Published online Feb 6, 2022. doi: 10.12998/wjcc.v10.i4.1401
Peer-review started: September 1, 2021
First decision: November 22, 2021
Revised: December 6, 2021
Accepted: December 23, 2021
Article in press: December 23, 2021
Published online: February 6, 2022
Processing time: 144 Days and 11.7 Hours
The endovascular repair of juxtarenal abdominal aortic aneurysms (JAAA) usually requires combination treatment with various stent graft modifications to preserve side branch patency. As a feasible technique, according to the situation, antegrade in situ laser fenestration still needs to be improved.
This report describes a case that was successfully treated with endovascular repair facilitated by antegrade in situ laser fenestration while maintaining renal arterial flow. Laser fenestration was performed using a steerable sheath positioned in the stent graft lumen in front of the renal artery ostium. With the bare stent region unreleased, renal artery perfusion could be maintained and accurate positioning could be achieved by angiography in real time.
This study suggests the feasibility and short-term safety of this novel antegrade in situ laser fenestration technique for select JAAA patients.
Core Tip: This report describes the feasibility and short-term safety of a novel antegrade in situ laser fenestration technique. During the operation, laser fenestration was performed using a steerable sheath positioned in the stent graft lumen in front of the renal artery ostium. With the bare stent region unreleased, renal artery perfusion could be maintained and accurate positioning could be achieved by angiography in real time. The technique is uncomplicated and has potential as an alternative approach when dealing with a hostile proximal aortic neck during endovascular aortic repair.
- Citation: Wang ZW, Qiao ZT, Li MX, Bai HL, Liu YF, Bai T. Antegrade in situ laser fenestration of aortic stent graft during endovascular aortic repair: A case report. World J Clin Cases 2022; 10(4): 1401-1409
- URL: https://www.wjgnet.com/2307-8960/full/v10/i4/1401.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i4.1401
An inadequate proximal neck is the most common anatomical challenge during the endovascular repair of juxtarenal abdominal aortic aneurysms (JAAAs)[1]. Retrograde in situ fenestration (ISF) has been reported to preserve the major aortic branches during thoracic endovascular aortic repair (TEVAR) effectively and safely[2]; however, because of the lack of downstream branch artery access, retrograde revascularization cannot be achieved easily in EVAR without a laparotomy or retroperitoneal incision[3-5]. If endovascular therapy is deemed to be the best treatment option, antegrade ISF may be an ideal method to preserve the patency of visceral arteries. Many animal experiments[6-9], benchtop studies[10-12], and clinical studies[13-17] have demon
A 55-year-old man presented to the emergency department of our hospital complai
The patient’s symptoms started a week prior with recurrent episodes of lower abdomen pain and distention, which had worsened in the last 4 h. The patient had no fever or diarrhea. Color Doppler ultrasound in a community hospital showed an abdominal aortic aneurysm (AAA).
The patient had a clear previous medical history.
However, he had smoked at least five cigarettes a day for ten years.
The abdomen was soft, with mild periumbilical tenderness and no rebound tenderness. A pulsatile abdominal mass could be palpated. The liver and spleen were impalpable.
The patient’s blood pressure was approximately 108/80 mmHg. The blood routine examination findings were normal. The prothrombin and partial thromboplastin times were normal, and the D-dimer level was slightly increased, at 0.47 mg/L. The serum C-reactive protein level was increased, at 66.48 mg/dL (normal range: < 5 mg/dL), and the erythrocyte sedimentation rate was 23 mm/h. The results of blood biochemistry, urinalysis, electrocardiography and arterial blood gas analysis were also normal.
