2 °C, and a pulse rate of 102 bpm. No dysmorphic features were observed.
Hematological tests showed normocytic anemia (Hb 60.7 g/L), normal MCV, and leukocyte and platelet counts. Vitamin B12 and folate levels were increased. The blood reticulocyte count was 2.4%, serum ferritin concentration was 405.2 ng/mL, ESR was 160 mm/h, and C-reactive protein (CRP) concentration was 145.42 mg/L. The Rous test and the direct and indirect antiglobulin Coombs tests were negative. The CD55 and CD95 expression on red blood cells and granulocytes, and lactic dehydrogenase and bilirubin values were normal. The blood coagulation panel and the urine test results were normal. Blood creatinine, alanine aminotransferase, and aspartate aminotransferase levels were normal. Furthermore, titers for antinuclear antibody, anti-extractable nuclear antigen antibody, antineutrophil cytoplasmic antibody, rheumatoid factor, immunoglobulin, and complement factors (C3/C4) were normal. Tests for human immunodeficiency virus, syphilis, hepatitis B, hepatitis C, and Parvovirus B19 were negative. Blood bacteria culture and the tuberculin purified protein derivative test were also negative. Tumor markers such as AFP, CEA, CA125, CA199, and PSA were all negative.
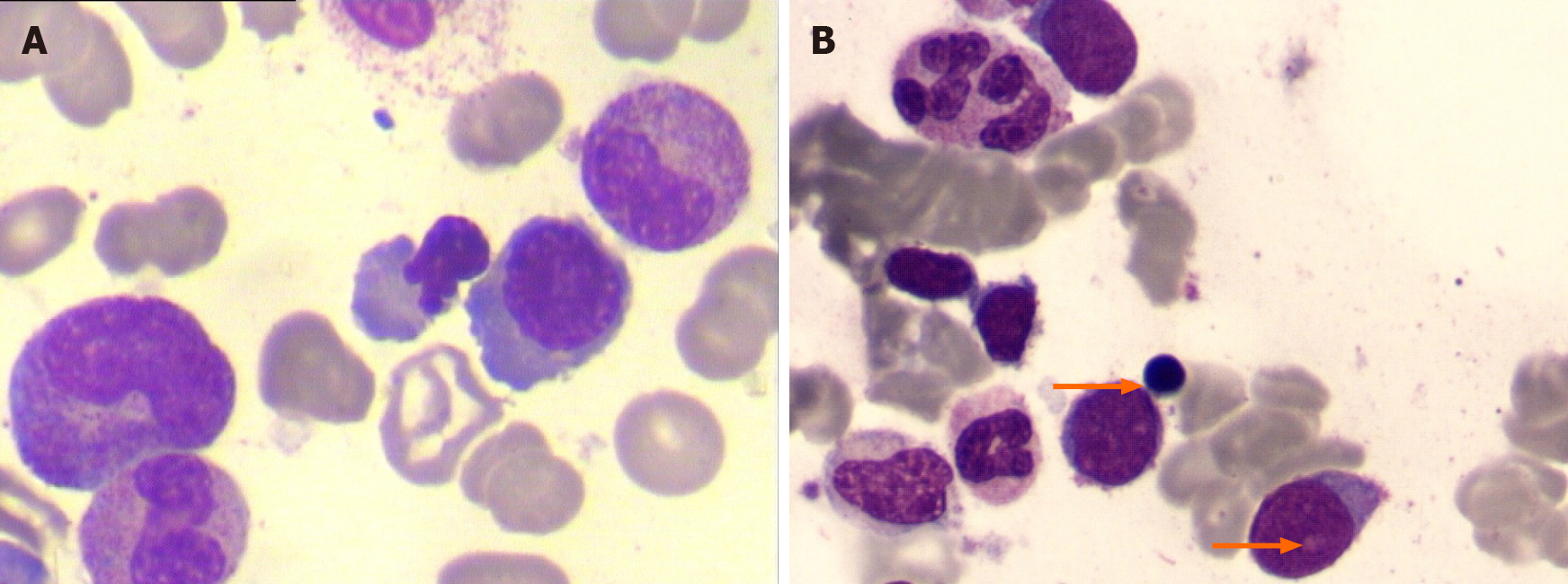
Bone marrow aspiration revealed hypocellular marrow with single erythroid dysplasia; however, ringed sideroblasts were absent in the sample obtained in August 2009 (Figure 1A). The cytogenetic test was normal. Therefore, a diagnosis of MDS could not be established. The patient received a blood transfusion, NSAIDs, analgesics, and antibiotics (cefuroxime, sulperazone, meropenem, imipenem/cilastatin, and azithromycin). However, the patient still experienced a low-grade fever and pain.
