Published online Feb 6, 2022. doi: 10.12998/wjcc.v10.i4.1296
Peer-review started: July 27, 2021
First decision: October 3, 2021
Revised: October 16, 2021
Accepted: December 25, 2021
Article in press: December 25, 2021
Published online: February 6, 2022
Processing time: 180 Days and 12.4 Hours
Research concerning postoperative outcomes of confirmed coronavirus disease 2019 (COVID-19) patients revealed unfavorable postoperative results with increased morbidity, pulmonary complications and mortality. Case reports have suggested that COVID-19 is associated with more aggressive presentation of acute cholecystitis. The aim of the present study is to describe the perioperative assessment and postoperative outcomes of ten patients with confirmed severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection with concomitant acute cholecystitis who underwent cholecystectomy.
We report a total of 10 SARS-CoV-2 positive patients with concomitant acute cholecystitis that underwent cholecystectomy. Six patients were males, the mean age was 47.1 years. Nine patients had moderate acute cholecystitis, and one patient had severe acute cholecystitis. All patients were treated with urgent/early laparoscopic cholecystectomy. Regarding the Parkland grading scale, two patients received a Parkland grade of 3, two patients received a Parkland grade of 4, and six patients received a Parkland grade of 5. Eight patients required a bail-out procedure. Four patients developed biliary leakage and required endoscopic retrograde cholangiopancreatography with biliary sphincterotomy. After surgery, five patients developed acute respiratory distress syndrome (ARDS) and required intensive care unit (ICU) admission. One patient died after cholecystectomy due to ARDS complications. The mean total length of stay (LOS) was 18.2 d. The histopathology demonstrated transmural necrosis (n = 5), vessel obliteration with ischemia (n = 3), perforation (n = 3), and acute peritonitis (n = 10).
COVID-19 patients with acute cholecystitis had difficult cholecystectomies, high rates of ICU admission, and a prolonged LOS.
Core Tip: Several studies have described multiple gastrointestinal complications in patients with coronavirus disease 2019, including advanced stages of cholecystitis. we found in the present study that patients with confirmed severe acute respiratory syndrome coronavirus 2 infections who presented with acute cholecystitis, tended to have a higher grade on the Parkland grading scale (including gallbladder perforation, empyema and total wall necrosis), had difficult laparoscopic cholecystectomies with an increased need for a bail-out procedure, had high rates of intensive care unit admission, and had a prolonged length of hospital stay.
- Citation: Bozada-Gutiérrez K, Trejo-Avila M, Chávez-Hernández F, Parraguirre-Martínez S, Valenzuela-Salazar C, Herrera-Esquivel J, Moreno-Portillo M. Surgical treatment of acute cholecystitis in patients with confirmed COVID-19: Ten case reports and review of literature. World J Clin Cases 2022; 10(4): 1296-1310
- URL: https://www.wjgnet.com/2307-8960/full/v10/i4/1296.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i4.1296
A novel coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) emerged in China, in December 2019, causing a new disease named coronavirus disease 2019 (COVID-19)[1].
Research concerning postoperative outcomes of confirmed COVID-19 patients revealed unfavorable postoperative results with increased morbidity, pulmonary complications and mortality[2-5].
Several studies have described gastrointestinal symptoms in patients with COVID-19, with nearly 25% of patients referring abdominal pain[6]. Association with other gastrointestinal complications for example, liver injury and late cholestasis, have been reported[7]. Concerning the gallbladder, some studies have described the presence of acute ischemic gangrenous cholecystitis, with or without perforation, and with or without associated cholelithiasis[8-30]. Studies have suggested that COVID-19 is associated with more aggressive presentation of acute cholecystitis[15,19,24,27,28].
The guidelines for the treatment of acute cholecystitis before the pandemic recommended early laparoscopic cholecystectomy (Lap-C) for patients with mild acute cholecystitis as the treatment of choice[31-33]. For patients with moderate acute cholecystitis the treatment of choice is urgent/early Lap-C when advanced laparoscopic techniques are available. The indications for urgent Lap-C in confirmed COVID-19 patients should not differ from those without COVID-19[19].
Since the beginning of the pandemic in 2020, several surgical societies have published their recommendations to treat acute cholecystitis[2,34]. The firsts recommendations were to avoid surgeries and to adopt a non-operative management when possible[28,35]. The finding of virus particles in the peritoneal fluid and the idea that pneumoperitoneum could allow the transmission of virus led many surgical societies to publish recommendations for gastrointestinal laparoscopic surgery[34,36-38]. More recent recommendations sustain that laparoscopy is not more likely to spread the COVID-19 infection than open surgery, and minimally invasive surgery provides better outcomes that laparotomy[2].
Since acute cholecystitis represents a very frequent cause of hospital admission worldwide[39], it is important to know the perioperative outcomes of patients with confirmed COVID-19 and cholecystitis, also it is important to know what to expect during surgeries.
The aim of the present study was to describe the perioperative assessment and postoperative outcomes of patients with confirmed SARS-CoV-2 infection with concomitant acute cholecystitis who underwent urgent cholecystectomy.
We included symptomatic and asymptomatic SARS-CoV-2 positive patients (asymptomatic carriers) who required urgent/early cholecystectomy. During the pandemic all the patients that were admitted to our hospital were screened for SARS-CoV-2 infection.
Regarding the chief complaints, patients presented with the characteristic right upper quadrant pain related to acute cholecystitis.
All patients had concomitant acute cholecystitis with SARS-CoV-2 infection. We included patients with the SARS-CoV-2 infection confirmed either by reverse-transcriptase polymerase chain reaction (RT-PCR) assay of a nasopharyngeal swab or a rapid antigenic test. Several variables were recorded including demographic parameters and preoperative quick Sequential Organ Failure Assessment (qSOFA) score[40]. Data is shown in Table 1.
| Classification | n = 10 |
| Sex, n (%) | |
| Female | 4 |
| Male | 6 |
| Age (yr), mean (range) | 47.1 (20-74) |
| BMI (kg/m2), mean (range) | 28.4 (20-43) |
| Current Smokers, n (%) | 3 |
| ASA classification, n (%) | |
| I | 2 |
| II | 4 |
| III | 4 |
| Comorbidities, n (%) | |
| Diabetes | 2 |
| Hypertension | 4 |
| CRD | 2 |
| Lupus | 1 |
| No | 6 |
| Preoperative qSOFA score, n (%) | |
| Not high risk (0-1) | 4 |
| High risk (> 2) | 6 |
| COVID-19 symtoms | |
| Yes | 6 |
| No | 4 |
| Preoperative studies | |
| Hemoglobin (g/dL) | 12.8 (2.8) |
| Platelets (n × 103/μL) | 284 (128.7) |
| Leucocytes (n/μL) | 11.95 (5.6) |
| CRP (mg/dL) | 20.1 (12.5) |
| Total Bilirubin (mg/dL) | 1.29 (1.7) |
| Gamma-glutamyl transferase (IU/L) | 163.1 (198.1) |
| Alanine aminotransferase (IU/L) | 86.1 (102.8) |
| Aspartate aminotransferase (IU/L) | 59.9 (46.7) |
| Alkaline fosfatase (IU/L) | 199 (189.7) |
| LDH (IU/L) | 215.1 (63.3) |
| Albumin (g/dL) | 4.17 (0.4) |
| Ferritin (ng/mL) | 565 (304.5) |
| Creatinine (md/dL) | 1.02 (0.5) |
Of the ten patients, four had history of past illness. The most frequent comorbidity was hypertension (n = 4). The complete list of comorbidities is presented in Table 1.