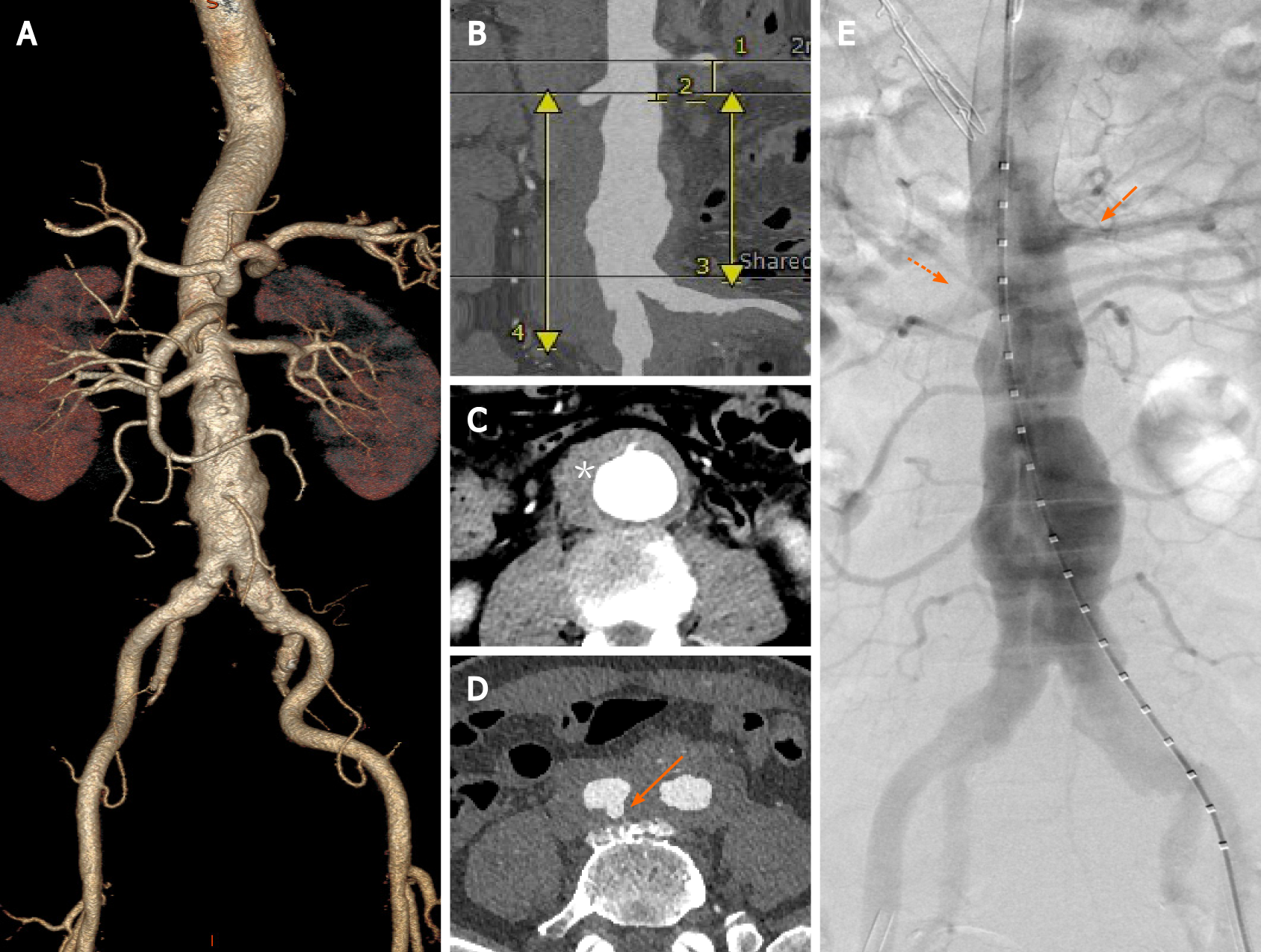
Preoperative computed tomography angiography (CTA) showed a JAAA with a diameter of 50.4 mm, a thick hematoma and multiple penetrating aortic ulcers (PAUs). Preoperative sizing showed an aortic ulcer with a hematoma below the right renal ostium, a short (4-mm-long) infrarenal aortic neck, and a proximal healthy landing zone of approximately 16.0 mm between the two renal arteries. The diameter of RRA was 6.1 mm. The diameter of the aortic bifurcation was 32 mm. The diameter of the vessel at the lower edge of the left renal artery was 20.9 mm. The diameter of the vessel at the lower edge of the RRA was 20.5 mm. The distance from the lower edge of the RRA to the aortic bifurcation was 91.2 mm. The distance from the lower edge of the RRA to the bifurcation of the right iliac artery was 124.3 mm (Figure 1A-D).