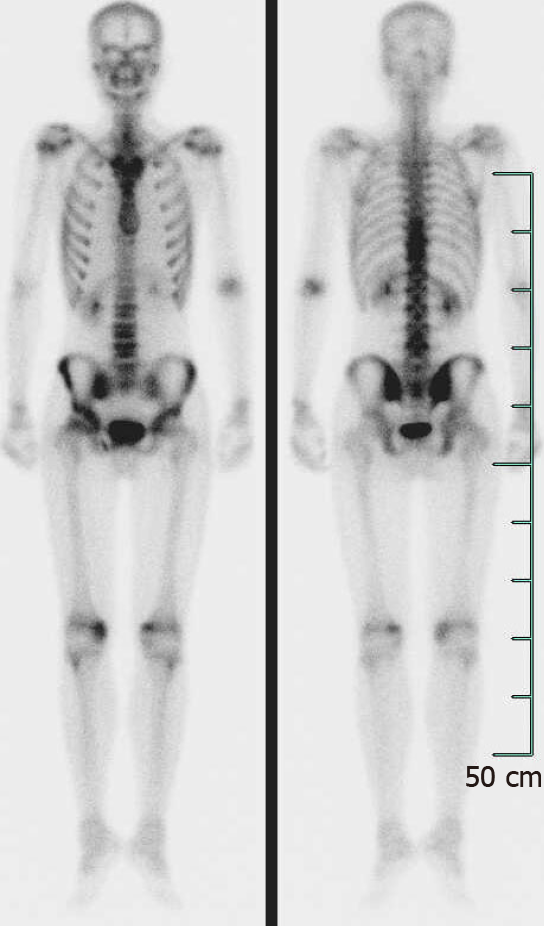
Abdominal ultrasonography showed splenomegaly (5.7 cm thickness). Computed tomography (CT) of the chest and the abdominal organs, and magnetic resonance imaging of the thoracic and lumbar vertebrae did not show any abnormalities. Tc-99m bone scintigraphy imaging showed active bone metabolism of the T9-11 and L4 vertebrae, the right sacroiliac joint, and both the knee joints (Figure 2).
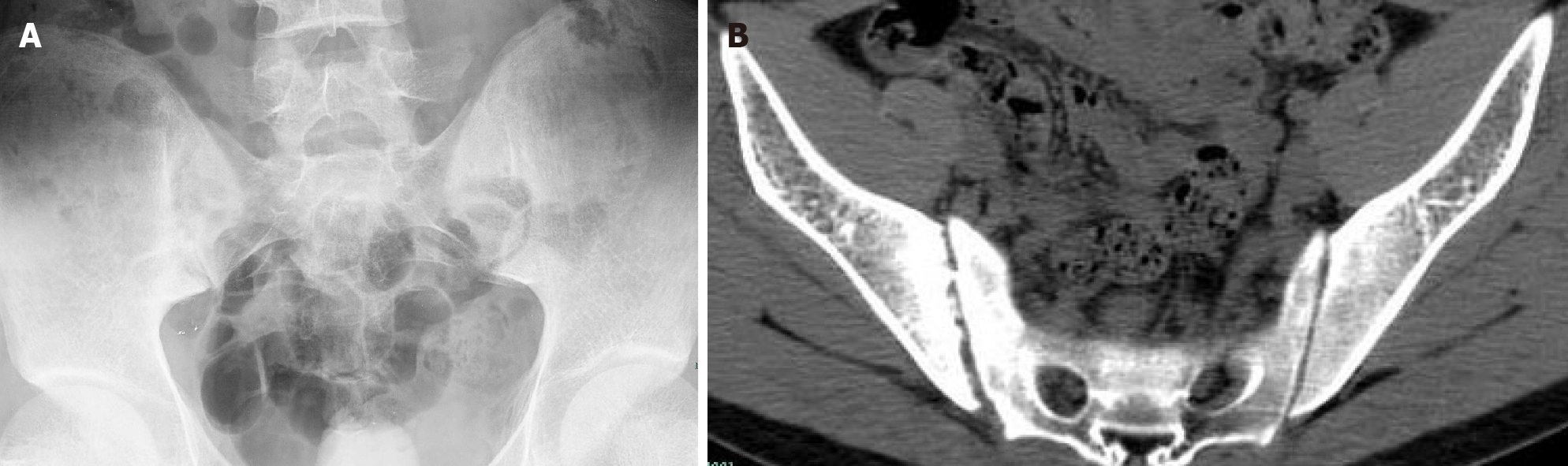
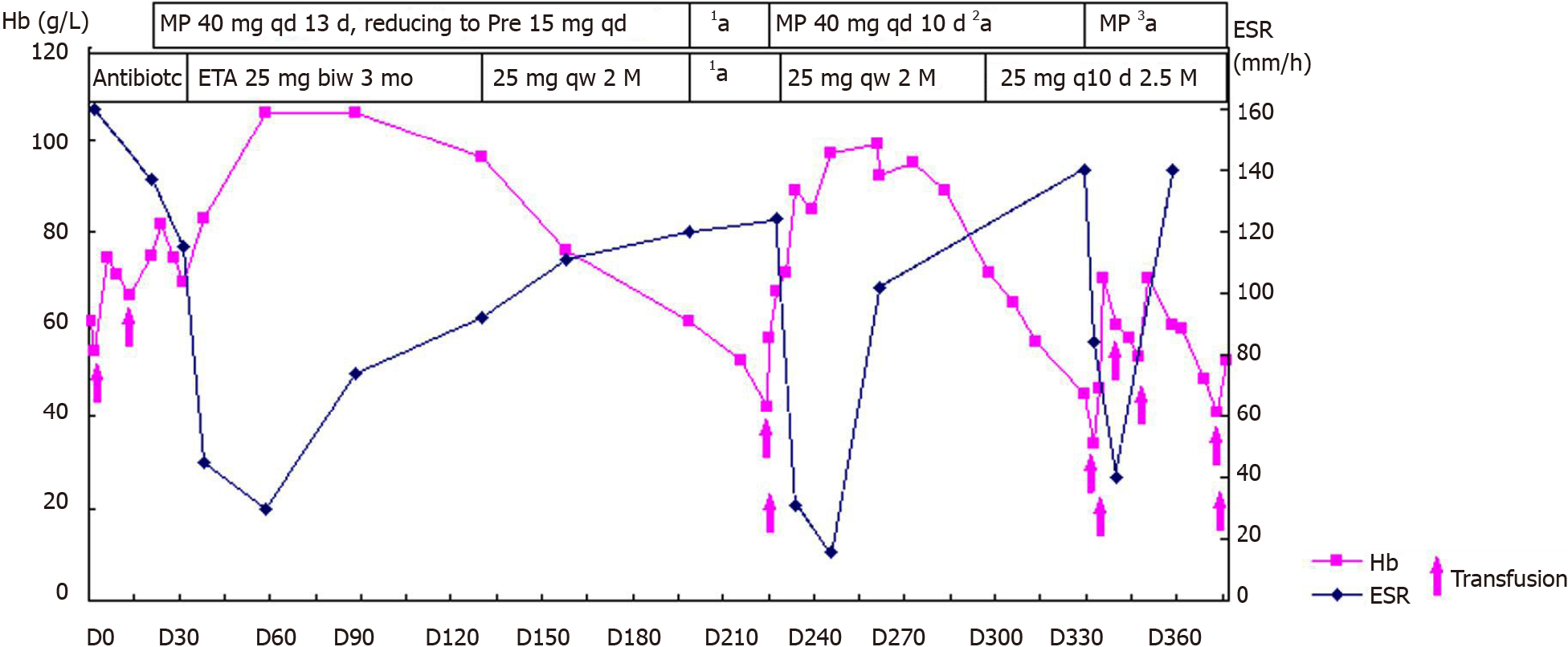
On the advice of rheumatologists, the patient was referred to the rheumatology department for further evaluation. The patient had a 1-year history of low back pain, which was especially painful at night when turning over. He had morning stiffness of the back and hip, swelling, and tenderness in the right knee. Physical examination revealed that the range of motion of the lumbar spine was limited; however, no enthesitis or uveitis was noted. Patrick’s maneuver elicited pain in the right sacroiliac joint. HLA-B27 was positive, and pelvis X-ray showed bilateral asymmetric sacroiliitis, grade 3 and grade 2 for the right side and left side, respectively, according to the modified New York criteria[5] (Figure 3A). Pelvis CT showed narrowing of the space of the sacroiliac joints, erosion, and subchondral sclerosis of the right iliac side with an irregular margin (Figure 3B). Hence, the diagnosis of AS was established. Anemia and fever were interpreted as manifestations of AS-related systemic inflammation. Although corticosteroids have a misleading effect on the bone marrow, he was treated with etanercept (25 mg twice a week) and methylprednisolone (40 mg