Published online Dec 26, 2022. doi: 10.12998/wjcc.v10.i36.13148
Peer-review started: July 20, 2022
First decision: November 5, 2022
Revised: November 20, 2022
Accepted: December 8, 2022
Article in press: December 8, 2022
Published online: December 26, 2022
Processing time: 159 Days and 13.2 Hours
Even in patients without a history of liver disease, liver injury caused by coronavirus disease 2019 (COVID-19) is gradually becoming more common. However, the precise pathophysiological mechanisms behind COVID-19's liver pathogenicity are still not fully understood. We hypothesize that inflammation may become worse by cytokine storms caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. Elevated ferritin levels can initiate ferritinophagy mediated by nuclear receptor coactivator 4 (NCOA4), which leads to iron elevation, and ferroptosis. In COVID-19 patients, ferroptosis can be restricted to reduce disease severity and liver damage by targeting NCOA4-mediated ferritinophagy. To confirm the role of ferritinophagy-mediated ferroptosis in SARS-CoV-2 infection, further research is required.
Core Tip: Liver injury in patients with coronavirus disease 2019 (COVID-19) has progressively emerged, yet the exact pathophysiological mechanisms to explain liver pathogenicity is presently not fully understood. We hypothesize that cytokine storms caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection may promote hyper-ferritinemia, which can further aggravate inflammation. Elevated ferritin levels can trigger nuclear receptor coactivator 4 (NCOA4)-mediated ferritinophagy, which leads to iron elevation, and ferroptosis. NCOA4-mediated ferritinophagy can be targeted to limit the ferroptosis and, therefore, prevent liver damage and disease severity in patients with COVID-19.
- Citation: Jia FJ, Han J. Liver injury in COVID-19: Holds ferritinophagy-mediated ferroptosis accountable. World J Clin Cases 2022; 10(36): 13148-13156
- URL: https://www.wjgnet.com/2307-8960/full/v10/i36/13148.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i36.13148
The coronavirus disease 2019 (COVID-19) pandemic is caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and exhibits a wide range of severity, from mild symptoms to severe presentation and death. Although respiratory symptoms are more frequent, signs of hepatic involvement have gradually come to light[1-4]. Aside from respiratory complications, the most common complication of SARS-CoV-2 infection is liver injury, which has been reported in up to 50% of cases[5-7]. Expanding prove shows a close relationship between anomalous liver function and the severity and mortality of the illness[8,9]. Derangement of alanine aminotransferase/aspartate aminotransferase levels is a key indicator of liver damage in COVID-19, accompanied by marginally elevated bilirubin levels, which are commonly used to diagnose hepatic injury such as acute hepatitis, steatosis, portal inflammation, granulomas, thrombotic bodies, and biliary pathology[10-12]. The raised liver catalysts are not only associated with severe disease and longer disease duration, but also common in the early stage of the COVID-19 pandemic[13]. However, the precise cause of liver damage is still unclear[14,15].
The following potential mechanisms have been suggested that may be responsible for hepatic injury: SARS-CoV-2 causing direct harm to hepatocytes and biliary epithelium, indirect damage prompted by an exaggerated cytokine storm, and/or drug-incited hepatoxicity[10]. Patients with COVID-19 may experience liver dysfunction as a direct result of viral infection. A previous study showed the liver pathology of SARS patients and found SARS-associated coronavirus in liver tissues, suggesting that hepatic impairment was caused by viral infection in the liver[16]. Angiotensin converting enzyme 2 (ACE2) has recently been discovered as the SARS-functional CoV's host cell receptor, facilitating the entry of SARS-CoV-2 into cells[17-20]. The spike (S) protein of SARS-CoV-2 may also be broken down by transmembrane protease serine 2 (TMPRSS2), which makes it easier for the virus to fuse with cellular membranes[21]. Although they are expressed in many organs, including the liver, ACE2 and TMPRSS2 are expressed at varying amounts in different cell types, with cholangiocytes expressing them at a higher level than hepatocytes[22,23]. It has been demonstrated that hepatocyte and cholangiocyte organoids are receptive to SARS-CoV-2 infection[24]; however, in vivo confirmation of this phenomenon is still pending[25]. Ultrastructural and histological analysis have shown that hepatocytes exhibit a characteristic SARS-CoV-2 infected lesion[26]. In-depth proteomic analysis of autopsy tissue recently produced new data that showed little evidence of virus replication in the liver[27]. Additionally, an autopsy of a COVID-19 patient demonstrated that the liver tissue was free of viral inclusions[28]. Direct viral damage to the liver is not considered to be the main culprit when multiorgan problems such cardiopulmonary insufficiency, renal impairment, systemic inflammatory status, and the use of several medicines are taken into account. Consequently, the mechanism of liver damage by SARS-CoV-2 infection remains unknown.
Although the majority of COVID-19 patients experience a moderate early disease onset, some patients' conditions quickly worsen and they may experience multiple organ failure as a result of an inflammatory "cytokine storm"[29,30]. Tumor necrosis factor-α, interleukin(IL)-2, IL-6, IL-7, IL-18, granulocyte-colony stimulating factor, interferon-γ, monocyte chemotactic protein 1, macrophage inflammatory protein 1 alpha, interferon-inducible protein-10, and ferritin are examples of inflammatory cytokines that are exuberantly released during an inflammatory cytokine storm[9,30]. Patients with severe disease had significantly higher peripheral blood levels of the aforementioned variables than patients with mild disease[31,32]. Hypercytokinemia that is deadly or fulminant may set off a series of events that damage the liver[33]. In patients with COVID-19, lymphopenia and high C-reactive protein levels may operate as independent predictors of hepatic damage, which may be caused by an inflammatory cytokine storm[34]. Through the activation of killer T cells and toll-like receptors (TLRs), SARS-CoV-2 can directly produce a number of proinflammatory signals[35]. Following SARS-CoV-2 infection, activated T lymphocytes attack the infected cells, causing them to die and become necrotic until there are no more T lymphocytes left. TLRs, chemicals associated with damage, that are generated by infected dead cells, can intensify the inflammatory signals. Failure to control viral and bacterial infections caused by T-lymphocyte depletion results in the activation of various inflammatory signaling pathways that activate macrophages thereby causing subsequent inflammatory reactions. In addition to the lungs, this vicious cycle can harm numerous organs, including the liver. Furthermore, it has been observed that COVID-19 patients show hepatic impairment due to a severe cytokine storm rather than the direct cytopathogenic effects of SARS-CoV-2 itself[36-39]. Patients with COVID-19 are more likely to have a number of hepatic illnesses, such as non-alcoholic fatty liver disease, liver cirrhosis, hepatocellular carcinoma, hepatitis B, and hepatitis C[36,40,41].