The personal and family history was noncontributory.
On physical examinations patients had right upper quadrant pain (n = 10), right upper quadrant mass (n = 6), and positive Murphy´s sign (n = 10).
Demographic data included age (years), gender, body mass index in kg/m2, comorbidities, smoking status, American Society of Anesthesiology classification. The preoperative qSOFA score was calculated and we divided the patients in high risk (> 2 point) or not high risk patients (0-1 points).
Preoperative laboratory examinations are shown in Table 1. Of relevance, the mean preoperative C-reactive protein level was 20.1 mg/dL, the mean total bilirubin was 1.29 mg/dL, and the mean ferritin level was 565 ng/mL.
All patients underwent chest computed tomography (CT) scans prior to surgery. Also, all patients underwent gallbladder ultrasound. We diagnosed acute cholecystitis according to Tokyo Guidelines (TG18) ultrasound criteria (thickened gallbladder wall, enlarged gallbladder, pericholecystic fluid collection)[32,41].
Regarding the timing of COVID-19 diagnosis, nine patients were diagnosed preoperatively and one was diagnosed postoperatively. Of the 10 patients, four were asymptomatic SARS-CoV-2 carriers. The rest of patients (n = 6) presented with symptomatic disease and preoperative CT scans with COVID-19 pneumonia (bilateral ground-glass opacities with consolidation).
We diagnosed and graded the severity of acute cholecystitis according to the 2018 TG18[32,41]. All patients were diagnosed with definite acute cholecystitis according to the TG18. Regarding the severity assessment, nine patients had grade II (moderate) acute cholecystitis and one patient had grade III (severe) acute cholecystitis. Additionally, one patient was diagnosed with grade II acute cholangitis, and the other patient had concomitant mild acute pancreatitis. Both patients were operated after resolution of cholangitis and pancreatitis, respectively.
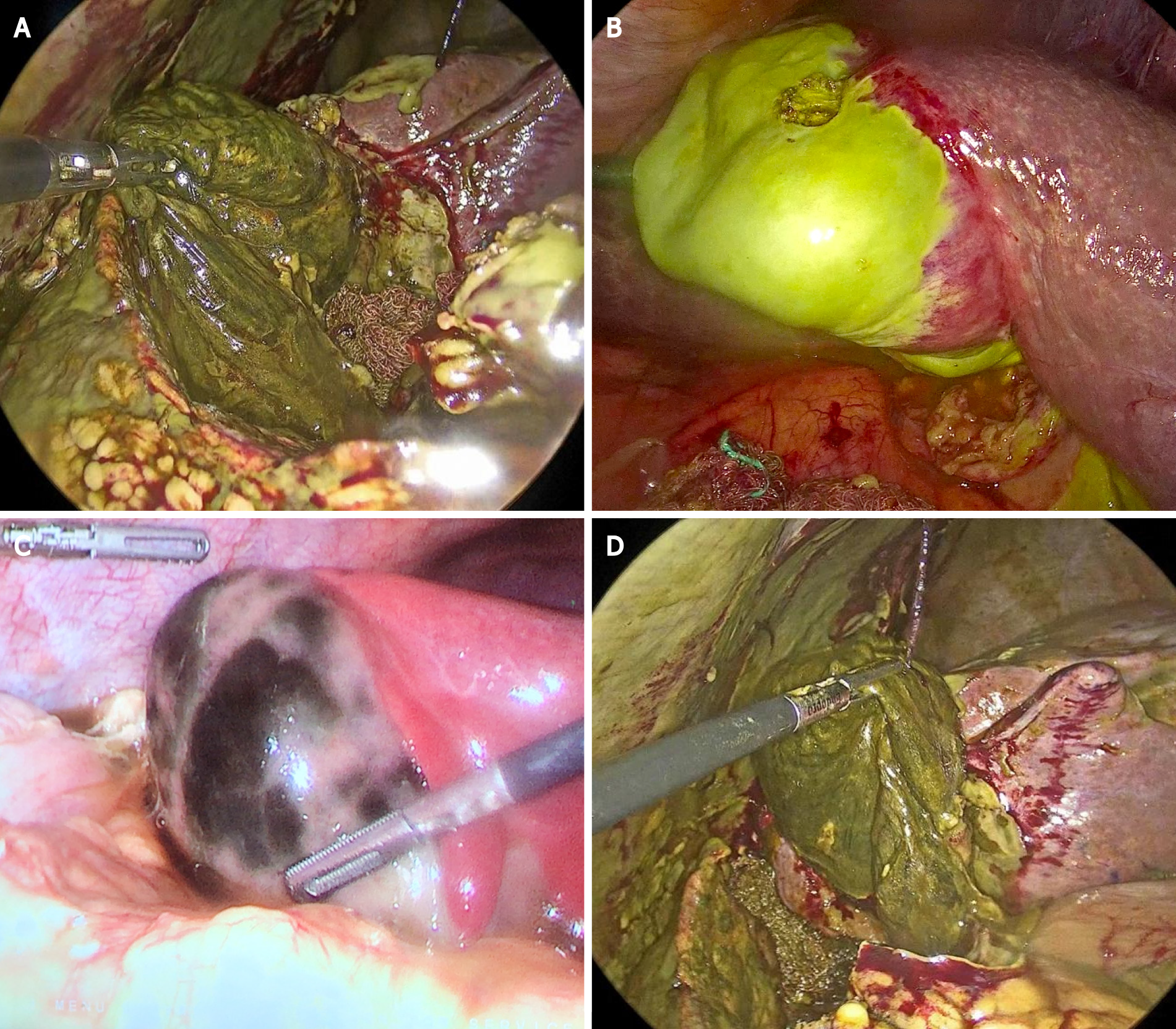
All surgeries were performed at a COVID-19 dedicated operating theater and all the medical staff were equipped with personal protective equipment. Laparoscopic cholecystectomies were performed with a 3 or 4 trocar technique depending the case, using a 12-mm umbilical trocar (optical), a 12-mm trocar in the sub-xiphoid area, and a 5-mm in the right flank. We performed diagnostic laparoscopy and we graded the intraoperative findings according with the Parkland scale. After that, bile and purulent collections were drained and intraabdominal adhesions were taken down. The decision to perform a bail-out procedure was done when the critical view of safety was difficult to achieve. In our hospital, we employ the reconstituting subtotal cholecystectomy or the open conversion as bailout procedures. The reconstituting subtotal cholecystectomy consisted in making an incision in the gallbladder, aspirating the contents including the stones, removing the peritonealized portion of the gallbladder, except the lowest portion (infundibulum and Hartmann´s pouch), and partially excising the posterior wall adherent to the liver. After that, the lowest part of the gallbladder is closed with sutures obliterating the cystic duct, leaving a closed gallbladder remnant[15,41].