After a thorough patient evaluation, we discussed the possible treatment alternatives. The PAUs involved the RRA, so open surgery was likely to be more traumatic and technically complex than traditional infrarenal repair[18]. EVAR has been widely accepted as the treatment of choice for patients who are unfit for open surgery. To achieve an adequate proximal seal, the RRA might need to be covered and then revascularized. "Off-the-shelf" techniques (such as chimney, periscope, and sandwich techniques) have a high risk of type IA endoleakage and reintervention and are thus not preferred. Customized fenestrated and branched endografts are expensive and not available for the treatment of acute syndromes. Even physician-modified stent grafts still need to be rotated and moved as needed for cannulation of the visceral vessels. In this case, the enhanced abdominal pain and thick perivascular hematoma suggested that the risk of aneurysm rupture was high. Therefore, for early and minimally invasive treatment, after obtaining informed consent, EVAR with antegrade ISF was planned.
Confirmed JAAA combined with PAUs.
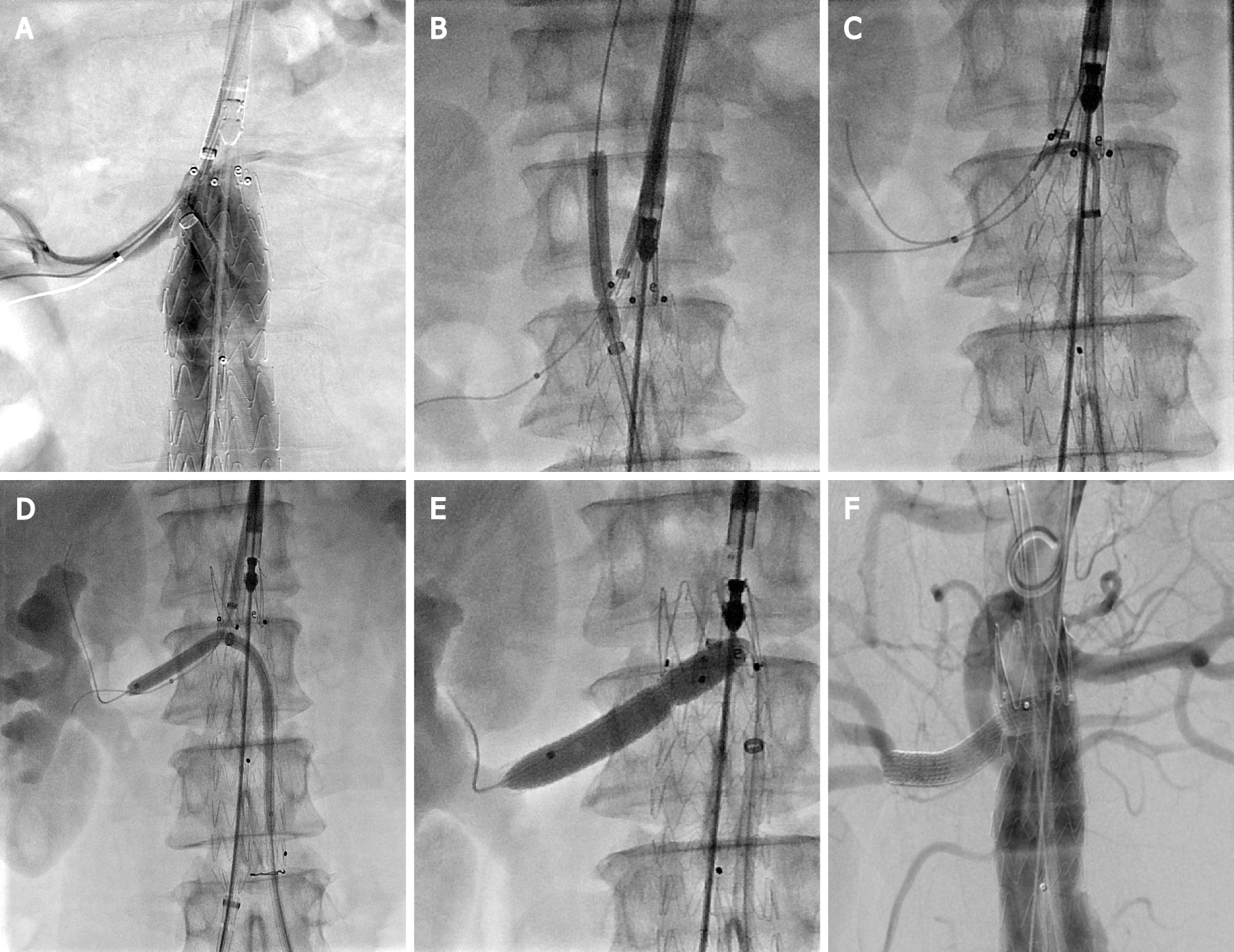
Emergency surgery was performed 24 h after admission. The surgical procedure was as follows. A sizing catheter was advanced via left femoral access, allowing for angiography and pertinent measurements to be achieved (Figure 1E). A long sheath (Flexor, Cook Medical) was used to deploy a guidewire and a balloon catheter into the RRA via brachial access to allow RRA salvage in case