daily) to suppress systemic inflammation. He also received a red blood cell transfusion. The patient reported no pain and fever, and his Hb level steadily increased to approximately 100 g/L. CRP concentration and ESR were nearly normal. Three months later, etanercept was reduced to 25 mg once a week with tapering of the glucocorticoid. Sulphasalazine (1500 mg daily) was introduced for a short time. However, this relatively safe Hb level was maintained for only 3 mo. Another month later, the Hb level was at 76 g/L.
At the same time, the patient self-discontinued etanercept and prednisone. His low back pain relapsed with fever; the Hb level decreased to 60.8 g/L. After another month, the Hb level was at 42 g/L, reticulocyte count was 3%, ESR increased to 124 mm/h, and CRP concentration increased to 63 mg/L. The patient was re-admitted to our rheumatology department in March 2010. Subsequently, the second bone marrow examination revealed trilineage dysplasia with 2.5% of blast cells. The bone marrow was hypercellular. Immunophenotyping showed that primitive myeloid cells were approximately 4.18%, and the suggested diagnosis was refractory cytopenia with multilineage dysplasia subtype of MDS[6].
We made a diagnosis of MDS based on the findings in bone marrow and cytogenetic abnormalities; and AS based on the clinical feature of low back pain and imaging finding of sacroiliitis.
Following a hematology consultation, it was suggested that the MDS might be related to the AS; however, cytogenetic testing was not offered. The patient still had back pain with high ESR and CRP levels. He had a high AS disease activity score. Subsequently, the patient received a