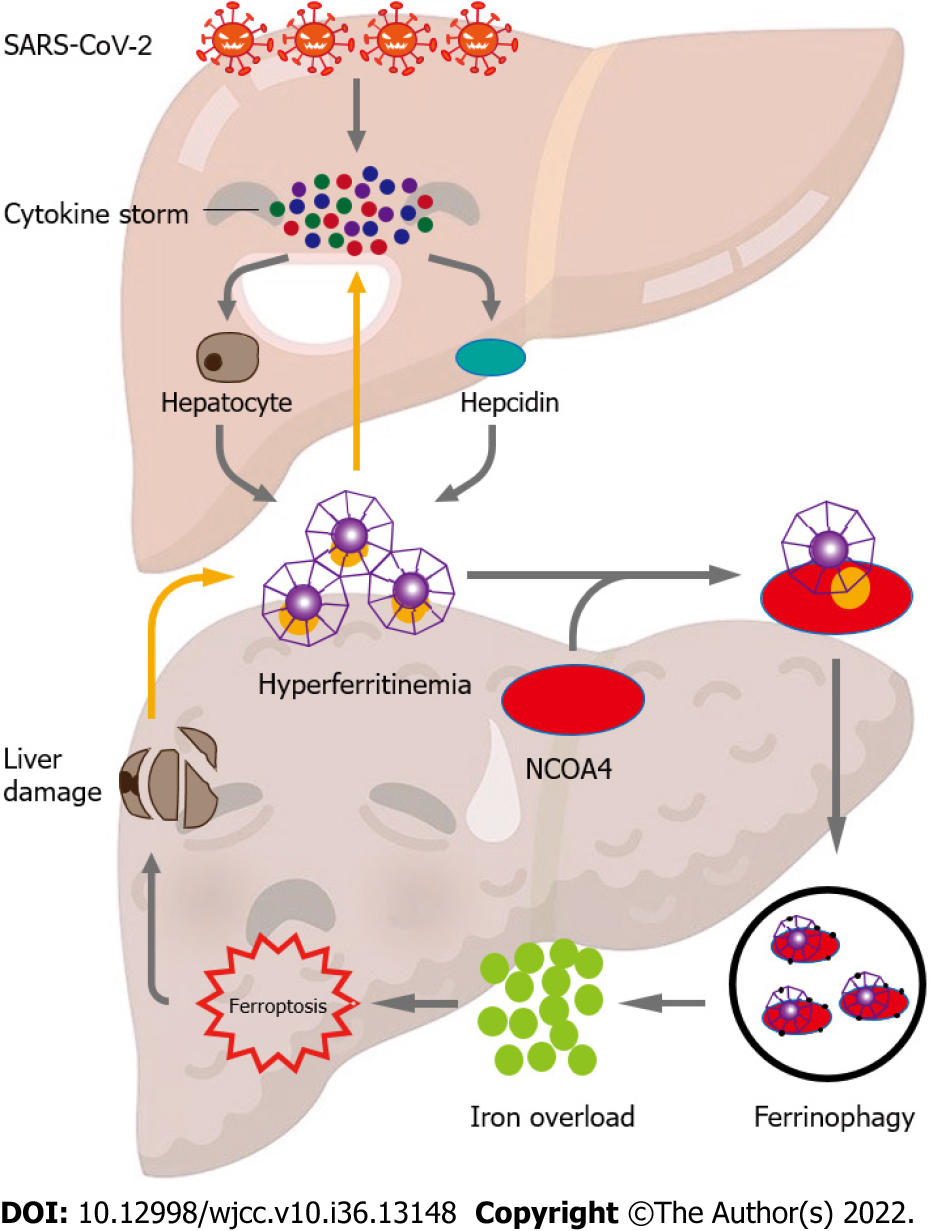
It has been established that hyperferritinemia is a distinctive symptom of severe COVID-19[42]. The severity and poor prognosis of patients with COVID-19 have been directly associated with ferritin levels[33]. Although the liver significantly contributes to the levels of circulating serum ferritin in COVID-19, proximal tubule cells of the kidney and splenic macrophages are also two potential biological sources. Hepatocytes not only actively secrete ferritin[43], but also release ferritin after hepatic cell death[44]. Furthermore, by producing iron in enterocytes and macrophages, the important iron-regulatory hormone hepcidin may raise intracellular ferritin levels[45]. Majority of patients had inflammation-dependent elevation of hepcidin levels to varying degrees in severe illnesses with hyperinflammation[46]. The severity of COVID-19 is associated with elevated serum hepcidin levels[46,47]. Interestingly, SARS-CoV-2 can imitate hepcidin without triggering an inflammatory response by causing ferroportin blockage, which results in high ferritin levels[48].
