Published online Feb 6, 2022. doi: 10.12998/wjcc.v10.i4.1172
Peer-review started: September 6, 2021
First decision: October 29, 2021
Revised: November 13, 2021
Accepted: December 31, 2021
Article in press: December 31, 2021
Published online: February 6, 2022
Processing time: 139 Days and 16.2 Hours
There are few studies regarding sequential changes in the sagittal alignment of the upper and lower cervical regions of the spine after occipitocervical fusion (OCF). In addition, no comparisons of cervical sagittal alignment (CSA) between patients with craniocervical junction disorders (CJDs) and normal populations have been reported.
To compare the CSA of patients with CJDs with that of normal controls and investigate the sequential changes in the CSA of the upper and lower cervical spine after OCF.
Eighty-four patients who underwent OCF (OCF group) and 42 asymptomatic volunteers (control group) were included. Radiographic parameters, including the occipital to C2 angle (O-C2a), occipital and external acoustic meatus to axis angle (O-EAa), C2–7 angle (C2-7a), and pharyngeal inlet angle (PIA), were measured and compared pre- and postoperatively. The correlations among the parameters were analyzed using Pearson’s correlation test.
The O-C2a and PIA of the OCF group were smaller than those of the control group, while their O-EAa and C2-7a values were larger than those of the normal controls. There were no significant differences in O-C2a, C2-7a, or PIA in the OCF group at baseline, 1 mo, or the final follow-up after surgery. The Pearson’s correlation results showed that there were significant correlations between the O-C2a and C2Ta, C2-7a, C2-7 sagittal vertical axis (SVA), and PIA at 1 mo after OCF surgery and between O-C2a and O-EAa, C2Ta, C2-7a, C2-7 SVA, and PIA at the final follow-up.
Patients with CJDs have a more kyphotic upper CSA and a more lordotic lower CSA than normal controls. The effectiveness of OCF surgery in restoring CSA may be limited by the realignment of the craniocervical junction being neglected. The reduction in O-C2a after OCF surgery may increase C2-7a and decrease PIA.
Core Tip: Patients with craniocervical junction disorders had a more kyphotic upper cervical sagittal alignment (CSA) and a more lordotic lower CSA than normal controls: The decreased lordosis of the upper cervical spine caused by the weakness of paraspinal muscles and ligaments (OC2a↓) led to the gravity center of the cranium moving forward (C2Ta↑). To maintain horizontal gaze and normal C2-7 sagittal vertical axis, the lordosis of the lower cervical spine was increased (C2-7a↑). Moreover, the restoration of CSA after occipitocervical fusion (OCF) may be limited by neglecting the realignment of craniocervical junction. The reduction of the O-C2a after OCF would increase the C2-7a and decrease the pharyngeal inlet angle and lead to postoperative dysphagia.
- Citation: Zhu C, Wang LN, Chen TY, Mao LL, Yang X, Feng GJ, Liu LM, Song YM. Sequential sagittal alignment changes in the cervical spine after occipitocervical fusion. World J Clin Cases 2022; 10(4): 1172-1181
- URL: https://www.wjgnet.com/2307-8960/full/v10/i4/1172.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i4.1172
The cervical spine can be classified into two parts: The upper cervical spine (C0-C2) and lower cervical spine (C3–C7). The sagittal alignments of the two parts are closely interrelated[1]. An imbalance in the upper and lower cervical regions of the spine can lead to poor cervical sagittal alignment (CSA), which correlates with headache, neck pain, and poor health-related quality of life (HRQOL)[2].
Several studies have explored the interrelationship between upper and lower CSA. Nojiri et al[1] and Lee et al[3] observed a significant negative correlation between the C0-2 and C2–7 angles in asymptomatic individuals. Huang et al[4] found that postoperative kyphosis in the lower cervical spine is associated with hyperlordotic atlantoaxial fusion. Kim et al[5] reported that anterior cervical discectomy and fusion in the lower cervical spine can induce improvements in regional lordosis at the surgical level and can subsequently cause changes in the upper cervical segment including upward inclinations of the C1 slope and C2 slope.
Occipitocervical fusion (OCF) was first described in 1927 and was suggested to be an effective and safe procedure for the surgical treatment of craniocervical junction disorders (CJDs) caused by congenital deformities, trauma, rheumatoid arthritis (RA), and degenerative processes[6]. Matsubayashi et al[7] found that the occipital to C7 angle (O-C7a) is regulated by the T1 slope and that the corresponding O-C7a is divided into the occipital to C2 angle (O-C2a) and C2–C7 angle (C2-7a), which have negative correlations with each other and then maintain horizontal gaze. Korovessis et al[8] demonstrated that postoperative O-C2a, pharyngeal inlet angle (PIA), and T1-slope safely predict HRQOL outcomes following OCF for fresh trauma.