During laparoscopy we filtered the pneumoperitoneum through filters able to remove most viral particles as suggested by several authors[2,42,43].
Two patients required preoperative endoscopic retrograde cholangiopancreatography (ERCP). One patient had cholestasis and a type I Mirizzi syndrome was found at ERCP. The other patient had grade II acute cholangitis and required early endoscopic treatment (ERCP biliary drainage).
All patients were treated with urgent/early Lap-C. Eight surgeries were completed via laparoscopy and two patients required conversion to open cholecystectomy due to operative difficulty.
Regarding the Parkland grading scale, all patients were found to have severe inflammation (grades 3-5): Two patients had pericholecystic fluid, adhesions to the gallbladder body, hyperemia and distended gallbladder (Parkland 3); One patient had adhesions obscuring the majority of the gallbladder and one patient had Mirizzi syndrome (Parkland 4); And six patients had Parkland 5 (six patients with complete necrosis of the gallbladder body infundibulum and cystic duct, three of them with gallbladder perforation) see Figure 1. Six cases were treated with subtotal reconstituting cholecystectomy, because a critical view of safety could not be achieved.
The mean estimated blood loss (EBL) was 258 mL, the mean operative time was 133.5 min, and eight patients required intraabdominal closed drainage.
Operative outcomes included modality of cholecystectomy (laparoscopic, open or converted), EBL in mL, operative time in minutes, and requirement of intraabdominal drainage. We graded the intraoperative findings according with the Parkland grading scale for cholecystitis[42]. Preoperative or postoperative need for ERCP and findings were registered.
Postoperative complications were classified and presented according with the Clavien-Dindo classification. The need for intensive care unit (ICU), vasopressors and invasive mechanical ventilation were recorded. The hospital length of stay (LOS) in days was registered.
Five patients developed biliary leak after subtotal cholecystectomy. Of these patients, two had low-output leak, while three patients had high-output biliary leak. Patients with low-output leaks were treated with closed suction drainage alone, while patients with high-output leaks needed ERCP with biliary sphincterotomy and biliary stent placement. The complete list of postoperative complications classified according to Clavien-Dindo is shown in Table 2.
| Grade | |
| Grade I | |
| Hydroelectrolytic imbalance | 8 |
| Antiemetics | 3 |
| Antipyretic (for fever ≥ 38.3) | 4 |
| Grade II | |
| Blood transfusions | 3 |
| Total parenteral nutrition | 3 |
| Postoperative Ileus | 1 |
| Pneumonia | 6 |
| Delirium | 4 |
| Biliar leak | 5 |
| Wound infection | 3 |
| Grade IIIb | |
| Evisceration | 1 |
| Bleeding | 1 |
| ERCP | 3 |
| Grade IVa | |
| Respiratory | 5 |
| Renal | 3 |
| Hepatic | 2 |
| Cardiovascular | 5 |
| Dialysis | 2 |
| Grade IVb | 5 |
| Multiorganic failure | |
| Grade V | 1 |
| Death of a patient |
After surgery five patients required ICU admission, and one patient was admitted preoperatively and remained in the ICU after surgery. The five patients were on invasive mechanical ventilation and vasopressor therapy. These patients developed acute respiratory distress syndrome (ARDS) related to SARS-CoV-2. One patient died after cholecystectomy and due to ARDS complications.
Considering the complete cohort of patients, the mean total LOS was 18.2 d.
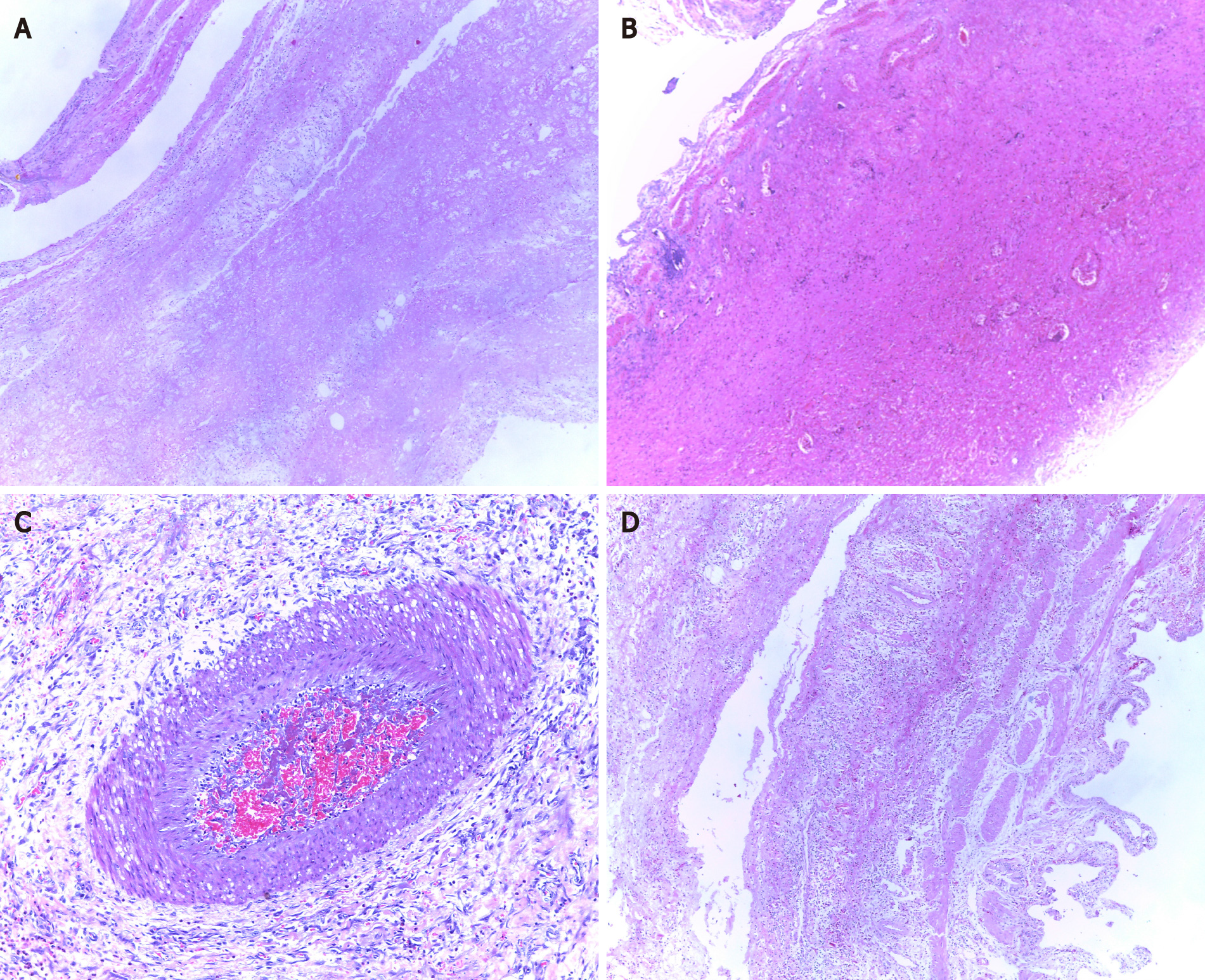
The histopathological diagnosis was performed using hematoxylin-eosin (H&E) stained slides (Table 3). As demonstrated in Figure 2, H&E slides displayed the inflammatory infiltration (n = 10), with transmural necrosis (n = 5), hemorrhagic infarction (n = 2), mucosal ulcerations (n = 1), vessel obliteration with ischemia (n = 3), gallbladder wall perforation (n = 3), and acute peritonitis (n = 10).