transfusion and was administered methylprednisolone (40 mg daily) again. There were significant improvements in the symptoms; the ESR and CRP concentrations decreased (16 mm/h and 15.6 mg/L, respectively). Subsequently, etanercept (25 mg weekly) was added with a tapering of the glucocorticoid. However, an Hb level of approximately 100 g/L could be maintained for only 1 mo.
After another 2 mo, the Hb level and platelet count decreased (34 g/L and 75 × 109/L, respectively), and ESR increased to 84 mm/h, and thus, the patient was admitted to the hospital for the third time in June 2010. He had no arthralgia. The etanercept and prednisone dosages were reduced to 25 mg once in 10 d and 25 mg per day, respectively. The third bone marrow examination showed hypercellular marrow with trilineage dysplasia, with an increased myeloblast count of 9%. Immunophenotyping showed primitive myeloid cells (10.26%) and abnormal mature or immature granulocytes (33.3%). The patient was diagnosed with refractory anemia with excess blasts-1 (RAEB-1)[6] (Figure 1B). Chromosome analysis revealed a karyotype of 47, XY, der(7)t(1;7)(q10;q10), +8 in all ten metaphases. Fluorescent in situ hybridization revealed del(7q) and trisomy 8 cytogenetic abnormalities in the bone marrow. The International Prognostic Scoring System (IPSS) score was 2, and it was categorized as intermediate-2 risk[7]. Even after more than six transfusions over 50 d (about three units of red blood cell each time), the Hb level remained approximately 50 g/L. Platelet count decreased to 24 × 109/L. The patient became transfusion-dependent and was referred to the hematology department for decitabine chemotherapy.