However, there are few studies regarding sequential changes in the sagittal alignment of the upper and lower cervical regions of the spine after OCF. In addition, to our knowledge, no comparisons of CSA between patients with CJDs and normal populations have been reported. Thus, this study had two purposes: (1) To compare the CSA of patients with craniocervical disorders with that of a normal control population; and (2) To investigate the sequential changes in and interrelationships of the sagittal alignment of the upper and lower cervical regions of the spine after OCF.
This was a retrospective study that was approved by the ethics committee of West China Hospital of Sichuan University and informed consent was obtained from all the patients. All methods were carried out in accordance with relevant guidelines and regulations.
Patients with CJDs who underwent OCF (OCF group) between April 2010 and May 2019 were included in the study. The inclusion criteria were as follows: (1) Age ≥ 18 years; (2) Surgical treatment with OCF; (3) No history of spine surgery; and (4) At least 1 year of radiographic follow-up data with adequate visualization of the cervical spine on pre- and postoperative films. The exclusion criteria included a history of spine surgery or oropharyngeal surgery, and the presence of preoperative dysphagia, and dysphagia resulting from esophageal disease. For comparison, asymptomatic volunteers with no history of cervical disease or trauma and no neck, shoulder, or arm symptoms were also included in the study as a control group. These people were matched by age and sex with the patients in the OCF group.
All surgical procedures were performed by two senior surgeons from one medical team. We routinely used autologous iliac bones for fusion. The patients in the OCF group were divided into dysphagia and nondysphagia subgroups according to whether they had suffered postoperative dysphagia, as determined by face-to-face questioning or telephone interviews. Patients were defined as having dysphagia if they needed swallowing agents or pureed foods to avoid choking[9].
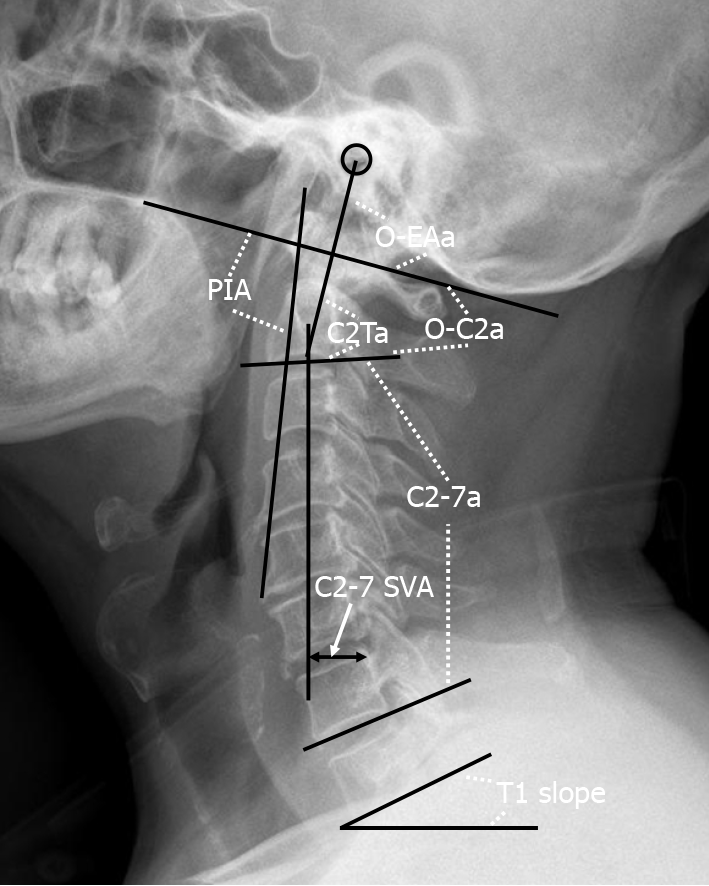
A lateral radiograph of the cervical spine was obtained at baseline, 1 mo, and the last follow-up after OCF surgery. The radiographic parameters assessed (Figure 1) were as follows based on previous studies[9-11]: O-C2a, which is the angle between the inferior end plate of C2 and the McGregor line that connects the hard palate and opisthion; the occipital and external acoustic meatus to axis angle (O-EAa), which is the angle formed by the McGregor line and the EA-line and connects the midpoint of the external acoustic meatuses and the midpoint of the inferior endplate of C2; the C2 tilting angle (C2Ta), which is the angle formed by the inferior endplate of C2 and the EA-line; C2-7a, which is the Cobb angle between the lower endplate of C2 and C7; the T1 slope, which is the angle between the horizontal and the T1 superior endplate; the C2-7 sagittal vertical axis (C2-7 SVA), which is the horizontal distance between the C2 plumb line and the posterior corner of C7; and the PIA[10], which is the angle between McGregor line and the line that links the center of the C1 anterior arch and the apex of the cervical sagittal curvature (or bottom when the cervical alignment is kyphotic). Positive values indicated lordosis, while negative values indicated kyphosis. The C2-7 SVA value was considered to be positive if the C2 plumb line was located in front of the posterior upper corner of the C7 vertebral body, and it was considered to be negative if the C2 plumb line was behind the posterior upper corner of the C7 vertebral body[12]. To avoid intraobserver bias, all radiological parameters were measured by two attending spinal surgeons who were not involved in the surgery, and the average value of their measurements was used for analysis.