The body's main organ for storing iron is the liver. According to growing evidence, lytic cell death processes like necroptosis, pyroptosis, and ferroptosis cause intense inflammatory reactions by releasing cellular components and permeating cell membranes. This results in the activation of hepatic stellate cells (HSCs) and the recruitment of immune cells[49]. It is without dispute that both hereditary and acquired iron in excess contribute to liver damage. Due to the active mobilization of cellular iron caused by the stimulation of ferritinophagy, ferroptosis may be used to evaluate excess iron. However, unrestrained free iron is harmful to the liver, promoting the development of hepatic disorders and producing serious side effects[49].
The most important cellular iron storing protein is ferritin. The liver is the primary organ for storing iron as a ferritin complex and plays a crucial role in maintaining iron homeostasis. It has been proven that excessive iron conditions may lead to liver damage. Different types of liver disorders are largely caused by iron-catalyzed oxidative damage[50,51]. The release of free iron from the broken hemoglobin and ferritin catabolism may cause COVID-19-related iron excess. High blood levels of free iron may result from ferritin, losing some of its internal iron content[52]. Ferritin abundance is a major determinant of iron homeostasis, as proved by the fact that iron deposits produced by ferritin result in a weakly labile iron pool. Contrarily, the release of iron into the labile iron pool, as a result of ferritin depletion, increases vulnerability to ferroptosis[53]. Studies have hypothesized that ferroptosis in fibroblasts and cancer cells is influenced by the selective autophagic turnover of ferritin (ferritinophagy)[54]. Ferritin's cargo receptor, nuclear receptor coactivator 4 (NCOA4), attaches and transports it to autophagosomes for ferritin breakdown and iron release[55,56]. Ferritinophagy has previously been shown to have important roles in the pathological processes of neurodegeneration, cancer, ischemia/reperfusion injury, and urinary tract infections[57], however, its possible involvement in COVID-19 remains unknown[58]. For the past 4 years, ferritinophagy has been involved in physiology and pathology process of liver, including hepatic insulin resistance[59], hepatocyte senescence[60], ferroptosis in hepatic stellate cells[61-63], hepatocellular carcinoma[64,65] and liver fibrosis[66] (Supplementary Table 1).
In COVID-19 patients, iron depletion or chelation has been suggested as a possible antiviral treatment to guard against severe inflammatory reactions and tissue damage by sequestering iron and inhibiting the generation of oxygen radicals and lipid peroxidation[67]. Poonkuzhi et al[68] mentioned that deferasirox administered orally, along with intravenous deferoxamine made iron chelation therapy effective for COVID-19 victims. A case-control study showed that iron chelators which reducd iron intake could be considered a therapeutic goal of COVID-19[69]. Additionally, the iron chelator and lactoferrin can block SARS-CoV-2 receptor binding for entrance into host cells[70,71].
Excess intracellular iron can produce reactive oxygen species (via Haber-Weiss and Fenton processes), reactive nitrogen species, and reactive sulfur species by reacting with molecular oxygen[72]. Redox injury favors mitochondrial malfunction, leading to ferroptosis, various tissue damages, and eventual fibrosis[73]. A new genetically encoded form of programmed cell death called ferroptosis is caused by iron-dependent lipid peroxidation[74]. Opportunities for diagnosis and treatment have been created by further functional understanding of ferroptosis' role in liver fibrosis development[74,75]. Artesunate reduces liver fibrosis by modulating the ferroptosis signaling system, according to a recent publication by Kong et al[66]. Additionally, Sui et al[76] demonstrated that by controlling the ferroptosis signaling pathway, magnesium isoglycyrrhizinate reduces liver fibrosis and HSC activation. Moreover, for artemether to reduce liver fibrosis and HSC activation brought on by carbon tetrachloride, p53-dependent induction of ferroptosis is required[77]. For artemether to effectively treat hepatic fibrosis via the ferroptosis pathway, iron regulatory protein 2 is necessary. Moreover, by inhibiting lipid peroxidation and glutathione depletion, ferrostatin-1, deferoxamine, and vitamin E may have a protective impact on hepatocytes[78].
Iron is abundant in HSCs, which is necessary for ferroptotic cell death[62]. Presumably, ferroptosis encourages the onset and progression of liver fibrosis. It has been found that liver fibrosis caused by acetaminophen in mice is amplified by excessive hepatic iron deposition and ferroptosis, which can be reversed by ferrostatin-1[79,80]. Ferroptosis was also shown by Zhou et al[81-83] to be a kind of autophagy-dependent cell death. Numerous studies have suggested that autophagy controls cellular iron homeostasis and the production of reactive oxygen species, thereby acting as an upstream mechanism in the activation of ferroptosis[81-83]. According to Wang et al[84], ferric citrate, a ferroptosis stimulant, potently causes ferroptosis in murine primary hepatocytes and bone marrow-derived macrophages, which prevents the healing of liver injury. Chen et al[85] proposed that ferroptosis caused by reactive oxygen species contributes to liver damage caused by COVID-19.
Notably, a number of recent investigations have shed light on controlled cell death and underlined the significance of autophagy as an emerging mechanism of ferroptosis[86,87]. Multiple routes, including ferritinophagy, which is NCOA4 dependent, may be involved in the molecular mechanisms[54]. Thus, during ferroptosis, ferritinophagy promotes iron accumulation and free radical damage. Dihydroartemisinin, according to Du et al[88], reduces leukemia cells' ability to proliferate and causes ferroptosis by degrading ferritin under the control of autophagy. Moreover, Kong et al[66] demonstrated that artesunate reduced HSC ferroptosis caused by ferritinophagy. Notably, the activation of ferritinophagy is required for the RNA-binding protein, embryonic lethal vision-like protein 1, to regulate ferroptosis in HSCs[62]. New diagnostic and therapeutic ways to control HSC survival and death in liver fibrosis may be provided by further investigations of the post-transcriptional regulating mechanisms of ferroptosis. However, as hepatocytes die, ferritin is further released. Due to the mutual stimulation of ferritin and hepatocyte destruction, a vicious cycle is created that continuously worsens liver damage.