| Perioperative outcomes | |
| ERCP result | |
| Preop Mirizzi syndrome 1 | 1 |
| Preop Cholangitis + CBD stones | 1 |
| Postoperative Biliary leak | 3 |
| ERCP Biliary stent | 3 |
| Modality of cholecystectomy, n (%) | |
| Laparoscopic | 8 |
| Lap converted to open | 2 |
| Type of cholecystectomy | |
| Total | 4 |
| Sub-total | 6 |
| Parkland grading scale, n (%) | |
| 3 | 2 |
| 4 | 2 |
| 5 | 6 |
| Estimated blood loss (mL), mean (range) | 258 (30-500) |
| Operative time (min), mean (range) | 133.5 (70-190) |
| Intraabdominal drainage, n (%) | 8 |
| ICU admission, n (%) | |
| Yes, Preoperative | 1 |
| Yes, Postoperative | 4 |
| ICU treatment, n (%) | |
| Invasive ventilation | 5 |
| Vasopressors | 5 |
| Hospital LOS (days), mean (range) | 18. 2 (3-50) |
| Histopathology results, n (%) | |
| Ischemic/segmental necrosis | 3 |
| Transmural necrosis | 5 |
| Perforated | 3 |
| Mucosal ulcerations | 1 |
| Acute peritonitis | 10 |
| GB empyema | 4 |
| Hemorrhagic | 2 |
We found in the present study that patients with confirmed SARS-CoV-2 infections with concomitant acute cholecystitis tended to have high grades on the Parkland grading scale (including gallbladder perforation, empyema and total wall necrosis), difficult Lap-C with an increased need for a bail-out procedure (open conversion or subtotal cholecystectomy), high ICU admission rates, high rates of postoperative biliary leaks that required ERCP (with biliary stent placement), and prolonged length of hospital stay.
Several studies have described the presentation of acute cholecystitis concomitant with a SARS-CoV-2 infection. A literature review of the research concerning COVID-19 and acute cholecystitis is summarized in Table 4. We have noticed that the majority of studies that have been published during the pandemic are case reports and letters to the editor (see Table 4). The first case report of histopathological findings of an acute ischemic gangrenous cholecystitis as a late complication in a COVID-19 patient was published by Bruni et al[21]. Since then, the description of gangrenous cholecystitis in patients with COVID-19 has been found in at least six other studies[15,18,19,24,27,28]. In our series, 3 patients had wall ischemia and segmental necrosis, and 5 patients had complete transmural necrosis. Of note, some of these case reports outlined the presence of gangrenous cholecystitis but without cholelithiasis (acalculous)[18,19,27]. In our series, only one patient presented with acalculous cholecystitis, and the rest of the patients had cholelithiasis. This could represent a different physiopathological pathway that should be further investigated. Nevertheless, patients with both etiologies required cholecystectomy.
| Ref. | Study design | Country | Sample size, n (%) | Age/sex (F:M) | COVID-19 diagnosis | Tokyo class | Treatment | Morbidity/PO complications | ICU, n (%) | LOS (d) | Mortality | Findings/histopathology |
| Çakır and Kabuli[8], 2021 | Retrospective study | Turkey | 18 | M: 14 (78%); F: 4 (22%); Age: 73.3 (67-81) | RT-PCR | GI: 3 (16.7%); GII: 9 (50%); GIII: 6 (33.3%) | THGD | No complications | 3 (16.6%) | 16 (3-32) | 3 (16.6%) | NR |
| Barabino et al[9], 2021 | Retrospective study | Italy | 37: 36 non-COVID; 1 COVID | 64 (38-94); Male: 21 (56.7%); Female 16 (43.3%) | RT-PCR | GI: 13 (35.1%); GII: 15 (40.5%); GIII: 8 (21.6%); COVID: GII | Antibiotic only 11 (29.7%); THGD 8 (21.6%); L 18 (48.7%); COVID: THGD 1 | Emergency LC 1; Bleeding 1; Cholangitis 2 | 2 | 9 (2-12) | - | - |
| Martínez Caballero et al[10], 2021 | Multicentre-combined (retrospective–prospective) cohort study | Spain | 42 | Age: COVID: 83 (65-87); COVID: 28 M/14 F | Clinics 10.9%; Imaging test 11.3%; RT-PCR 12.5% | GI: 112 (43.6%); GII: 121 (47.1%); GIII: 24 (9.3%) | Antibiotic therapy 47.9%; Surgical treatment 31.5%; THGD 20.6%. COVID: 93.3% non surgical treatment | Gallblader perforation 8.4%; Biliar setic shock 8.4% | 23% | Non-COVID: 5 d (3–8). COVID: 11.0 d (7.5–27.5) | Non-COVID: 3.25%; COVID: 11.9% | - |
| Çiyiltepe et al[11], 2021 | Retrospective study | Turkey | 65 non-COVID; 7 COVID | Age: 57.3; F: 40 (55.6)/M: 32 (44.4) | GI: 35 (48.6%); GII: 37 (51.3%) | 11 THGD | - | - | 9.2 (6-20) | - | - | |
| Somuncu et al[12], 2021 | Retrospective study | Turkey | 4 COVID; 32 non-COVID | Age: 53 (26-78); M: 17/F: 19 | Thorax CT | - | Antibiotic therapy 14; THGD 14 (39%); LC 8 | - | - | 7 (2-20) | 1: Cardiac arrest | - |
| Puig et al[13], 2021 | Case report | Spain | 2 | M: 65/57 | RT-PCR | GIII: 2 | Percutaneous cholecystostomy 2 | Pulmonary tromboemboly 2 | 2 | 34 | 0 | - |
| Abaleka et al[14], 2021 | Case report | United States | 1 | Age: 76; F | RT-PCR | Grade II | Antibiotics | - | - | - | - | - |
| Lovece et al[15], 2020 | Case report | Italy | 1 | Age: 42/M | RT-PCR | Grade III | LC | Gallblader perforation | - | - | - | - |
| Famularo and Spada[16], 2021 | Letter/case report | Italy | 1 | 90/M | RT-PCR + | NR | THGD | No | No | 26 | No | NR |
| Vaishnav and Patel[17], 2021 | Observational/prospective | India | 16 | 50/F: 7 (29%); M: 17 (70%) | RT-PCR + CT + | GIII | LC | No | NR | 4.9 | NR | NR |
| Alhassan et al[18], 2020 | Case report | Qatar | 1 | 40/F | Confirmed 14 d prior | AAC | Antibiotics | No | Yes (1, 100%) | NR | No | - |
| Asti et al[19], 2020 | Letter/case report | Italy | 3 | 40-86/F: 1 (33%); M: 2 (66%) | Confirmed | AAC | LC | NR | NR | NR | NR | Acalculous, gangrene |
| Balaphas et al[20], 2020 | Letter/case report | Switzerland | 2 | 83-84/F: 1 (50%); M: 1 (50%) | RT-PCR + | AAC | LC/Antibiotics | NR | Yes (1, 50%) | NR | Yes (1, 50%) | qRT-PCR revealed the presence of SARS-CoV-2 in the gallbladder wall |
| Bruni et al[21], 2020 | Case report | Italy | 1 | 59/M | RT-PCR + | AC/GIII | OC | NR | Yes (1, 100%) | 44 | No | Gangrenous, Hemorrhagic, vasculitis |