Analyses were performed with SPSS software (version 22.0; IBM Corp., Armonk, NY, United States). Values are presented as the mean ± SD. Quantitative data were analyzed using the Student’s t test or Mann–Whitney U test, as appropriate. Cate
A total of 84 patients were included in the OCF group. The average patient age was 50.5 ± 15.0 years, and 45.2% (38/84) of patients were male. CJDs were caused by deformities (n = 63), trauma (n = 12), and RA (n = 9). The fusion levels were O-C2 (n = 28), O-C3 (n = 31), O-C4 (n = 18), and O-C5 (n = 7). The postoperative follow-up period in the OCF group ranged from 12 to 78 mo (mean, 22.8 ± 16.6 mo).
For comparison, 42 asymptomatic volunteers were enrolled in the control group. The average age was 51.4 ± 12.4 years, and 47.6% (20/42) of patients were male. The differences in age and sex between the OCF group and control group were not significant (P = 0.738 and P = 0.851, respectively). The radiographic parameters in these two groups are listed in Table 1. The O-C2a and PIA of patients in the OCF group were significantly smaller than those of the patients in the control group (P < 0.05), while the O-EAa, C2Ta, and C2-7a of the OCF patients were significantly larger than those of the normal controls (P < 0.05). Significant correlations were found between O-C2a and C2Ta (r = -0.872), C2-7a (r = -0.585), and PIA (r = 0.757) in the OCF group and between O-C2a and O-EAa (r = 0.309), C2Ta (r = -0.802), C2-7a (r = -0.385), C2-7 SVA (r = 0.331), and PIA (r = 0.579) in the control group (Table 2).
| OCF (n = 84) | Control (n = 42) | |||
| Pre-op | Post-op | Final follow-up | ||
| O-C2a (°) | 4.7 ± 15.3 | 4.6 ± 12.6 | 4.7 ± 13.3 | 12.0 ± 8.2a,b,c |
| O-EAa (°) | 101.1 ± 8.5 | 99.4 ± 7.7 | 99.0 ± 9.1 | 92.2 ± 5.4a,b,c |
| C2Ta (°) | 96.5 ± 17.3 | 94.8 ± 13.5 | 94.2 ± 13.2 | 80.0 ± 8.6a,b,c |
| C2-7a (°) | 24.8 ± 18.1 | 21.7 ± 16.1 | 21.6 ± 15.8 | 16.2 ± 9.6a,b,c |
| T1 slope (°) | 23.2 ± 9.5 | 23.4 ± 8.2 | 21.5 ± 8.2 | 21.9 ± 7.2 |
| C2-7 SVA (cm) | 1.4 ± 1.1c | 1.5 ± 1.1c | 1.0 ± 1.0a,b | 1.5 ± 0.8c |
| PIA (°) | 91.6 ± 10.3 | 89.3 ± 10.1 | 88.8 ± 12.5 | 97.3 ± 7.5a,b,c |
| OCF (n = 84) | r value | P value | Control (n = 42) | r value | P value |
| O-C2a | |||||
| O-EAa | 0.039 | 0.728 | O-EAa | 0.309 | 0.046 |
| C2Ta | -0.872 | 0.000 | C2Ta | -0.802 | 0.000 |
| C2-7a | -0.585 | 0.000 | C2-7a | -0.385 | 0.012 |
| T1 slope | -0.174 | 0.214 | T1 slope | -0.080 | 0.615 |
| C2-7 SVA | 0.067 | 0.545 | C2-7 SVA | 0.331 | 0.032 |
| PIA | 0.757 | 0.000 | PIA | 0.579 | 0.000 |
| C2-7a | |||||
| O-C2a | -0.585 | 0.000 | O-C2a | -0.385 | 0.012 |
| O-EAa | 0.023 | 0.837 | O-EAa | -0.100 | 0.530 |
| C2Ta | 0.540 | 0.000 | C2Ta | 0.326 | 0.035 |
| T1 slope | 0.490 | 0.000 | T1 slope | 0.523 | 0.000 |
| C2-7 SVA | -0.013 | 0.909 | C2-7 SVA | -0.239 | 0.127 |
| PIA | -0.546 | 0.000 | PIA | -0.156 | 0.324 |