In light of this, we speculate that cytokine storms caused by SARS-COV-2 infection can encourage hyper-ferritinemia, exacerbating inflammation. Elevated ferritin levels can cause ferroptosis, cell death, and liver damage by inducing NCOA4-mediated ferritinophagy. Therefore, in COVID-19 patients, ferroptosis can be addressed to reduce liver damage and disease severity by limiting ferroptosis (Figure 1). To validate the role of ferritinophagy-mediated ferroptosis in SARS-CoV-2 infection, further research is required. Drug designing to target hepatic cells ferritinophagy-mediated ferroptosis would be a novel approach in the treatment of COVID-19-induced liver injury.
| 1. | Mao R, Qiu Y, He JS, Tan JY, Li XH, Liang J, Shen J, Zhu LR, Chen Y, Iacucci M, Ng SC, Ghosh S, Chen MH. Manifestations and prognosis of gastrointestinal and liver involvement in patients with COVID-19: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2020;5:667-678. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 741] [Cited by in RCA: 760] [Article Influence: 126.7] [Reference Citation Analysis (2)] |
| 2. | Wu HHL, Athwal VS, Kalra PA, Chinnadurai R. COVID-19 and hepatorenal syndrome. World J Gastroenterol. 2022;28:5666-5678. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 3. | Shih AR, Misdraji J. COVID-19: gastrointestinal and hepatobiliary manifestations. Hum Pathol. 2022;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 4. | Payus AO, Mohd Noh M, Azizan N, Muthukaruppan Chettiar R. SARS-CoV-2-induced liver injury: A review article on the high-risk populations, manifestations, mechanisms, pathological changes, management, and outcomes. World J Gastroenterol. 2022;28:5723-5730. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 3] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (1)] |
| 5. | Fan Z, Chen L, Li J, Cheng X, Yang J, Tian C, Zhang Y, Huang S, Liu Z, Cheng J. Clinical Features of COVID-19-Related Liver Functional Abnormality. Clin Gastroenterol Hepatol. 2020;18:1561-1566. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 559] [Cited by in RCA: 560] [Article Influence: 93.3] [Reference Citation Analysis (0)] |
| 6. | Bloom PP, Meyerowitz EA, Reinus Z, Daidone M, Gustafson J, Kim AY, Schaefer E, Chung RT. Liver Biochemistries in Hospitalized Patients With COVID-19. Hepatology. 2021;73:890-900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 148] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 7. | Sun J, Aghemo A, Forner A, Valenti L. COVID-19 and liver disease. Liver Int. 2020;40:1278-1281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 222] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 8. | Henry BM, Vikse J. Clinical Characteristics of Covid-19 in China. N Engl J Med. 2020;382:1860-1861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 260] [Article Influence: 43.3] [Reference Citation Analysis (0)] |
| 9. | Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497-506. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35178] [Cited by in RCA: 30478] [Article Influence: 5079.7] [Reference Citation Analysis (13)] |
| 10. | Yang RX, Zheng RD, Fan JG. Etiology and management of liver injury in patients with COVID-19. World J Gastroenterol. 2020;26:4753-4762. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 58] [Cited by in RCA: 53] [Article Influence: 8.8] [Reference Citation Analysis (2)] |
| 11. | Papadopoulos N, Vasileiadi S, Deutsch M. COVID-19 and liver injury: where do we stand? Ann Gastroenterol. 2020;33:459-464. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 12. | Lagana SM, Kudose S, Iuga AC, Lee MJ, Fazlollahi L, Remotti HE, Del Portillo A, De Michele S, de Gonzalez AK, Saqi A, Khairallah P, Chong AM, Park H, Uhlemann AC, Lefkowitch JH, Verna EC. Hepatic pathology in patients dying of COVID-19: a series of 40 cases including clinical, histologic, and virologic data. Mod Pathol. 2020;33:2147-2155. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 130] [Cited by in RCA: 184] [Article Influence: 30.7] [Reference Citation Analysis (1)] |
| 13. | Chen D, Ning M, Feng Y, Liu J. The early stage of COVID-19 pandemic: Gastrointestinal manifestations and liver injury in COVID-19 patients in Wuhan, China. Front Med (Lausanne). 2022;9:997000. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 14. | Li D, Ding X, Xie M, Tian D, Xia L. COVID-19-associated liver injury: from bedside to bench. J Gastroenterol. 2021;56:218-230. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 15. | Brizawasi A, Ahirwar AK, Prabhat, Kaim K, Ahirwar P, Kumawat R, Prasad J. COVID-19: a viewpoint from hepatic perspective. Horm Mol Biol Clin Investig. 2022;. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 16. | Chau TN, Lee KC, Yao H, Tsang TY, Chow TC, Yeung YC, Choi KW, Tso YK, Lau T, Lai ST, Lai CL. SARS-associated viral hepatitis caused by a novel coronavirus: report of three cases. Hepatology. 2004;39:302-310. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 307] [Cited by in RCA: 305] [Article Influence: 13.9] [Reference Citation Analysis (1)] |
| 17. | Li W, Moore MJ, Vasilieva N, Sui J, Wong SK, Berne MA, Somasundaran M, Sullivan JL, Luzuriaga K, Greenough TC, Choe H, Farzan M. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450-454. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4113] [Cited by in RCA: 4644] [Article Influence: 201.9] [Reference Citation Analysis (0)] |
| 18. | Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203:631-637. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3643] [Cited by in RCA: 4193] [Article Influence: 190.6] [Reference Citation Analysis (1)] |