| Cirillo et al[22], 2020 | Letter/case report | Italy | 1 | 79/M | Confirmed | AAC | Cholecystectomy | No | NR | NR | No | Perforated acalculous cholecystitis |
| Giulio et al[23], 2020 | Letter/case report | Italy | 1 | 45/F | RT-PCR + | AC/GI | LC | No | NR | 30 | No | NR |
| Gupta et al[24], 2020 | Retrospective original article | India | 5 | 53.2/NR | Confirmed | AC | OC | Bile leak | NR | 4-9 | No | Acute on chronic calculous cholecystitis, gangrenous acalculous cholecystitis |
| Kabir et al[25], 2020 | Letter/case report | Singapore | 1 | Middle-aged/M | RT-PCR + | Gangrenous cholecystitis | Subtotal reconstituting OC | NR | NR | NR | NR | NR |
| Lisotti et al[26], 2020 | Case report | Italy | 1 | 80/F | CT suspicious | AC/GII | EUS-GBD | No | No | 1 | NR | NR |
| Mattone et al[27], 2020 | Case report | Italy | 1 | 66/M | RT-PCR + | AAC | Initially THGDLC | No | Yes | NR | No | Gangrenous gallbladder |
| F Narvaez et al[28], 2020 | Brief report/review | United States | 1 | NR/F | Confirmed | AC | LC | No | No | NR | No | Near-gangrenous gallbladder |
| Safari et al[29], 2020 | Case report | Iran | 1 | 75/F | RT-PCR +CT + | AC/GII | LC | NR | Yes (1, 100%) | 9 | Yes | NR |
| Ying et al[30], 2020 | Case report | China | 1 | 68/F | RT-PCR + | AC/GII | THGD | No | No | 25 | No | NR |
An editorial by Cirillo et al[22], reported the finding of acalculous hemorrhagic cholecystitis in a patient with a SARS-CoV-2 infection who needed emergent cholecystectomy. In our histopathological analysis we found that 2 patients had hemorrhagic changes in the gallbladder wall after surgery. Our report differs from the report of Cirillo et al[22], in the fact that they preoperatively diagnosed the hemorrhage by CT scan with active contrast extravasation around and inside a perforated gallbladder. The presence of hemorrhage from an inflamed gallbladder is rare and larger studies are needed to confirm an association with SARS-CoV-2 infection.
Considering the potential association of SARS-CoV-2 with gallbladder disease, several hypothesis have been formulated. One hypothesis is that the systemic inflammation, the immune system changes induced by SARS-CoV-2, and the immunotherapy employed to treat it, may contribute to the late onset of cholecystitis by pro-inflammatory pathways[15]. Also, the findings of small-vessel thrombosis and gallbladder wall ischemia suggested a correlation with the coagulopathy and pro-thrombotic state induced by this coronavirus[21,44]. Furthermore, it has been suggested that due to the expression of angiotensin-converting enzyme 2 receptor in gallbladder epithelial cells, SARS-CoV-2 could target that cells[45]. Taking into account these inflammatory changes, we found on the histopathological examination of our patients, acute peritonitis, acute inflammatory infiltrates, as well as ischemic and necrosis associated with small vessel thrombi, and hemorrhagic changes in the gallbladder wall of our patients.
Regarding the treatment of acute cholecystitis, some authors reported the treatment with antibiotics[46], others reported percutaneous cholecystostomy[16,26], and others published their outcomes after laparoscopic or open cholecystectomy[46,47] (Table 4). The relevance of our study is that we described the outcomes of 10 patients with positive COVID-19 tests who required urgent cholecystectomy. Urgent/early cholecystectomies were performed due to gallbladder perforation (n = 3) and gangrenous cholecystitis (n = 8), where medical treatment with antibiotics only or cholecystostomy were considered insufficient treatments[19,32,41].
Concerning the intraoperative findings, we found that patients were operated on at a very advanced stage of acute cholecystitis with severe inflammation (a Parkland score > 3); thus, the critical view of safety was very difficult to achieve. The majority of our patients required a bail-out procedure for a safe cholecystectomy. As mentioned in the results section, 2 underwent conversion to laparotomy and 6 required sub-total reconstituting cholecystectomy. Of note, 3 of the 6 patients who needed sub-total cholecystectomy required ERCP due to biliary leak. Leaks developed in patients with complete gangrenous cholecystitis that extended to the infundibulum and to the cystic duct. Therefore, it is important to consider that in patients with suspected gallbladder necrosis, ischemia could extend to the cystic duct, thereby increasing the risk of postoperative biliary leakage or fistula. In our series, patients who develop postoperative bile leakage where treated with ERCP. Endoscopic management of biliary leaks (sphincterotomy with or without biliary stent) is associated with more than 90% of biliary leak healing or closure[48]. ERCP is currently considered the first-line treatment option for biliary leaks, specially cystic stump leaks[48]. Surgeons should be aware that when treating patients with difficult cholecystectomies, the goals are to resolve the septic process and to prevent secondary damage. As reported in previous COVID-19 cases[19,21,24], as well as in our series, operated patients during this pandemic tended to have severe inflammation of the gallbladder thus increasing the risks of postoperative complications including bile duct injury. As suggested by international guidelines[41], choosing a bail-out procedure (subtotal cholecystectomy or open conversion) based on intraoperative findings is recommended to avoid a secondary damage. Both bail-out procedures have been reported to reduce bile duct injury and overall postoperative complications, although it has been recognized that laparoscopic subtotal cholecystectomy is associated with increased rates of postoperative bile leakage in comparison with open conversion[41,49].