The radiological parameters of patients in the OCF group before surgery, 1 mo after surgery, and at the last follow-up after OCF surgery are reported in Table 1. There were no significant differences in O-C2a, C2-7a, or PIA at baseline, 1 mo after surgery, or the final follow-up after surgery (P > 0.05). Compared with the asymptomatic volunteers in the control group, the patients in the OCF group had significantly smaller O-C2a and PIA values after OCF surgery (P < 0.05) and significantly larger O-EAa, C2Ta, and C2-7a values (P < 0.05). The results of the Pearson’s correlation analysis of O-C2a and the other parameters showed that there were significant correlations between O-C2a and C2Ta (r = -0.840, P = 0.000), C2-7a (r = -0.333, P = 0.002), C2-7 SVA (r = 0.218, P = 0.046), and PIA (r = 0.744, P = 0.000) at 1 mo after OCF surgery and between O-C2a and -EAa (r = 0.346, P = 0.001), C2Ta (r = -0.764, P = 0.000), C2-7a (r = -0.314, P = 0.004), C2-7 SVA (r = 0.293, P = 0.007), and PIA (r = 0.495, P = 0.000) at the final follow-up.
There were 20 patients with dysphagia and 64 patients without dysphagia in the OCF group. No significant intergroup differences were found in terms of age (52.2 ± 12.8 vs 49.9 ± 15.7, P = 0.555), fusion level (≤ C3/> C3: 16/4 vs 44/20, P = 0.405), the proportion of patients with RA (4/20 vs 6/64, P = 0.185), or the proportion of patients with AS (13/20 vs 40/60, P = 0.840). However, the proportion of female patients was significantly higher in the patients with dysphagia (16/20) than in the patients without (30/64) (P = 0.019). The details of the radiological parameters of the patients with and without dysphagia at baseline, 1 mo after the surgery, and the final follow-up after OCF surgery are shown in Table 3.
| Dysphagia (n = 20) | Without dysphagia (n = 64) | P value | ||
| O-C2a (°) | Pre-op | 9.7 ± 10.7 | 3.2 ± 16.2 | 0.046 |
| Post-op | 0.9 ± 9.3a | 5.8 ± 13.3 | 0.128 | |
| Final follow-up | 1.3 ± 9.6a | 5.8 ± 14.1 | 0.182 | |
| O-EAa (°) | Pre-op | 104.2 ± 7.8 | 100.2 ± 8.6 | 0.070 |
| Post-op | 95.1 ± 8.3a | 100.8 ± 6.6 | 0.003 | |
| Final follow-up | 96.9 ± 6.5a | 99.7 ± 9.7 | 0.240 | |
| C2Ta (°) | Pre-op | 94.5 ± 13.1 | 97.1 ± 18.5 | 0.478 |
| Post-op | 94.3 ± 14.1 | 94.9 ± 13.4 | 0.851 | |
| Final follow-up | 95.6 ± 10.6 | 93.8 ± 14.0 | 0.598 | |
| C2-7a (°) | Pre-op | 19.5 ± 14.3 | 26.4 ± 19.0 | 0.136 |
| Post-op | 26.9 ± 13.5a | 20.0 ± 16.6 | 0.100 | |
| Final follow-up | 27.9 ± 12.6a | 19.6 ± 16.2 | 0.041 | |
| T1 slope (°) | Pre-op | 21.5 ± 5.7 | 21.2 ± 11.1 | 0.340 |
| Post-op | 21.5 ± 6.8 | 24.4 ± 8.9 | 0.226 | |
| Final follow-up | 22.7 ± 6.5 | 20.9 ± 9.1 | 0.436 | |
| C2-7 SVA (cm) | Pre-op | 0.9 ± 0.9 | 1.6 ± 1.1 | 0.028 |
| Post-op | 1.1 ± 1.3 | 1.6 ± 1.1 | 0.088 | |
| Final follow-up | 0.4 ± 1.1b | 1.2 ± 0.9 | 0.002 | |
| PIA (°) | Pre-op | 95.0 ± 6.7 | 90.6 ± 11.0 | 0.035 |
| Post-op | 82.0 ± 5.7a | 91.6 ± 10.2 | 0.000 | |
| Final follow-up | 86.3 ± 6.8a,b | 89.5 ± 13.7 | 0.321 |