of fenestration failure. First, the right internal iliac artery was embolized. An Endurant II stent graft (25 mm × 13 mm × 166 mm, Medtronic Vascular, Inc.) was advanced through the right femoral sheath and deployed just below the left renal artery. The short leg of the stent graft was released while leaving the bare stent in place. Thereafter, a 10-F steerable sheath (FuStar, Lifetech Scientific Corp.) was inserted into the stent graft from the left groin through the short leg. The ostium of the RRA was displayed in real time by contrast injection from the brachial access point. Meanwhile, the tip of the steerable sheath was adjusted to align with the ostium of the RRA (Figure 2A), and the site of perforation was determined via 2 different fluoroscopic projections. A 4-mm balloon catheter (Bard Rival, Bard Peripheral Vascular) was passed via the sheath followed by a laser fiber (GIGAA Laser, Wuhan, China), which was calibrated to deliver energy pulses of 18 W in 3 s, to fenestrate the membrane of the thoracic aortic stent graft[2]. After fene
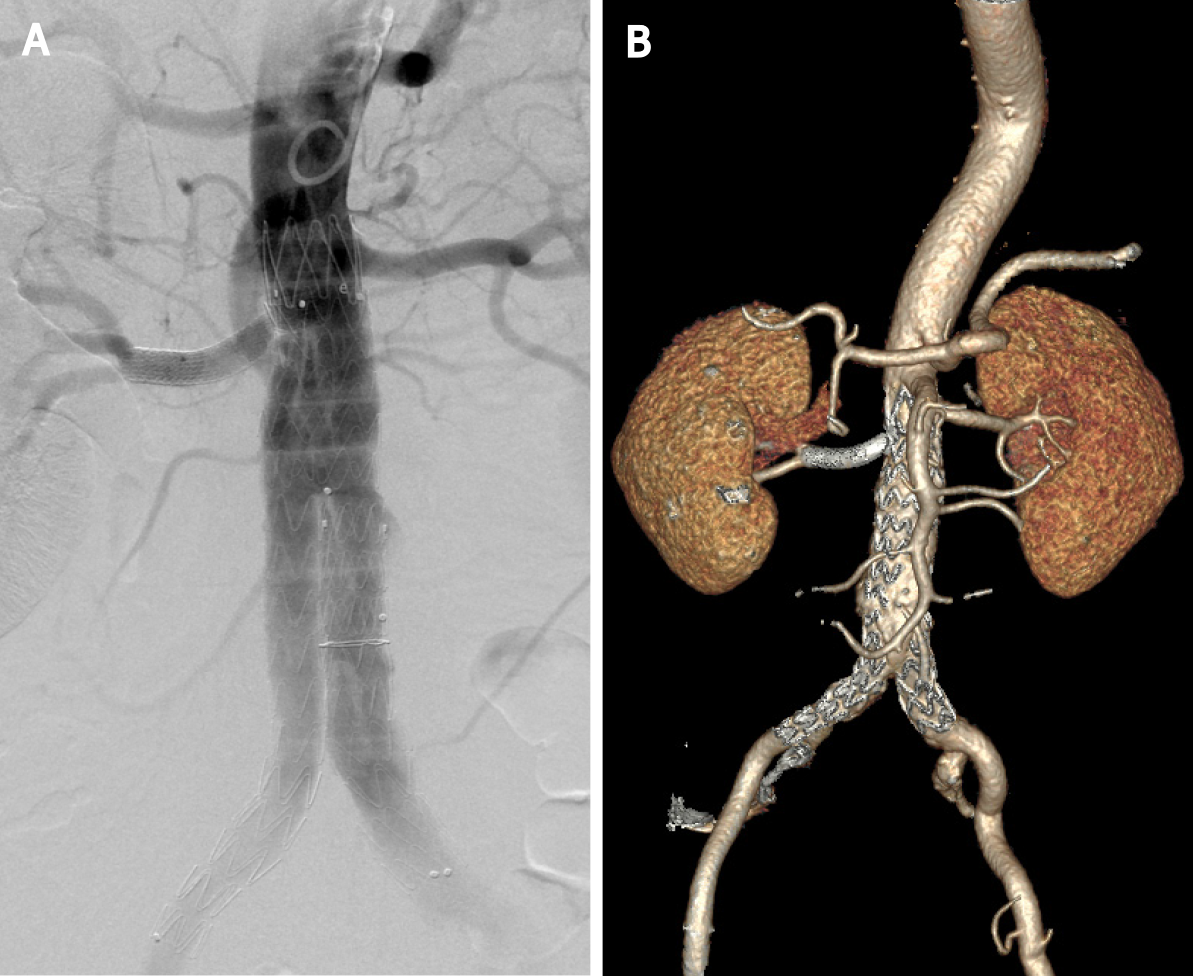
The patient had an uneventful recovery and was continued on aspirin daily. The total procedure time was 2 h. The fluoroscopy time was 45 min, and the total amount of contrast used was 100 mL. The estimated blood loss was 20 cc. Because the left renal artery was not involved, RRA perfusion was maintained during the operation. Postoperatively, there was no abnormal renal function, and the patient was discharged home on postoperative day 7. The 1-year follow-up CTA confirmed patency the of both renal arteries and the absence of endoleakage (Figure 3B).
The endovascular repair of complex aortic aneurysms involving renal and/or visceral branches usually requires combination treatment with various types of stent graft modifications to preserve side branch patency. These techniques feature devices with parallel grafts, fenestrations, or branches. The parallel graft technique has a high risk of type 1A endoleakage, especially when multiple arteries require reconstruction[17]. The use of such customized systems, including those involving fenestrated grafts and branched endografts, requires accurate preoperative imaging and planning. During the procedure, alignment of the vessel ostia with the fenestrations or branches can be difficult[3-5,19]. In addition, the high cost and long waiting time reduce the accessibility of these technologies to patients, particularly in emergent cases. In retrograde ISF, as the site is approached from within the target vessel, it is easy to place the stent graft at the correct site, and this method has been confirmed to be effective and safe for use in major aortic branches[2]. Unfortunately, retrograde endovascular revascularization cannot be easily achieved in visceral arteries[3,4,20]. Since Tse et al[6] first reported the feasibility of antegrade ISF in the placement of abdominal aortic stent grafts to preserve the patency of the renal arteries in a canine model, many studies have explored this technique (Tables 1 and 2).