| 19. | Walls AC, Park YJ, Tortorici MA, Wall A, McGuire AT, Veesler D. Structure, Function, and Antigenicity of the SARS-CoV-2 Spike Glycoprotein. Cell. 2020;181:281-292.e6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4743] [Cited by in RCA: 6275] [Article Influence: 1045.8] [Reference Citation Analysis (1)] |
| 20. | Domovitz T, Ayoub S, Werbner M, Alter J, Izhaki Tavor L, Yahalom-Ronen Y, Tikhonov E, Meirson T, Maman Y, Paran N, Israely T, Dessau M, Gal-Tanamy M. HCV Infection Increases the Expression of ACE2 Receptor, Leading to Enhanced Entry of Both HCV and SARS-CoV-2 into Hepatocytes and a Coinfection State. Microbiol Spectr. 2022;e0115022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 21. | Dong M, Zhang J, Ma X, Tan J, Chen L, Liu S, Xin Y, Zhuang L. ACE2, TMPRSS2 distribution and extrapulmonary organ injury in patients with COVID-19. Biomed Pharmacother. 2020;131:110678. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 189] [Cited by in RCA: 187] [Article Influence: 31.2] [Reference Citation Analysis (0)] |
| 22. | Osorio Martínez A, González-Razo VT, Navarro-Sánchez V, Souto Meiriño CA, Ahumada-Ayala M. SARS-CoV-2-Related Subacute Thyroiditis, Myocarditis, and Hepatitis After Full Resolution of COVID-19 Serum Markers. Am J Case Rep. 2021;22:e932321. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 23. | Pirola CJ, Sookoian S. SARS-CoV-2 virus and liver expression of host receptors: Putative mechanisms of liver involvement in COVID-19. Liver Int. 2020;40:2038-2040. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 97] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 24. | Zhao B, Ni C, Gao R, Wang Y, Yang L, Wei J, Lv T, Liang J, Zhang Q, Xu W, Xie Y, Wang X, Yuan Z, Zhang R, Lin X. Recapitulation of SARS-CoV-2 infection and cholangiocyte damage with human liver ductal organoids. Protein Cell. 2020;11:771-775. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 268] [Cited by in RCA: 318] [Article Influence: 53.0] [Reference Citation Analysis (2)] |
| 25. | Philips CA, Ahamed R, Augustine P. SARS-CoV-2 related liver impairment - perception may not be the reality. J Hepatol. 2020;73:991-992. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 26. | Wang Y, Liu S, Liu H, Li W, Lin F, Jiang L, Li X, Xu P, Zhang L, Zhao L, Cao Y, Kang J, Yang J, Li L, Liu X, Li Y, Nie R, Mu J, Lu F, Zhao S, Lu J, Zhao J. SARS-CoV-2 infection of the liver directly contributes to hepatic impairment in patients with COVID-19. J Hepatol. 2020;73:807-816. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 353] [Cited by in RCA: 468] [Article Influence: 78.0] [Reference Citation Analysis (1)] |
| 27. | Nie X, Qian L, Sun R, Huang B, Dong X, Xiao Q, Zhang Q, Lu T, Yue L, Chen S, Li X, Sun Y, Li L, Xu L, Li Y, Yang M, Xue Z, Liang S, Ding X, Yuan C, Peng L, Liu W, Yi X, Lyu M, Xiao G, Xu X, Ge W, He J, Fan J, Wu J, Luo M, Chang X, Pan H, Cai X, Zhou J, Yu J, Gao H, Xie M, Wang S, Ruan G, Chen H, Su H, Mei H, Luo D, Zhao D, Xu F, Zhu Y, Xia J, Hu Y, Guo T. Multi-organ proteomic landscape of COVID-19 autopsies. Cell. 2021;184:775-791.e14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 358] [Cited by in RCA: 326] [Article Influence: 65.2] [Reference Citation Analysis (0)] |
| 28. | Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, Liu S, Zhao P, Liu H, Zhu L, Tai Y, Bai C, Gao T, Song J, Xia P, Dong J, Zhao J, Wang FS. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420-422. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5228] [Cited by in RCA: 5831] [Article Influence: 971.8] [Reference Citation Analysis (3)] |
| 29. | Prompetchara E, Ketloy C, Palaga T. Immune responses in COVID-19 and potential vaccines: Lessons learned from SARS and MERS epidemic. Asian Pac J Allergy Immunol. 2020;38:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 351] [Cited by in RCA: 658] [Article Influence: 109.7] [Reference Citation Analysis (1)] |
| 30. | Tisoncik JR, Korth MJ, Simmons CP, Farrar J, Martin TR, Katze MG. Into the eye of the cytokine storm. Microbiol Mol Biol Rev. 2012;76:16-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1171] [Cited by in RCA: 1355] [Article Influence: 96.8] [Reference Citation Analysis (1)] |
| 31. | Cai Q, Huang D, Ou P, Yu H, Zhu Z, Xia Z, Su Y, Ma Z, Zhang Y, Li Z, He Q, Liu L, Fu Y, Chen J. COVID-19 in a designated infectious diseases hospital outside Hubei Province, China. Allergy. 2020;75:1742-1752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 331] [Article Influence: 55.2] [Reference Citation Analysis (0)] |
| 32. | Li H, Chen K, Liu M, Xu H, Xu Q. The profile of peripheral blood lymphocyte subsets and serum cytokines in children with 2019 novel coronavirus pneumonia. J Infect. 2020;81:115-120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 59] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 33. | Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ; HLH Across Speciality Collaboration, UK. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033-1034. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6366] [Cited by in RCA: 6842] [Article Influence: 1140.3] [Reference Citation Analysis (1)] |