Previous studies have explored the relations between the alignments of the upper and subaxial cervical regions of the spine in asymptomatic volunteers[1,13,14]. Nojiri et al[1] enrolled 313 asymptomatic individuals in their study and investigated the relationships between the alignments of the upper and lower cervical regions of the spine. They observed significant negative correlations between O-C2a and C2-7a and between C1-2a and C2-7a. The correlation coefficient between O-C2a and C2–7a was larger than that between C1–2a and C2–7a. Sherekar et al[13] also reported that O-C2a was negatively correlated with C2-7a. Guo et al[14] found that O-C2a was larger in females than in males, whereas C2–7a was significantly larger in males. Both C2–7a and O-C2a correlated significantly with age. In our study, the mean values of O-C2a and C2-7a in the asymptomatic volunteers were 12.0° and 16.2°, respectively, which were comparable with the results of previous studies[1,13,14]. A statistically signi
Compared to the patients with CJDs, the normal controls had a larger OC2a (12.0° vs 4.7°), while the O-EAa, C2Ta, and C2-7a were smaller (92.2° vs 101.1°, 80.0° vs 96.5°, 16.2° vs 24.8°, respectively). Based on these results, we speculated that the degree of lordosis of the upper cervical spine was markedly lower in the patients with these disorders because of weaknesses in vertebrae, muscles, and ligaments caused by craniocervical lesions (OC2a↓). Hypolordosis in the upper cervical spine caused the center of gravity of the cranium to move forward (C2Ta↑). Finally, the degree of lordosis of the lower cervical spine increased to compensate for this change (C2-7a↑) so that the individuals could maintain a horizontal gaze and normal C2–7 SVA.
OCF can provide immediate rigidity and fusion in the treatment of CJDs. Although the best result that surgeons expect from OCF surgery is the restoration of normal physiologic occipitocervical alignment in patients, optimal craniocervical alignment has not been confirmed until now. Matsunaga et al[16] suggested that the position of fixation of the occipital bone and the axis should be within the range of 0-30° according to the O-C2a measurements of 240 healthy volunteers. Inada et al[17] indicated that excessive O–C2a lordotic correction should be avoided because it might induce a mid-to-lower cervical compensatory decrease in lordosis. Bagley et al[18] suggested that preoperative halo immobilization before OCF can guarantee physiologic craniocervical neutrality and prevent related complications. This method allows patients to be in a comfortable position when they are awake and allows surgeons to adjust the craniocervical alignment before final fixation and fusion.
However, the results of our study showed that there were no significant changes in O-C2a or C2-7a from before to after OCF surgery. The O-C2a of the patients with craniocervical disorders at the last follow-up was still significantly smaller than that of the asymptomatic volunteers, while the C2-7a of those patients was larger. We considered the main reason to be that we focused on decompression, reduction, and fusion for the treatment of craniocervical disorders but neglected the importance of restoring craniocervical sagittal alignment.