There are some limitations to our study that need to be mentioned. The most important limitation is that this was a single-center study with a small sample, which predisposes the study to all the biases inherent to the design (selection, information and confusion biases). Further prospective and multi-center studies should be performed and published, in order to better understand the effects of COVID-19 on acute cholecystitis. However, despite these limitations we consider that the results of this study could help us to describe some of the implications of SARS-CoV-2 infections in patients who require urgent/early Lap-C.
In conclusion, we found in the present study that patients with confirmed SARS-CoV-2 infections who presented with acute cholecystitis, tended to have a higher grade on the Parkland grading scale (including gallbladder perforation, empyema and total wall necrosis), had difficult laparoscopic cholecystectomies with an increased need for a bail-out procedure, had high rates of ICU admission, and had a prolonged length of hospital stay. As suggested by our case series and previously published literature, we advise to surgeons performing cholecystectomy in confirmed SARS-CoV-2 patients to be prepared for a difficult surgery and to consider a bail-out procedure to prevent secondary damage.
We would like to thank all medical and nurse staff dedicated to COVID-19 surgical care at our Hospital. Special thanks to Dr. Karina Flores, for sharing her surgical images.
| 1. | World Health Organisation (WHO). Coronavirus disease (COVID-19) outbreak webpage. [cited 10 June 2021]. Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019. |
| 2. | Campanile FC, Podda M, Arezzo A, Botteri E, Sartori A, Guerrieri M, Cassinotti E, Muttillo I, Pisano M, Brachet Contul R, D'Ambrosio G, Cuccurullo D, Bergamini C, Allaix ME, Caracino V, Petz WL, Milone M, Silecchia G, Anania G, Agrusa A, Di Saverio S, Casarano S, Cicala C, Narilli P, Federici S, Carlini M, Paganini A, Bianchi PP, Salaj A, Mazzari A, Meniconi RL, Puzziello A, Terrosu G, De Simone B, Coccolini F, Catena F, Agresta F. Acute cholecystitis during COVID-19 pandemic: a multisocietary position statement. World J Emerg Surg. 2020;15:38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 45] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 3. | COVIDSurg Collaborative. Mortality and pulmonary complications in patients undergoing surgery with perioperative SARS-CoV-2 infection: an international cohort study. Lancet. 2020;396:27-38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1203] [Cited by in RCA: 1244] [Article Influence: 207.3] [Reference Citation Analysis (4)] |
| 4. | COVIDSurg Collaborative. Surgery during the COVID-19 pandemic - Authors' reply. Lancet. 2020;396:e79. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 5. | Ozada-Gutiérrez K, Trejo-Avila M, Valenzuela-Salazar C, Herrera-Esquivel J, Moreno-Portillo M. Outcomes of COVID-19 patients that needed emergency general surgery: predictors of mortality and postoperative complications. Res Square. 2021;. [DOI] [Full Text] |
| 6. | Luo S, Zhang X, Xu H. Don't Overlook Digestive Symptoms in Patients With 2019 Novel Coronavirus Disease (COVID-19). Clin Gastroenterol Hepatol. 2020;18:1636-1637. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 234] [Cited by in RCA: 235] [Article Influence: 39.2] [Reference Citation Analysis (0)] |
| 7. | Nardo AD, Schneeweiss-Gleixner M, Bakail M, Dixon ED, Lax SF, Trauner M. Pathophysiological mechanisms of liver injury in COVID-19. Liver Int. 2021;41:20-32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 281] [Cited by in RCA: 277] [Article Influence: 55.4] [Reference Citation Analysis (2)] |
| 8. | Çakır Ç, Kabuli HA. Percutaneous cholecystostomy in the treatment of acute calculous cholecystitis in elderly patients with COVID-19 and high comorbidity. Ulus Travma Acil Cerrahi Derg. 2021;27:296-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 9. | Barabino M, Piccolo G, Trizzino A, Fedele V, Ferrari C, Nicastro V, Pisani Ceretti A, De Nicola E, Mariani NM, Giovenzana M, Scifo G, Mazza M, Vercelli R, Santambrogio R, Luigiano C, Opocher E. COVID-19 outbreak and acute cholecystitis in a Hub Hospital in Milan: wider indications for percutaneous cholecystostomy. BMC Surg. 2021;21:180. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 10. | Martínez Caballero J, González González L, Rodríguez Cuéllar E, Ferrero Herrero E, Pérez Algar C, Vaello Jodra V, Pérez Díaz MD, Dziakova J, San Román Romanillos R, Di Martino M, de la Hoz Rodríguez Á, Galán Martín M, Sánchez López D, García Virosta M, de la Fuente Bartolomé M, Pardo de Lama MM, Gutiérrez Samaniego M, Díaz Pérez D, Alias Jiménez D, de Nicolás Navas L, Pérez Alegre JJ, García-Quijada García J, Guevara-Martínez J, Villadoniga A, Martínez Fernández R. Multicentre cohort study of acute cholecystitis management during the COVID-19 pandemic. Eur J Trauma Emerg Surg. 2021;47:683-692. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 11. | Çiyiltepe H, Yıldırım G, Fersahoğlu MM, Aydın MT, Özcabı Y, Bulut NE, Taşdelen İ, Fersahoğlu AT, Yananlı ZD, Aydın İ, Ağca B, Karakaş HM, Akyüz U, Memisoğlu K. Clinical approach to patients admitted to the emergency room due to acute cholecystitis during the COVID-19 pandemic and percutaneous cholecystostomy experience. Ulus Travma Acil Cerrahi Derg. 2021;27:34-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 12. | Somuncu E, Kara Y, Kızılkaya MC, Bozdağ E, Yıldız ZB, Özkan C, Şener A, Gökay R, Aydın MO, Bozkurt MA, Kocataş A. Percutaneous cholecystostomy instead of laparoscopy to treat acute cholecystitis during the COVID-19 pandemic period: single center experience. Ulus Travma Acil Cerrahi Derg. 2021;27:89-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 13. | Puig G, Giménez-Milà M, Campistol E, Caño V, Valcarcel J, Colomina MJ. Development of concomitant diseases in COVID-19 critically ill patients. Rev Esp Anestesiol Reanim (Engl Ed). 2021;68:37-40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | Abaleka FI, Nigussie B, Bedanie G, Mohammed A, Galiboglu S. Acute Acalculous Cholecystitis Due to COVID-19, an Unusual Presentation. Cureus. 2021;13:e15431. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 15. | Lovece A, Asti E, Bruni B, Bonavina L. Subtotal laparoscopic cholecystectomy for gangrenous gallbladder during recovery from COVID-19 pneumonia. Int J Surg Case Rep. 2020;72:335-338. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 16. | Famularo G, Spada PL. COVID-19-related cholecystitis. Clin Res Hepatol Gastroenterol. 2021;45:101635. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Vaishnav D, Patel B. Laparoscopic Gastrointestinal Surgery During COVID-19 Pandemic: Single-Center Experience. J Laparoendosc Adv Surg Tech A. 2021;31:455-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 18. | Alhassan SM, Iqbal P, Fikrey L, Mohamed Ibrahim MI, Qamar MS, Chaponda M, Munir W. Post COVID 19 acute acalculous cholecystitis raising the possibility of underlying dysregulated immune response, a case report. Ann Med Surg (Lond). 2020;60:434-437. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 19. | Asti E, Lovece A, Bonavina L. Gangrenous cholecystitis during hospitalization for SARS-CoV2 infection. Updates Surg. 2020;72:917-919. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 20. | Balaphas A, Gkoufa K, Meyer J, Peloso A, Bornand A, McKee TA, Toso C, Popeskou SG. COVID-19 can mimic acute cholecystitis and is associated with the presence of viral RNA in the gallbladder wall. J Hepatol. 2020;73:1566-1568. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 46] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 21. | Bruni A, Garofalo E, Zuccalà V, Currò G, Torti C, Navarra G, De Sarro G, Navalesi P, Longhini F, Ammendola M. Histopathological findings in a COVID-19 patient affected by ischemic gangrenous cholecystitis. World J Emerg Surg. 2020;15:43. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 22. | Cirillo B, Brachini G, Crocetti D, Sapienza P, Mingoli A. Acalcolous Hemorrhagic Cholecystitis and SARS-CoV-2 Infection. Br J Surg. 2020;107:e524. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 23. | Giulio M, Achilli P, Dario M. An underestimated "false negative COVID cholecystitis" in Northern Italy and the contagion of a surgical ward: it can happen everywhere. Updates Surg. 2020;72:315-316. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 24. | Gupta P, Chauhan V, Baraiya Y. A Study on Cases of Gall Bladder Perforation during COVID-19 Pandemic. GCSMC J Med Sci. 2020;9:70-73. [DOI] [Full Text] |
| 25. | Kabir T, Kam JH, Chew MH. Cholecystectomy during the COVID-19 pandemic: Current evidence and an understanding of the 'new' critical view of safety: Correspondence. Int J Surg. 2020;79:307-308. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 26. | Lisotti A, Bacchilega I, Linguerri R, Fusaroli P. Endoscopic ultrasound-guided gallbladder drainage as a strategy to overcome shortage of operating rooms and intensive care unit beds during Covid-19 crisis. Endoscopy. 2020;52:E263-E264. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 27. | Mattone E, Sofia M, Schembari E, Palumbo V, Bonaccorso R, Randazzo V, La Greca G, Iacobello C, Russello D, Latteri S. Acute acalculous cholecystitis on a COVID-19 patient: a case report. Ann Med Surg (Lond). 2020;58:73-75. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 28. | F Narvaez JR, Cooper C, Brewer JJ, Schwaitzberg SD, Guo WA. Do We "Do No Harm" in the Management of Acute Cholecystitis in COVID-19 Patients? Am Surg. 2020;86:748-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 29. | Safari S, Keyvani H, Malekpour Alamdari N, Dehghanian A, Razavi Hashemi M, Nemati Honar B, Aminian A. Abdominal Surgery in Patients With COVID-19: Detection of SARS-CoV-2 in Abdominal and Adipose Tissues. Ann Surg. 2020;272:e253-e256. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 30. | Ying M, Lu B, Pan J, Lu G, Zhou S, Wang D, Li L, Shen J, Shu J; From the COVID-19 Investigating and Research Team. COVID-19 with acute cholecystitis: a case report. BMC Infect Dis. 2020;20:437. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 31. | Ansaloni L, Pisano M, Coccolini F, Peitzmann AB, Fingerhut A, Catena F, Agresta F, Allegri A, Bailey I, Balogh ZJ, Bendinelli C, Biffl W, Bonavina L, Borzellino G, Brunetti F, Burlew CC, Camapanelli G, Campanile FC, Ceresoli M, Chiara O, Civil I, Coimbra R, De Moya M, Di Saverio S, Fraga GP, Gupta S, Kashuk J, Kelly MD, Koka V, Jeekel H, Latifi R, Leppaniemi A, Maier RV, Marzi I, Moore F, Piazzalunga D, Sakakushev B, Sartelli M, Scalea T, Stahel PF, Taviloglu K, Tugnoli G, Uraneus S, Velmahos GC, Wani I, Weber DG, Viale P, Sugrue M, Ivatury R, Kluger Y, Gurusamy KS, Moore EE. 2016 WSES guidelines on acute calculous cholecystitis. World J Emerg Surg. 2016;11:25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 176] [Cited by in RCA: 222] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 32. | Yokoe M, Hata J, Takada T, Strasberg SM, Asbun HJ, Wakabayashi G, Kozaka K, Endo I, Deziel DJ, Miura F, Okamoto K, Hwang TL, Huang WS, Ker CG, Chen MF, Han HS, Yoon YS, Choi IS, Yoon DS, Noguchi Y, Shikata S, Ukai T, Higuchi R, Gabata T, Mori Y, Iwashita Y, Hibi T, Jagannath P, Jonas E, Liau KH, Dervenis C, Gouma DJ, Cherqui D, Belli G, Garden OJ, Giménez ME, de Santibañes E, Suzuki K, Umezawa A, Supe AN, Pitt HA, Singh H, Chan ACW, Lau WY, Teoh AYB, Honda G, Sugioka A, Asai K, Gomi H, Itoi T, Kiriyama S, Yoshida M, Mayumi T, Matsumura N, Tokumura H, Kitano S, Hirata K, Inui K, Sumiyama Y, Yamamoto M. Tokyo Guidelines 2018: diagnostic criteria and severity grading of acute cholecystitis (with videos). J Hepatobiliary Pancreat Sci. 2018;25:41-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 769] [Cited by in RCA: 782] [Article Influence: 97.8] [Reference Citation Analysis (0)] |
| 33. | Kiriyama S, Kozaka K, Takada T, Strasberg SM, Pitt HA, Gabata T, Hata J, Liau KH, Miura F, Horiguchi A, Liu KH, Su CH, Wada K, Jagannath P, Itoi T, Gouma DJ, Mori Y, Mukai S, Giménez ME, Huang WS, Kim MH, Okamoto K, Belli G, Dervenis C, Chan ACW, Lau WY, Endo I, Gomi H, Yoshida M, Mayumi T, Baron TH, de Santibañes E, Teoh AYB, Hwang TL, Ker CG, Chen MF, Han HS, Yoon YS, Choi IS, Yoon DS, Higuchi R, Kitano S, Inomata M, Deziel DJ, Jonas E, Hirata K, Sumiyama Y, Inui K, Yamamoto M. Tokyo Guidelines 2018: diagnostic criteria and severity grading of acute cholangitis (with videos). J Hepatobiliary Pancreat Sci. 2018;25:17-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 468] [Cited by in RCA: 487] [Article Influence: 60.9] [Reference Citation Analysis (0)] |