| Ref. | Year | Model | Fenestration device | Guidance/landmarks of visceral arteries | Stent graft | Technical success rate1 | Experimental results |
| Tse et al[6] | 2007 | Canine | Needle | (1) Intravascular ultrasound; and (2) Preliminary stenting of all visceral arteries | Endurant | (1) 0/2; and (2) 2/2 | (1) The experiment was terminated; and (2) At the 1-mo follow-up, both renal arteries were patent. Stent fractures were observed bilaterally |
| Riga et al[7] | 2009 | Porcine | Needle | On a fixed fluoroscopic projection, the position of these target vessels was marked on the fluoroscopy screen | Valiant | 2/2 | There were no immediate complications. Both renal arteries were patent |
| Tse et al[8] | 2010 | Canine | Radiofrequency | Target vessels were marked with detachable coils or with hydrophilic catheters | Zenith | 2/3 | Thrombosis was seen in one stent during 1 mo of follow-up, while the other fenestrated artery remained patent |
| Saari et al[9] | 2012 | Porcine | Needle | Target vessels were marked with balloon catheters | Endurant, Talent | 2/6 | In one pig, the kidney showed clear signs of ischemic injury when autopsied |
| Ruthrauff et al[10] | 2015 | Benchtop | Radiofrequency | NA | Zenith, Endurant | 8/8 | NA |
| Piazza et al[11] | 2017 | Benchtop | Laser | A guiding catheter with EM sensor | NA | NA | NA |
| Condino et al[12] | 2020 | Benchtop | Laser | Simultaneous EM-guided navigation of guidewires and catheters | Zenith | 22/22 | NA |
| Ref. | Year | No. of patients | Puncture method | Guidance/landmarks | Stent graft | Technical success rate1 | Clinical outcomes |
| Bismuth et al[13] | 2012 | 1 | Needle | NA | C3 Excluder | 1/1 | At the 1-mo follow-up, the patient remained without complications. There was no evidence of endoleakage, migration or stent occlusion, and the bilateral renal arteries remained patent |
| Kölbel et al[19] | 2013 | 1 | Needle | Angiography and the marker at the proximal graft edge | Zenith | 1/1 | On CTA 4 d postoperatively, all side branches were fully preserved. Renal function was completely restored |
| Le Houérou et al[14] | 2018 | 16 | Laser | Preliminary stenting of all visceral arteries | Valiant, Endurant, Zenith | 33/35 | During a mean follow up of 17 mo, no deaths occurred. Four secondary procedures were required: Two related to fenestrations; one for stent dislocation; and one for stent stenosis. The follow up CTA demonstrated 97% primary patency |
| Leger et al[15] | 2019 | 20 | Laser | Image fusion guidance | Endurant | 48/50 | The 30-d safety rate was 90% (n = 2 deaths). At the one-week follow-up, all target vessels were patents, and two patients (15%) required a secondary procedure for endoleakage |
| Zhang et al[16] | 2020 | 1 | Laser | Preliminary stenting of all visceral arteries | Valiant | 4/4 | The 3-mo CTA demonstrated a decreased aneurysm sac, patent stent branches, and no endoleakage |
| Salib et al[17] | 2021 | 1 | Laser | Image fusion guidance | Endurant | 3/3 | The postoperative course was uneventful. The 6-mo CTA demonstrated an excluded aneurysm, patent stent branches, and no endoleakage |
Fenestration devices are important components of antegrade ISF. Graft perforation is achieved easily with most devices, including mechanical and energetic fenestration devices. Mechanical fenestration requires rigidity to transfer force from the operator’s hands to the graft. If the angulation of the needle is not sufficiently acute, target vessel cannulation becomes difficult[6,9]. Additionally, the rigidity of the needle may make it difficult to cross the typically tortuous and diseased iliac vessels. However, the occ
When using an antegrade approach in visceral arteries, finding the right puncture site can be challenging. To allow for fluoroscopic visualization, various landmarks can be deployed in target arteries prior to endograft deployment. In Tse et al[6]’s first report, bare stents were deployed in both renal arteries; however, stent fractures were observed bilaterally. No renal stent fractures were observed in a second study by the same team[8], in which the modified stent grafts had an unsupported portion in the fenestration area. In this study, they marked target vessels with detachable coils or hydrophilic catheters. However, thrombosis of the renal artery was observed after the procedure, which was likely related to partial coil deployment. Le Houérou et al[14] confirmed the feasibility of preliminarily stenting each target artery in a large clinical study[14]. Hsiao et al[21] found that overlapping stents exhibited lower fatigue resistance during respiration than a single stent. In Saari et al[9]’s study, deflation of the balloon in the renal artery indicated successful puncture, but a long warm ischemia time for the left kidney caused infarction. Furthermore, stents, balloons, and coils result in additional costs.
To avoid using invasive landmarks, Riga et al[7] marked target vessels on a fluoroscopy screen on a fixed projection with robot-assisted antegrade ISF. Leger et al[15] and Salib et al[17] succeeded under fused CT guidance. However, the configuration of the aorta may change after stent graft insertion[5]. To accurately position stent grafts in real time, various navigation devices have been tested for guidance during antegrade ISF. Intravascular ultrasound probes were not able to visualize the renal orifices for fenestration both within the stent graft and within the inferior vena cava[6]. However, electromagnetically guided endovascular instrumentation was successful in in vitro trials[11,12]. Therefore, in our case, fenestration was performed while the stent graft was positioned well with the bare stent region unreleased, allowing real-time positioning by angiography.