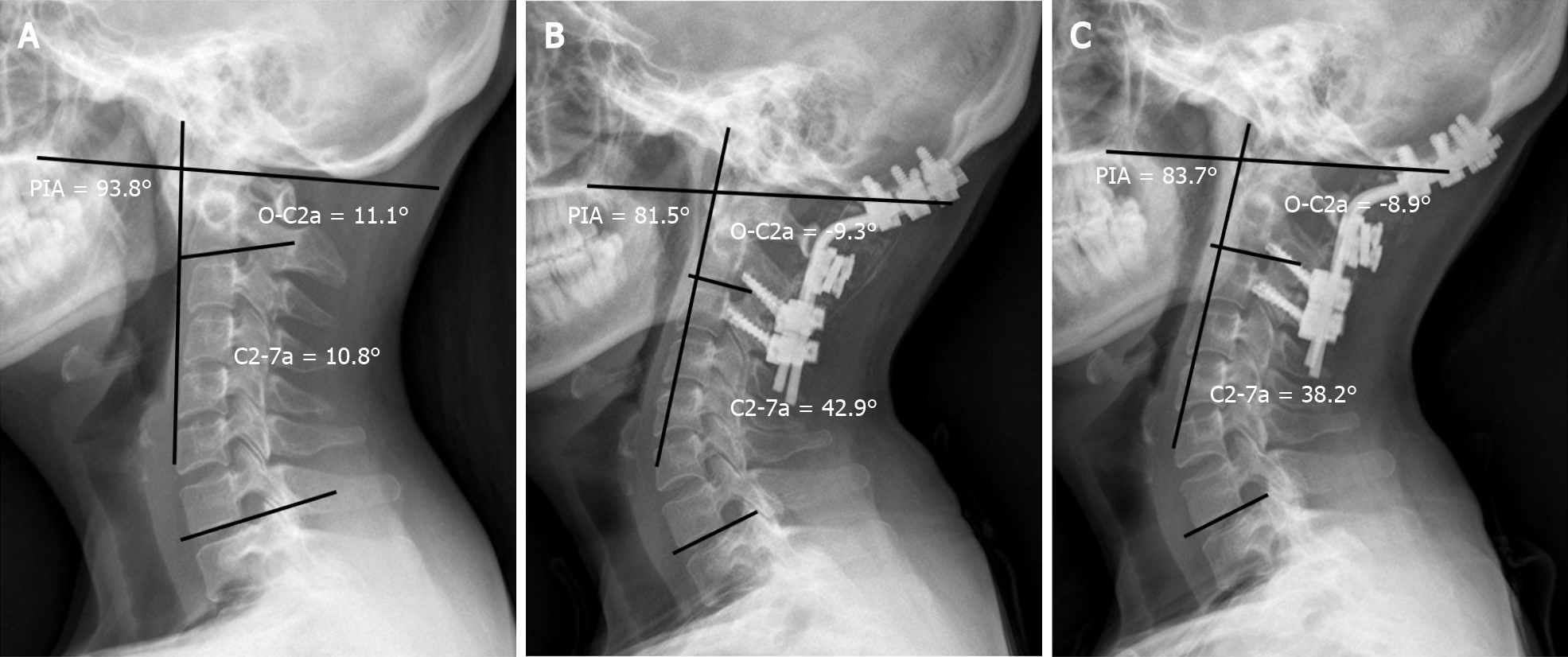
Dysphagia was reported to be one of the most common complications caused by cervical sagittal malalignment after OCF, with an incidence ranging from 9.4% to 26.6%[9,10,19,20]. The mechanisms of postoperative dysphagia after OCF remain unclear and are speculated to be multifactorial. In the present study, O-C2a and PIA decreased significantly in the patients with dysphagia from before surgery to 1 mo after surgery, and the effect remained at the final follow-up, while C2-7a increased 1 mo postoperatively and remained the same at the last follow-up. However, the O-C2a, C2-7a, and PIA of the patients without dysphagia were comparable pre- and postoperatively. Pearson’s correlation test showed that postoperative O-C2a values correlated significantly with C2-7a and PIA values. Our results were consistent with those of previous reports describing the relationship between CSA and postoperative dysphagia after OCF[10,19,20]. Based on these results, we assume that the mechanism by which postoperative dysphagia is caused by a reduction in O-C2a is as follows: When O-C2a decreases after OCF surgery, the degree of subaxial lordosis (C2-7a) increases to compensate for the decrease in occipitocervical lordosis so that the individual can maintain a horizontal gaze. Then, the apex of cervical spine lordosis protrudes anteriorly (PIA↓), which can compress and narrow the oropharyngeal space directly and lead to dysphagia (Figure 2).