| 34. | Francis N, Dort J, Cho E, Feldman L, Keller D, Lim R, Mikami D, Phillips E, Spaniolas K, Tsuda S, Wasco K, Arulampalam T, Sheraz M, Morales S, Pietrabissa A, Asbun H, Pryor A. SAGES and EAES recommendations for minimally invasive surgery during COVID-19 pandemic. Surg Endosc. 2020;34:2327-2331. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 125] [Cited by in RCA: 167] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 35. | Tebala GD, Bond-Smith G. Guidelines and recommendations during the COVID-19 pandemic: A word of caution. Am J Surg. 2020;220:1526-1527. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 36. | Gu J, Han B, Wang J. COVID-19: Gastrointestinal Manifestations and Potential Fecal-Oral Transmission. Gastroenterology. 2020;158:1518-1519. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 954] [Cited by in RCA: 950] [Article Influence: 158.3] [Reference Citation Analysis (2)] |
| 37. | Xiao F, Tang M, Zheng X, Liu Y, Li X, Shan H. Evidence for Gastrointestinal Infection of SARS-CoV-2. Gastroenterology. 2020;158:1831-1833.e3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1985] [Cited by in RCA: 2016] [Article Influence: 336.0] [Reference Citation Analysis (3)] |
| 38. | Coccolini F, Tartaglia D, Puglisi A, Giordano C, Pistello M, Lodato M, Chiarugi M. SARS-CoV-2 Is Present in Peritoneal Fluid in COVID-19 Patients. Ann Surg. 2020;272:e240-e242. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 112] [Cited by in RCA: 104] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 39. | Tazuma S, Unno M, Igarashi Y, Inui K, Uchiyama K, Kai M, Tsuyuguchi T, Maguchi H, Mori T, Yamaguchi K, Ryozawa S, Nimura Y, Fujita N, Kubota K, Shoda J, Tabata M, Mine T, Sugano K, Watanabe M, Shimosegawa T. Evidence-based clinical practice guidelines for cholelithiasis 2016. J Gastroenterol. 2017;52:276-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 155] [Article Influence: 17.2] [Reference Citation Analysis (1)] |
| 40. | Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, Hotchkiss RS, Levy MM, Marshall JC, Martin GS, Opal SM, Rubenfeld GD, van der Poll T, Vincent JL, Angus DC. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315:801-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15803] [Cited by in RCA: 18871] [Article Influence: 1887.1] [Reference Citation Analysis (4)] |
| 41. | Wakabayashi G, Iwashita Y, Hibi T, Takada T, Strasberg SM, Asbun HJ, Endo I, Umezawa A, Asai K, Suzuki K, Mori Y, Okamoto K, Pitt HA, Han HS, Hwang TL, Yoon YS, Yoon DS, Choi IS, Huang WS, Giménez ME, Garden OJ, Gouma DJ, Belli G, Dervenis C, Jagannath P, Chan ACW, Lau WY, Liu KH, Su CH, Misawa T, Nakamura M, Horiguchi A, Tagaya N, Fujioka S, Higuchi R, Shikata S, Noguchi Y, Ukai T, Yokoe M, Cherqui D, Honda G, Sugioka A, de Santibañes E, Supe AN, Tokumura H, Kimura T, Yoshida M, Mayumi T, Kitano S, Inomata M, Hirata K, Sumiyama Y, Inui K, Yamamoto M. Tokyo Guidelines 2018: surgical management of acute cholecystitis: safe steps in laparoscopic cholecystectomy for acute cholecystitis (with videos). J Hepatobiliary Pancreat Sci. 2018;25:73-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 318] [Article Influence: 39.8] [Reference Citation Analysis (0)] |
| 42. | Madni TD, Leshikar DE, Minshall CT, Nakonezny PA, Cornelius CC, Imran JB, Clark AT, Williams BH, Eastman AL, Minei JP, Phelan HA, Cripps MW. The Parkland grading scale for cholecystitis. Am J Surg. 2018;215:625-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 85] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 43. | Mintz Y, Arezzo A, Boni L, Chand M, Brodie R, Fingerhut A; and the Technology Committee of the European Association for Endoscopic Surgery. A Low-cost, Safe, and Effective Method for Smoke Evacuation in Laparoscopic Surgery for Suspected Coronavirus Patients. Ann Surg. 2020;272:e7-e8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 59] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 44. | Zhang Y, Xiao M, Zhang S, Xia P, Cao W, Jiang W, Chen H, Ding X, Zhao H, Zhang H, Wang C, Zhao J, Sun X, Tian R, Wu W, Wu D, Ma J, Chen Y, Zhang D, Xie J, Yan X, Zhou X, Liu Z, Wang J, Du B, Qin Y, Gao P, Qin X, Xu Y, Zhang W, Li T, Zhang F, Zhao Y, Li Y. Coagulopathy and Antiphospholipid Antibodies in Patients with Covid-19. N Engl J Med. 2020;382:e38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1731] [Cited by in RCA: 1618] [Article Influence: 269.7] [Reference Citation Analysis (0)] |
| 45. | Zong H, Yin B, Zhou H, Cai D, Ma B, Xiang Y. Loss of angiotensin-converting enzyme 2 promotes growth of gallbladder cancer. Tumour Biol. 2015;36:5171-5177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 46. | Ielpo B, Prieto M, Ortega I, Balibrea JM, Rubio-Pérez I, Juvany M, Gómez-Bravo MÁ, Ramia JM. National survey on the treatment of cholelitiasis in Spain during the initial period of the COVID-19 pandemic. Cir Esp (Engl Ed). 2021;99:346-353. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 47. | Munavalli GG, Guthridge R, Knutsen-Larson S, Brodsky A, Matthew E, Landau M. "COVID-19/SARS-CoV-2 virus spike protein-related delayed inflammatory reaction to hyaluronic acid dermal fillers: a challenging clinical conundrum in diagnosis and treatment". Arch Dermatol Res. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 90] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 48. | Rio-Tinto R, Canena J. Endoscopic Treatment of Post-Cholecystectomy Biliary Leaks. GE Port J Gastroenterol. 2021;28:265-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 49. | Elshaer M, Gravante G, Thomas K, Sorge R, Al-Hamali S, Ebdewi H. Subtotal cholecystectomy for "difficult gallbladders": systematic review and meta-analysis. JAMA Surg. 2015;150:159-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 257] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: Mexico
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Abdolusefi MS, Osorno JF, Tazegul G S-Editor: Fan JR L-Editor: A P-Editor: Fan JR