Blocking a visceral artery may cause visceral ischemia[9]. Le Houérou et al[14] implanted an undersized stent graft at the level of the visceral aorta to avoid temporary visceral ischemia. Tse et al[8] thought that, in theory, the marking catheter could serve to cold-perfuse renal arteries during the fenestration procedure. Bismuth et al[13] performed antegrade ISF with a constrained stent graft. In our case, fenestration was performed while the bare stent region was unreleased, and RRA perfusion was maintained during the procedure.
The stability of supporting devices is also important for correct positioning of the perforation site. Tse et al[6] described that support for the needle was provided by inflating a balloon catheter against the contralateral aortic wall. However, this catheter does not provide sufficient needle angulation. In most studies, a steerable catheter or sheath has been used to support fenestration devices. Riga et al[7] used a remotely controlled robotic steerable catheter system to improve accuracy and stability. Piazza et al[11] also proposed some design solutions for catheter stabilization. In this study, a FuStar steerable sheath was used. In addition, the main body of the stent graft was fixed, and the space in the proximal part was limited, which improved the stability of the fenestration system.
Other aspects, such as fatigue resistance[21], are also important issues in antegrade ISF. Our technique may be suitable for use in select JAAA patients with the involvement of only one renal artery. Due to the length limitation of the bare area, whether other visceral arteries can be cannulated in the same fashion needs further study.
This study suggests the feasibility and short-term safety of this novel antegrade ISF technique as an off-label technique for use in select JAAA patients. Renal artery perfusion was maintained during the procedure, and accurate positioning was achieved using angiography. This technique could serve as an alternative approach for the management of hostile proximal aortic necks during EVAR. However, the long-term effects of this method require further study in a larger cohort with long-term follow-up.
| 1. | Chaikof EL, Dalman RL, Eskandari MK, Jackson BM, Lee WA, Mansour MA, Mastracci TM, Mell M, Murad MH, Nguyen LL, Oderich GS, Patel MS, Schermerhorn ML, Starnes BW. The Society for Vascular Surgery practice guidelines on the care of patients with an abdominal aortic aneurysm. J Vasc Surg. 2018;67:2-77.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1099] [Cited by in RCA: 1971] [Article Influence: 246.4] [Reference Citation Analysis (1)] |
| 2. | Qin J, Zhao Z, Wang R, Ye K, Li W, Liu X, Liu G, Cui C, Shi H, Peng Z, Yuan F, Yang X, Lu M, Huang X, Jiang M, Wang X, Yin M, Lu X. In Situ Laser Fenestration Is a Feasible Method for Revascularization of Aortic Arch During Thoracic Endovascular Aortic Repair. J Am Heart Assoc. 2017;6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 80] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 3. | McGarry JG, McEvoy SH, Brophy DP. Endovascular recanalisation of an acute superior mesenteric artery occlusion. A case report and review of the literature. Ann Med Surg (Lond). 2014;4:76-79. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 4. | Veraldi GF, Mezzetto L, Scorsone L, Gennai S, Macrì M, Silingardi R. Hybrid rescue technique after failure of a standard multibranched stent graft. J Vasc Surg Cases Innov Tech. 2019;5:338-342. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 5. | Pineda FP, Pathmarajah T, Sieunarine K. Retrograde Catheterization of the Superior Mesenteric Artery and Celiac Axis as a Bailout Technique in a Complex FEVAR. Vasc Endovascular Surg. 2021;55:645-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 6. | Tse LW, Bui BT, Lerouge S, Salazkin I, Therasse E, Benko A, Héon H, Oliva VL, Soulez G. In vivo antegrade fenestration of abdominal aortic stent-grafts. J Endovasc Ther. 2007;14:158-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 7. | Riga CV, Bicknell CD, Wallace D, Hamady M, Cheshire N. Robot-assisted antegrade in-situ fenestrated stent grafting. Cardiovasc Intervent Radiol. 