Previous studies evaluated many factors that might lead to postoperative dysphagia, such as O-C2a, fused segments, age, pathologies, and subaxial cervical positioning[19,21]. However, O-C2a was the only significant independent variable correlated with dysphagia. Consequently, operative positioning of O-C2 is the most effective way to avoid postoperative dysphagia. Bagley et al[18] advocated that preoperative halo immobilization might allow patients to have their head fixed in a particular position and prevent dysphagia. Wang et al[9] and Meng et al[11] recom
Our study has limitations. Although we found that the O-C2a, C2-7a, and PIA could not be corrected to the normal range by OCF while neglecting craniocervical realignment, we did not find a good method of restoring physiologic occipitocervical alignment in patients with CJDs. To date, there is no consensus on the best method to reestablish occipitocervical sagittal alignment because of its complexity. Many factors should be taken into account to restore optimal alignment, such as the duration of the craniocervical disease, cervical motions in different directions, the influence of the intraoperative position, variations across different techniques, and the impact on adjacent segments[19]. Therefore, more studies focusing on the basic biomechanics of the occipitocervical junction, the development of new instrumentations and techniques, and individualized treatment to restore ideal occipitocervical alignment are needed in the future. Moreover, our study was also limited by the retrospective nature and short follow-up period. Therefore, additional prospective studies with more patients and longer follow-up periods are needed to investigate both the clinical and radiographic outcomes of OCF patients.
Compared to a normal age-matched control population, patients with CJDs have a more kyphotic upper CSA and a more lordotic lower CSA. The effectiveness of the restoration of CSA provided by OCF surgery may be limited by the realignment of the craniocervical junction being neglected. The reduction in O-C2a after OCF surgery may increase C2-7a and decrease PIA.
The studies regarding sequential changes of cervical sagittal alignment (CSA) after occipitocervical fusion (OCF) were limited.
The comprehension of sequential changes of CSA after OCF can help surgeons prevent postoperative complications after OCF.
To compare the CSA of patients with craniocervical junction disorders (CJDs) with that of normal controls and investigate the sequential changes in the CSA of the upper and lower cervical spine after OCF.
Radiographic parameters including the occipital to C2 angle (O-C2a), occipital and external acoustic meatus to axis angle (O-EAa), C2–7 angle (C2-7a), and pharyngeal inlet angle (PIA) of the selected patients were measured and compared pre- and postoperatively.
The O-C2a and PIA of the OCF group were smaller than those of the control group, while their O-EAa and C2-7a values were larger than those of the normal controls. There were significant correlations between the O-C2a and C2Ta, C2-7a, C2-7 sagittal vertical axis (SVA), and PIA at 1 mo after OCF surgery and between O-C2a and O-EAa, C2Ta, C2-7a, C2-7 SVA, and PIA at the final follow-up.
Patients with CJDs have a more kyphotic upper CSA and a more lordotic lower CSA than normal controls. The effectiveness of OCF surgery in restoring CSA may be limited by the realignment of the craniocervical junction being neglected. The reduction in O-C2a after OCF surgery may increase C2-7a and decrease PIA.
This study provides novel insights for sequential changes of CSA after OCF.
We are grateful to Yi Zhu from McMaster University for his kind help in editing the language in this paper.
| 1. | Nojiri K, Matsumoto M, Chiba K, Maruiwa H, Nakamura M, Nishizawa T, Toyama Y. Relationship between alignment of upper and lower cervical spine in asymptomatic individuals. J Neurosurg. 2003;99:80-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 52] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 2. | Patwardhan AG, Khayatzadeh S, Havey RM, Voronov LI, Smith ZA, Kalmanson O, Ghanayem AJ, Sears W. Cervical sagittal balance: a biomechanical perspective can help clinical practice. Eur Spine J. 2018;27:25-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 107] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 3. | Lee SH, Kim KT, Seo EM, Suk KS, Kwack YH, Son ES. The influence of thoracic inlet alignment on the craniocervical sagittal balance in asymptomatic adults. J Spinal Disord Tech. 2012;25:E41-E47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 259] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 4. | Huang JC, Qian BP, Qiu Y, Yu Y, Ni HB. Surgical overreduction and hyperlordotic fusion of C1-C2 joint are associated with cervical sagittal malalignment. Arch Orthop Trauma Surg. 2017;137:1631-1639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 5. | Kim JT, Lee HJ, Choi DY, Shin MH, Hong JT. Sequential alignment change of the cervical spine after anterior cervical discectomy and fusion in the lower cervical spine. Eur Spine J. 2016;25:2223-2232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 6. | Kunakornsawat S, Pluemvitayaporn T, Pruttikul P, Punpichet S, Piyasakulkaew C, Arirachakaran A, Kongtharvonskul J. A new method for measurement of occipitocervical angle by occiput-C3 angle. Eur J Orthop Surg Traumatol. 