| 34. | Wu Y, Li H, Guo X, Yoshida EM, Mendez-Sanchez N, Levi Sandri GB, Teschke R, Romeiro FG, Shukla A, Qi X. Incidence, risk factors, and prognosis of abnormal liver biochemical tests in COVID-19 patients: a systematic review and meta-analysis. Hepatol Int. 2020;14:621-637. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 93] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 35. | Tartey S, Takeuchi O. Pathogen recognition and Toll-like receptor targeted therapeutics in innate immune cells. Int Rev Immunol. 2017;36:57-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 163] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 36. | Ali FEM, Mohammedsaleh ZM, Ali MM, Ghogar OM. Impact of cytokine storm and systemic inflammation on liver impairment patients infected by SARS-CoV-2: Prospective therapeutic challenges. World J Gastroenterol. 2021;27:1531-1552. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (3)] |
| 37. | Fix OK, Hameed B, Fontana RJ, Kwok RM, McGuire BM, Mulligan DC, Pratt DS, Russo MW, Schilsky ML, Verna EC, Loomba R, Cohen DE, Bezerra JA, Reddy KR, Chung RT. Clinical Best Practice Advice for Hepatology and Liver Transplant Providers During the COVID-19 Pandemic: AASLD Expert Panel Consensus Statement. Hepatology. 2020;72:287-304. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 338] [Cited by in RCA: 425] [Article Influence: 70.8] [Reference Citation Analysis (0)] |
| 38. | Liu J, Li S, Liu J, Liang B, Wang X, Wang H, Li W, Tong Q, Yi J, Zhao L, Xiong L, Guo C, Tian J, Luo J, Yao J, Pang R, Shen H, Peng C, Liu T, Zhang Q, Wu J, Xu L, Lu S, Wang B, Weng Z, Han C, Zhu H, Zhou R, Zhou H, Chen X, Ye P, Zhu B, Wang L, Zhou W, He S, He Y, Jie S, Wei P, Zhang J, Lu Y, Wang W, Zhang L, Li L, Zhou F, Wang J, Dittmer U, Lu M, Hu Y, Yang D, Zheng X. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. EBioMedicine. 2020;55:102763. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1171] [Cited by in RCA: 1222] [Article Influence: 203.7] [Reference Citation Analysis (0)] |
| 39. | Gu J, Han B, Wang J. COVID-19: Gastrointestinal Manifestations and Potential Fecal-Oral Transmission. Gastroenterology. 2020;158:1518-1519. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 954] [Cited by in RCA: 950] [Article Influence: 158.3] [Reference Citation Analysis (2)] |
| 40. | Sachdeva S, Khandait H, Kopel J, Aloysius MM, Desai R, Goyal H. NAFLD and COVID-19: a Pooled Analysis. SN Compr Clin Med. 2020;2:2726-2729. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 65] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 41. | Sarin SK, Choudhury A, Lau GK, Zheng MH, Ji D, Abd-Elsalam S, Hwang J, Qi X, Cua IH, Suh JI, Park JG, Putcharoen O, Kaewdech A, Piratvisuth T, Treeprasertsuk S, Park S, Wejnaruemarn S, Payawal DA, Baatarkhuu O, Ahn SH, Yeo CD, Alonzo UR, Chinbayar T, Loho IM, Yokosuka O, Jafri W, Tan S, Soo LI, Tanwandee T, Gani R, Anand L, Esmail ES, Khalaf M, Alam S, Lin CY, Chuang WL, Soin AS, Garg HK, Kalista K, Batsukh B, Purnomo HD, Dara VP, Rathi P, Al Mahtab M, Shukla A, Sharma MK, Omata M; APASL COVID Task Force, APASL COVID Liver Injury Spectrum Study (APCOLIS Study-NCT 04345640). Pre-existing liver disease is associated with poor outcome in patients with SARS CoV2 infection; The APCOLIS Study (APASL COVID-19 Liver Injury Spectrum Study). Hepatol Int. 2020;14:690-700. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 217] [Cited by in RCA: 216] [Article Influence: 36.0] [Reference Citation Analysis (1)] |
| 42. | Bellmann-Weiler R, Lanser L, Barket R, Rangger L, Schapfl A, Schaber M, Fritsche G, Wöll E, Weiss G. Prevalence and Predictive Value of Anemia and Dysregulated Iron Homeostasis in Patients with COVID-19 Infection. J Clin Med. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 148] [Cited by in RCA: 157] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 43. | Ghosh S, Hevi S, Chuck SL. Regulated secretion of glycosylated human ferritin from hepatocytes. Blood. 2004;103:2369-2376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 99] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 44. | Colafrancesco S, Alessandri C, Conti F, Priori R. COVID-19 gone bad: A new character in the spectrum of the hyperferritinemic syndrome? Autoimmun Rev. 2020;19:102573. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 205] [Cited by in RCA: 188] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 45. | Daher R, Manceau H, Karim Z. Iron metabolism and the role of the iron-regulating hormone hepcidin in health and disease. Presse Med. 2017;46:e272-e278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 77] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 46. | Nai A, Lorè NI, Pagani A, De Lorenzo R, Di Modica S, Saliu F, Cirillo DM, Rovere-Querini P, Manfredi AA, Silvestri L. Hepcidin levels predict Covid-19 severity and mortality in a cohort of hospitalized Italian patients. Am J Hematol. 2021;96:E32-E35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 61] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 47. | Zhou C, Chen Y, Ji Y, He X, Xue D. Increased Serum Levels of Hepcidin and Ferritin Are Associated with Severity of COVID-19. Med Sci Monit. 2020;26:e926178. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 83] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 48. | Cavezzi A, Troiani E, Corrao S. COVID-19: hemoglobin, iron, and hypoxia beyond inflammation. A narrative review. Clin Pract. 2020;10:1271. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 283] [Cited by in RCA: 286] [Article Influence: 47.7] [Reference Citation Analysis (0)] |