Unfortunately, 6 mo later, the patient died due to cerebral hemorrhage. The main cause of hemorrhage was a very low platelet count due to MDS progression.
Anemia in AS is not rare but is generally mild[8]. The exact prevalence of anemia in patients with AS is unknown. There are multiple potential causes of anemia in patients with AS, including chronic disease and the use of NSAIDs that can cause gastrointestinal bleeding[9]. In our patient, severe anemia could not be attributed to the aforementioned reasons. Furthermore, the anemia was accompanied by mild to severe thrombocytopenia. White blood cell and platelet counts are usually normal in AS, and the bone marrow is normal or hypercellular[10]. Radiotherapy, which was once adopted for treating AS patients, might produce extensive chromosomal damage, probably increasing the risk of leukemia in these patients; hence, its use has been discontinued[11]. When etanercept was administered to our patient, he had serious anemia. Phenylbutazone used to treat AS may increase the incidence rate of acute myeloid leukemia (AML)[12]. However, our patient did not receive phenylbutazone. There were no other related drugs, such as cyclophosphamide or radioactive agents, that could lead to anemia or MDS.
As the disease developed, single erythroid dysplasia progressed to trilineage dysplasia in our patient. The myeloblast counts rapidly increased from 2.5% to 9%. Del(7q) and trisomy 8 of the typical chromosome abnormalities were found in the bone marrow. The presence of del(7q) and trisomy 8 simultaneously suggested the diagnosis of de novo MDS.
Thus, there was no evidence that MDS was secondary to AS. However, autoimmune manifestations are often reported in MDS[2,13,14]. Sacroiliitis was described as a paraneoplastic phenomenon of de novo acute leukemia[15-17]. In our case, severe anemia appeared from the beginning. As noted, the anemia that occurs in patients with AS is generally mild. This may suggest a diagnosis of MDS from the beginning; however, the first bone marrow examination in our case did not confirm a diagnosis of MDS. We suspect that AS may be an early autoimmune phenomenon related to MDS. Recently, a paper reports that the mutation of an epigenetic regulator may increase the risk of autoimmune diseases, such as AS in patients with MDS[18].
One study reported that HLA-B27 carriers might have an increased risk of hematological malignancies[19]. Although they were uncertain about the relationship between AS and MDS, Lee et al[20] believed that HLA-B27 might provide a link between AS and MDS. However, HLA-B27 positivity did not affect the outcome of patients with leukemia who received an allogeneic transplant[21]. Thus, the possibility of a coincidental association of MDS and AS cannot be excluded.
Considering the patient’s young age, there is a high probability of an underlying germline cancer-predisposing syndrome[22]. However, our patient did not have any dysmorphic features. His mother and father were healthy. Regrettably, we did not finish a high throughput sequencing analysis to find any germline mutation.
Our patient had a good response to the combination therapy of etanercept, glucocorticoid, and transfusion in the early stage, but his Hb level remained unstable. The lifespan of red blood cells is about 120 da, and our patient experienced a transient improvement of anemia mainly due to the transfusion. Furthermore, his transfusion dependence increased with the disease progression.
The efficacy of the etanercept and glucocorticoid therapy in MDS cannot be denied. Sufficient doses of the etanercept (25 mg twice a week for 3 mo) and glucocorticoid in the early phase may be useful in maintaining the Hb level. When etanercept was reduced to 25 mg once a week and then once every 10 d, the joint symptoms im
Some patients with refractory anemia or refractory anemia with ring sideroblasts may benefit from glucocorticoid therapy; however, the result is not encouraging. A combination of anti-thymocyte globulin plus etanercept can offer effective therapy for some patients with MDS, with an overall response rate of 56%[24]. Striking hematological improvements and loss of transfusion dependence were found in the responding patients.