2009;32:522-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 43] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 8. | Tse LW, Lerouge S, Bui BT, Therasse E, Héon H, Soulez G. Radiofrequency perforation system for in vivo antegrade fenestration of aortic stent-grafts. J Endovasc Ther. 2010;17:192-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Saari P, Lähteenvuo M, Honkonen K, Manninen H. Antegrade in situ fenestration of aortic stent graft: in-vivo experiments using a pig model. Acta Radiol. 2012;53:754-758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 10. | Ruthrauff AA, King MW, Soulez G, Tan KT, Crawford SA, Roche-Nagle G, Cloutier G, Tse LW. Effects of Pulsatile Fatigue on In Situ Antegrade Fenestrated Polyester Stent Grafts Deployed in a Patient-Specific Phantom Model of Juxtarenal Aortic Aneurysm. J Vasc Interv Radiol. 2015;26:1551-1558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 11. | Piazza R, Condino S, Alberti A, Berchiolli RN, Coppi G, Gesi M, Ferrari V, Ferrari M. Design of a sensorized guiding catheter for in situ laser fenestration of endovascular stent. Comput Assist Surg (Abingdon). 2017;22:27-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 12. | Condino S, Piazza R, Viglialoro RM, Mocellin DM, Turini G, Berchiolli RN, Micheletti F, Rossi F, Pini R, Ferrari V, Ferrari M. Novel EM Guided Endovascular Instrumentation for In Situ Endograft Fenestration. IEEE J Transl Eng Health Med. 2020;8:1900208. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 13. | Bismuth J, Duran C, Hassoun HT. In situ fenestration for branch vessel preservation during EVAR. Methodist Debakey Cardiovasc J. 2012;8:33-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 14. | Le Houérou T, Fabre D, Alonso CG, Brenot P, Bourkaib R, Angel C, Amsallem M, Haulon S. In Situ Antegrade Laser Fenestrations During Endovascular Aortic Repair. Eur J Vasc Endovasc Surg. 2018;56:356-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 62] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 15. | Leger T, Tacher V, Majewski M, Touma J, Desgranges P, Kobeiter H. Image Fusion Guidance for In Situ Laser Fenestration of Aortic Stent graft for Endovascular Repair of Complex Aortic Aneurysm: Feasibility, Efficacy and Overall Functional Success. Cardiovasc Intervent Radiol. 2019;42:1371-1379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 16. | Zhang LL, Weaver FA, Rowe VL, Ziegler KR, Magee GA, Han SM. Antegrade in situ fenestrated endovascular repair of a ruptured thoracoabdominal aortic aneurysm. J Vasc Surg Cases Innov Tech. 2020;6:416-421. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 17. | Salib P, Majewski M, Touma J, Tacher V, Kobeiter H, Desgranges P. Combination of Quadruple Antegrade and Retrograde In Situ Stent-Graft Laser Fenestration in the Management of a Complex Abdominal Aortic Aneurysm. Ann Vasc Surg. 2021;71:533.e7-533.e12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 18. | Chiesa R, Tshomba Y, Mascia D, Rinaldi E, Logaldo D, Civilini E. Open repair for juxtarenal aortic aneurysms. J Cardiovasc Surg (Torino). 2013;54:35-45. [PubMed] |
| 19. | Kölbel T, Carpenter SW, Diener H, Wipper S, Debus ES, Larena-Avellaneda A. Antegrade in situ stent-graft fenestration for the renal artery following inadvertent coverage during EVAR. J Endovasc Ther. 2013;20:289-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (2)] |
| 20. | Verhoeven ELG, Marques de Marino P, Katsargyris A. Increasing Role of Fenestrated and Branched Endoluminal Techniques in the Thoracoabdominal Segment Including Supra- and Pararenal AAA. Cardiovasc Intervent Radiol. 2020;43:1779-1787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 21. | Hsiao HM, Nikanorov A, Prabhu S, Razavi MK. Respiration-induced kidney motion on cobalt-chromium stent fatigue resistance. J Biomed Mater Res B Appl Biomater. 2009;91:508-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Mezzetto L, Spiliopoulos S S-Editor: Fan JR L-Editor: A P-Editor: Fan JR