2017;27:1051-1056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 7. | Matsubayashi Y, Shimizu T, Chikuda H, Takeshita K, Oshima Y, Tanaka S. Correlations of Cervical Sagittal Alignment before and after Occipitocervical Fusion. Global Spine J. 2016;6:362-369. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 8. | Korovessis P, Mpountogianni E, Papaioannou I. Predictive value of sagittal craniocervical roentgenographic parameters for HRQOL after craniocervical fusion. Eur J Orthop Surg Traumatol. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 9. | Wang LN, Hu BW, Song YM, Liu LM, Zhou CG, Wang L, Zhou ZJ, Xiu P, Chen TY, Yang X. Predictive abilities of O-C2a and O-EAa for the development of postoperative dysphagia in patients undergoing occipitocervical fusion. Spine J. 2020;20:745-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 10. | Kaneyama S, Sumi M, Takabatake M, Kasahara K, Kanemura A, Hirata H, Darden BV. The Prediction and Prevention of Dysphagia After Occipitospinal Fusion by Use of the S-line (Swallowing Line). Spine (Phila Pa 1976). 2017;42:718-725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 11. | Meng Y, Wu T, Liu Z, Wen D, Rong X, Chen H, Lou J, Liu H. The impact of the difference in O-C2 angle in the development of dysphagia after occipitocervical fusion: a simulation study in normal volunteers combined with a case-control study. Spine J. 2018;18:1388-1397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 12. | Kong W, Yang X, Li Z, Hu B, Song Y. Analysis of the Cervical Sagittal Alignment in Patients with Unstable Hangman Fracture Under C2∼3 Anterior Discectomy and Fusion. World Neurosurg. 2020;137:e1-e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 13. | Sherekar SK, Yadav YR, Basoor AS, Baghel A, Adam N. Clinical implications of alignment of upper and lower cervical spine. Neurol India. 2006;54:264-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 42] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 14. | Guo Q, Ni B, Yang J, Liu K, Sun Z, Zhou F, Zhang J. Relation between alignments of upper and subaxial cervical spine: a radiological study. Arch Orthop Trauma Surg. 2011;131:857-862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 59] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 15. | Morizane K, Takemoto M, Neo M, Fujibayashi S, Otsuki B, Kawata T, Matsuda S. Occipital and external acoustic meatus to axis angle as a predictor of the oropharyngeal space in healthy volunteers: a novel parameter for craniocervical junction alignment. Spine J. 2018;18:811-817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 16. | Matsunaga S, Onishi T, Sakou T. Significance of occipitoaxial angle in subaxial lesion after occipitocervical fusion. Spine (Phila Pa 1976). 2001;26:161-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 91] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 17. | Inada T, Furuya T, Kamiya K, Ota M, Maki S, Suzuki T, Takahashi K, Yamazaki M, Aramomi M, Mannoji C, Koda M. Postoperative Increase in Occiput-C2 Angle Negatively Impacts Subaxial Lordosis after Occipito-Upper Cervical Posterior Fusion Surgery. Asian Spine J. 2016;10:744-747. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 18. | Bagley CA, Witham TF, Pindrik JA, Davis RF, Bydon A, Gokaslan ZL, Wolinsky JP. Assuring optimal physiologic craniocervical alignment and avoidance of swallowing-related complications after occipitocervical fusion by preoperative halo vest placement. J Spinal Disord Tech. 2009;22:170-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 19. | Miyata M, Neo M, Fujibayashi S, Ito H, Takemoto M, Nakamura T. O-C2 angle as a predictor of dyspnea and/or dysphagia after occipitocervical fusion. Spine (Phila Pa 1976). 2009;34:184-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 123] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 20. | Tian W, Yu J. The Role of C2-C7 Angle in the Development of Dysphagia After Anterior and Posterior Cervical Spine Surgery. Clin Spine Surg. 2017;30:E1306-E1314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 21. | Gonda DD, Huang M, Briceño V, Lam SK, Luerssen TG, Jea A. Protecting Against Postoperative Dyspnea and Dysphagia After Occipitocervical Fusion. Oper Neurosurg (Hagerstown). 2020;18:254-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 22. | Huang M, Gonda DD, Briceño V, Lam SK, Luerssen TG, Jea A. Dyspnea and dysphagia from upper airway obstruction after occipitocervical fusion in the pediatric age group. Neurosurg Focus. 2015;38:E13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Byeon H, Chhabra HS S-Editor: Fan JR L-Editor: Wang TQ P-Editor: Fan JR