The prognosis of MDS patients with autoimmune manifestations appeared to be closely related to the IPSS subcategory of the underlying hematological malignancy[25]. Thus, our patient with intermediate-2-risk MDS as defined by the IPSS category had a poor prognosis. Considering the patient’s young age and the presence of more than three cytogenetic aberrations, an allogeneic transplant would have been the gold standard for treatment[26]. The transplant plan was discussed with the patient and his family, but they did not accept it at that time. They wanted to see the effects of chemotherapy before deciding on the transplant. However, the severe thrombocytopenia resulted in bleeding and death, and the patient missed the opportunity for treatment with an allogeneic transplant.
In summary, we present a rare case of simultaneous presentation of AS and MDS in the same patient with positive HLA-B27. We suspect that the AS may be an early autoimmune phenomenon related to MDS; however, the possibility of a coincidence cannot be excluded. AS can cause anemia, but it is usually mild. Therefore, if a patient with AS presents with severe anemia, it must be diagnosed as a hematopoietic system pathology.
| 1. | Giannouli S, Voulgarelis M, Zintzaras E, Tzioufas AG, Moutsopoulos HM. Autoimmune phenomena in myelodysplastic syndromes: a 4-yr prospective study. Rheumatology (Oxford). 2004;43:626-632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 98] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 2. | Enright H, Miller W. Autoimmune phenomena in patients with myelodysplastic syndromes. Leuk Lymphoma. 1997;24:483-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 61] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 3. | Banerjee T, Calvi LM, Becker MW, Liesveld JL. Flaming and fanning: The Spectrum of inflammatory influences in myelodysplastic syndromes. Blood Rev. 2019;36:57-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 4. | Chamuleau ME, Westers TM, van Dreunen L, Groenland J, Zevenbergen A, Eeltink CM, Ossenkoppele GJ, van de Loosdrecht AA. Immune mediated autologous cytotoxicity against hematopoietic precursor cells in patients with myelodysplastic syndrome. Haematologica. 2009;94:496-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 55] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 5. | van der Linden S, Valkenburg HA, Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum. 1984;27:361-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3782] [Cited by in RCA: 4194] [Article Influence: 99.9] [Reference Citation Analysis (1)] |
| 6. | Vardiman JW, Thiele J, Arber DA, Brunning RD, Borowitz MJ, Porwit A, Harris NL, Le Beau MM, Hellström-Lindberg E, Tefferi A, Bloomfield CD. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009;114:937-951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2981] [Cited by in RCA: 3222] [Article Influence: 189.5] [Reference Citation Analysis (0)] |
| 7. | Greenberg P, Cox C, LeBeau MM, Fenaux P, Morel P, Sanz G, Sanz M, Vallespi T, Hamblin T, Oscier D, Ohyashiki K, Toyama K, Aul C, Mufti G, Bennett J. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood. 1997;89:2079-2088. [PubMed] |
| 8. | Zviahina OV, Shevchuk SV, Kuvikova IP, Segeda IS. Anemia in patients with ankylosing spondylitis, association with the activity of the inflammatory process and the severity of the disease. Wiad Lek. 2020;73:715-721. [PubMed] |
| 9. | Fraenkel PG. Anemia of Inflammation: A Review. Med Clin North Am. 2017;101:285-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 140] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 10. | Boyraz I, Koç B, Boyacı A, Tutoğlu A, Sarman H, Ozkan H. Ratio of neutrophil/Lymphocyte and platelet/Lymphocyte in patient with ankylosing spondylitis that are treating with anti-TNF. Int J Clin Exp Med. 2014;7:2912-2915. [PubMed] |
| 11. | Wick RR, Nekolla EA, Gaubitz M, Schulte TL. Increased risk of myeloid leukaemia in patients with ankylosing spondylitis following treatment with radium-224. Rheumatology (Oxford). 2008;47:855-859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 12. | Calabro JJ, Maltz BA. Leukemia and ankylosing spondylitis. N Engl J Med. 1970;282:1324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 13. | Hamblin TJ. Myelodysplasia. Br J Hosp Med. 1987;38:558-561. [PubMed] |
| 14. | Marisavljević D, Kraguljac N, Rolović Z. Immunologic abnormalities in myelodysplastic syndromes: clinical features and characteristics of the lymphoid population. Med Oncol. 2006;23:385-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 15. | Hoshino T, Matsushima T, Saitoh Y, Yamane A, Takizawa M, Irisawa H, Saitoh T, Handa H, Tsukamoto N, Karasawa M, Murakami H, Nojima Y. Sacroiliitis as an initial manifestation of acute myelogenous leukemia. Int J Hematol. 2006;84:421-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 16. | Xu D, Xu G, Xu L, Cao H, Xu B, Chen W, Sun C, Yue L, Lin J. Acute lymphocytic leukemia mimicking spondyloarthritis in an adolescent: A case report and review of the literature. Oncol Lett. 2016;11:1143-1145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 17. | Liu W, Chen G, Xu B, Sun S, Tian J, Zhang Y. Early stage Acute B lymphocytic leukemia presenting with symptoms of ankylosing spondylitis (AS): A case report. Medicine (Baltimore). 2020;99:e19806. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 18. | Oh YJ, Shin DY, Hwang SM, Kim SM, Im K, Park HS, Kim JA, Song YW, Márquez A, Martín J, Lee DS, Park JK. Mutation of ten-eleven translocation-2 is associated with increased risk of autoimmune disease in patients with myelodysplastic syndrome. Korean J Intern Med. 2020;35:457-464. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 19. | Au WY, Hawkins BR, Cheng N, Lie AK, Liang R, Kwong YL. Risk of haematological malignancies in HLA-B27 carriers. Br J Haematol. 2001;115:320-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 20. | Lee J, Kim H, Ahn JK, Hwang JW, Jang JH, Koh EM, Cha HS. Ankylosing spondylitis in a patient with myelodysplastic syndrome: an association with HLA-B27 or coincidence? Rheumatol Int. 2009;29:689-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 21. | Huang SJ, Chan J, Bruyère H, Allan LL, Gerrie AS, Toze CL. Chronic lymphocytic leukemia patients with HLA-B27 referred for allogeneic hematopoietic stem cell transplantation do not have worse outcomes: Results of a population-based case series analysis in British Columbia, Canada. Leuk Res. 2019;84:106193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 22. | Klco JM, Mullighan CG. Advances in germline predisposition to acute leukaemias and myeloid neoplasms. Nat Rev Cancer. 2021;21:122-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 117] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 23. | Braun J, van der Heijde D, Doyle MK, Han C, Deodhar A, Inman R, de Vlam K, Burmester GR, Van den Bosch F, Xu S, Visvanathan S, Rahman MU. Improvement in hemoglobin levels in patients with ankylosing spondylitis treated with infliximab. Arthritis Rheum. 2009;61:1032-1036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 26] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 24. | Scott BL, Ramakrishnan A, Fosdal M, Storer B, Becker P, Petersdorf S, Deeg HJ. Anti-thymocyte globulin plus etanercept as therapy for myelodysplastic syndromes (MDS): a phase II study. Br J Haematol. 2010;149:706-710. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 43] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 25. | Enright H, Jacob HS, Vercellotti G, Howe R, Belzer M, Miller W. Paraneoplastic autoimmune phenomena in patients with myelodysplastic syndromes: response to immunosuppressive therapy. Br J Haematol. 1995;91:403-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 152] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 26. | de Witte T, Bowen D, Robin M, Malcovati L, Niederwieser D, Yakoub-Agha I, Mufti GJ, Fenaux P, Sanz G, Martino R, Alessandrino EP, Onida F, Symeonidis A, Passweg J, Kobbe G, Ganser A, Platzbecker U, Finke J, van Gelder M, van de Loosdrecht AA, Ljungman P, Stauder R, Volin L, Deeg HJ, Cutler C, Saber W, Champlin R, Giralt S, Anasetti C, Kröger N. Allogeneic hematopoietic stem cell transplantation for MDS and CMML: recommendations from an international expert panel. Blood. 2017;129:1753-1762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 287] [Article Influence: 31.9] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: Member of Chinese Society of Immunology, M200622928M.
Specialty type: Rheumatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Son TQ, Tsilivigkos C S-Editor: Liu JH L-Editor: Wang TQ P-Editor: Liu JH