| 49. | Gautheron J, Gores GJ, Rodrigues CMP. Lytic cell death in metabolic liver disease. J Hepatol. 2020;73:394-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 172] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 50. | Bloomer SA, Brown KE. Iron-Induced Liver Injury: A Critical Reappraisal. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 60] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 51. | Philippe MA, Ruddell RG, Ramm GA. Role of iron in hepatic fibrosis: one piece in the puzzle. World J Gastroenterol. 2007;13:4746-4754. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 67] [Cited by in RCA: 67] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 52. | Pretorius E, Kell DB. Diagnostic morphology: biophysical indicators for iron-driven inflammatory diseases. Integr Biol (Camb). 2014;6:486-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 119] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 53. | Stockwell BR, Jiang X, Gu W. Emerging Mechanisms and Disease Relevance of Ferroptosis. Trends Cell Biol. 2020;30:478-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 852] [Article Influence: 142.0] [Reference Citation Analysis (0)] |
| 54. | Hou W, Xie Y, Song X, Sun X, Lotze MT, Zeh HJ 3rd, Kang R, Tang D. Autophagy promotes ferroptosis by degradation of ferritin. Autophagy. 2016;12:1425-1428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1171] [Cited by in RCA: 1808] [Article Influence: 180.8] [Reference Citation Analysis (0)] |
| 55. | Dowdle WE, Nyfeler B, Nagel J, Elling RA, Liu S, Triantafellow E, Menon S, Wang Z, Honda A, Pardee G, Cantwell J, Luu C, Cornella-Taracido I, Harrington E, Fekkes P, Lei H, Fang Q, Digan ME, Burdick D, Powers AF, Helliwell SB, D'Aquin S, Bastien J, Wang H, Wiederschain D, Kuerth J, Bergman P, Schwalb D, Thomas J, Ugwonali S, Harbinski F, Tallarico J, Wilson CJ, Myer VE, Porter JA, Bussiere DE, Finan PM, Labow MA, Mao X, Hamann LG, Manning BD, Valdez RA, Nicholson T, Schirle M, Knapp MS, Keaney EP, Murphy LO. Selective VPS34 inhibitor blocks autophagy and uncovers a role for NCOA4 in ferritin degradation and iron homeostasis in vivo. Nat Cell Biol. 2014;16:1069-1079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 395] [Cited by in RCA: 626] [Article Influence: 52.2] [Reference Citation Analysis (0)] |
| 56. | Mancias JD, Wang X, Gygi SP, Harper JW, Kimmelman AC. Quantitative proteomics identifies NCOA4 as the cargo receptor mediating ferritinophagy. Nature. 2014;509:105-109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 728] [Cited by in RCA: 1614] [Article Influence: 134.5] [Reference Citation Analysis (0)] |
| 57. | Tang M, Chen Z, Wu D, Chen L. Ferritinophagy/ferroptosis: Iron-related newcomers in human diseases. J Cell Physiol. 2018;233:9179-9190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 232] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 58. | Jia F, Liu H, Kang S. NCOA4-Mediated Ferritinophagy: A Vicious Culprit in COVID-19 Pathogenesis? Front Mol Biosci. 2021;8:761793. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 59. | Jiang C, Zhang S, Li D, Chen L, Zhao Y, Mei G, Liu J, Tang Y, Gao C, Yao P. Impaired ferritinophagy flux induced by high fat diet mediates hepatic insulin resistance via endoplasmic reticulum stress. Food Chem Toxicol. 2020;140:111329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 60. | Qi X, Song A, Ma M, Wang P, Zhang X, Lu C, Zhang J, Zheng S, Jin H. Curcumol inhibits ferritinophagy to restrain hepatocyte senescence through YAP/NCOA4 in non-alcoholic fatty liver disease. Cell Prolif. 2021;54:e13107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 65] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 61. | Zhang Z, Guo M, Li Y, Shen M, Kong D, Shao J, Ding H, Tan S, Chen A, Zhang F, Zheng S. RNA-binding protein ZFP36/TTP protects against ferroptosis by regulating autophagy signaling pathway in hepatic stellate cells. Autophagy. 2020;16:1482-1505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 365] [Article Influence: 60.8] [Reference Citation Analysis (1)] |
| 62. | Zhang Z, Yao Z, Wang L, Ding H, Shao J, Chen A, Zhang F, Zheng S. Activation of ferritinophagy is required for the RNA-binding protein ELAVL1/HuR to regulate ferroptosis in hepatic stellate cells. Autophagy. 2018;14:2083-2103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 384] [Article Influence: 48.0] [Reference Citation Analysis (0)] |
| 63. | Tan Y, Huang Y, Mei R, Mao F, Yang D, Liu J, Xu W, Qian H, Yan Y. HucMSC-derived exosomes delivered BECN1 induces ferroptosis of hepatic stellate cells via regulating the xCT/GPX4 axis. Cell Death Dis. 2022;13:319. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 116] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 64. | Li ZJ, Dai HQ, Huang XW, Feng J, Deng JH, Wang ZX, Yang XM, Liu YJ, Wu Y, Chen PH, Shi H, Wang JG, Zhou J, Lu GD. Artesunate synergizes with sorafenib to induce ferroptosis in hepatocellular carcinoma. Acta Pharmacol Sin. 2021;42:301-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 232] [Article Influence: 46.4] [Reference Citation Analysis (2)] |
| 65. | Wang K, Zhang Z, Tsai HI, Liu Y, Gao J, Wang M, Song L, Cao X, Xu Z, Chen H, Gong A, Wang D, Cheng F, Zhu H. Branched-chain amino acid aminotransferase 2 regulates ferroptotic cell death in cancer cells. Cell Death Differ. 2021;28:1222-1236. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 179] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 66. | Kong Z, Liu R, Cheng Y. Artesunate alleviates liver fibrosis by regulating ferroptosis signaling pathway. Biomed Pharmacother. 2019;109:2043-2053. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 296] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 67. | Perricone C, Bartoloni E, Bursi R, Cafaro G, Guidelli GM, Shoenfeld Y, Gerli R. COVID-19 as part of the hyperferritinemic syndromes: the role of iron depletion therapy. Immunol Res. 2020;68:213-224. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 144] [Cited by in RCA: 135] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 68. | Poonkuzhi Naseef P, Elayadeth-Meethal M, Mohammed Salim KT, Anjana A, Muhas C, Abdul Vajid K, Saheer Kuruniyan M. Therapeutic potential of induced iron depletion using iron chelators in Covid-19. Saudi J Biol Sci. 2022;29:1947-1956. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 69. | Bastin A, Shiri H, Zanganeh S, Fooladi S, Momeni Moghaddam MA, Mehrabani M, Nematollahi MH. Iron Chelator or Iron Supplement Consumption in COVID-19? Biol Trace Elem Res. 2022;200:4571-4581. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 70. | Rainey NE, Moustapha A, Saric A, Nicolas G, Sureau F, Petit PX. Iron chelation by curcumin suppresses both curcumin-induced autophagy and cell death together with iron overload neoplastic transformation. Cell Death Discov. 2019;5:150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 64] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 71. | Chang R, Ng TB, Sun WZ. Lactoferrin as potential preventative and adjunct treatment for COVID-19. Int J Antimicrob Agents. 2020;56:106118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 120] [Article Influence: 20.0] [Reference Citation Analysis (1)] |
| 72. | Oexle H, Gnaiger E, Weiss G. Iron-dependent changes in cellular energy metabolism: influence on citric acid cycle and oxidative phosphorylation. Biochim Biophys Acta. 1999;1413:99-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 230] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 73. | Le Lan C, Loréal O, Cohen T, Ropert M, Glickstein H, Lainé F, Pouchard M, Deugnier Y, Le Treut A, Breuer W, Cabantchik ZI, Brissot P. Redox active plasma iron in C282Y/C282Y hemochromatosis. Blood. 2005;105:4527-4531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 117] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 74. | Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS, Morrison B 3rd, Stockwell BR. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060-1072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4711] [Cited by in RCA: 13336] [Article Influence: 952.6] [Reference Citation Analysis (2)] |
| 75. | Hassannia B, Vandenabeele P, Vanden Berghe T. Targeting Ferroptosis to Iron Out Cancer. Cancer Cell. 2019;35:830-849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 900] [Cited by in RCA: 1801] [Article Influence: 257.3] [Reference Citation Analysis (0)] |
| 76. | Sui M, Jiang X, Chen J, Yang H, Zhu Y. Magnesium isoglycyrrhizinate ameliorates liver fibrosis and hepatic stellate cell activation by regulating ferroptosis signaling pathway. Biomed Pharmacother. 2018;106:125-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 127] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 77. | Wang L, Zhang Z, Li M, Wang F, Jia Y, Zhang F, Shao J, Chen A, Zheng S. P53-dependent induction of ferroptosis is required for artemether to alleviate carbon tetrachloride-induced liver fibrosis and hepatic stellate cell activation. IUBMB Life. 2019;71:45-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 132] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 78. | Yamada N, Karasawa T, Kimura H, Watanabe S, Komada T, Kamata R, Sampilvanjil A, Ito J, Nakagawa K, Kuwata H, Hara S, Mizuta K, Sakuma Y, Sata N, Takahashi M. Ferroptosis driven by radical oxidation of n-6 polyunsaturated fatty acids mediates acetaminophen-induced acute liver failure. Cell Death Dis. 2020;11:144. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 269] [Article Influence: 44.8] [Reference Citation Analysis (0)] |
| 79. | Aldrovandi M, Conrad M. Ferroptosis: the Good, the Bad and the Ugly. Cell Res. 2020;30:1061-1062. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 80. | Yu Y, Jiang L, Wang H, Shen Z, Cheng Q, Zhang P, Wang J, Wu Q, Fang X, Duan L, Wang S, Wang K, An P, Shao T, Chung RT, Zheng S, Min J, Wang F. Hepatic transferrin plays a role in systemic iron homeostasis and liver ferroptosis. Blood. 2020;136:726-739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 418] [Cited by in RCA: 471] [Article Influence: 78.5] [Reference Citation Analysis (0)] |
| 81. | Zhou B, Liu J, Kang R, Klionsky DJ, Kroemer G, Tang D. Ferroptosis is a type of autophagy-dependent cell death. Semin Cancer Biol. 2020;66:89-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 790] [Article Influence: 112.9] [Reference Citation Analysis (0)] |
| 82. | Bai Y, Meng L, Han L, Jia Y, Zhao Y, Gao H, Kang R, Wang X, Tang D, Dai E. Lipid storage and lipophagy regulates ferroptosis. Biochem Biophys Res Commun. 2019;508:997-1003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 378] [Article Influence: 47.3] [Reference Citation Analysis (0)] |
| 83. | Yang M, Chen P, Liu J, Zhu S, Kroemer G, Klionsky DJ, Lotze MT, Zeh HJ, Kang R, Tang D. Clockophagy is a novel selective autophagy process favoring ferroptosis. Sci Adv. 2019;5:eaaw2238. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 289] [Cited by in RCA: 396] [Article Influence: 56.6] [Reference Citation Analysis (0)] |
| 84. | Wang H, An P, Xie E, Wu Q, Fang X, Gao H, Zhang Z, Li Y, Wang X, Zhang J, Li G, Yang L, Liu W, Min J, Wang F. Characterization of ferroptosis in murine models of hemochromatosis. Hepatology. 2017;66:449-465. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 309] [Cited by in RCA: 543] [Article Influence: 60.3] [Reference Citation Analysis (0)] |
| 85. | Chen Y, Xu Y, Zhang K, Shen L, Deng M. Ferroptosis in COVID-19-related liver injury: A potential mechanism and therapeutic target. Front Cell Infect Microbiol. 2022;12:922511. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 86. | Sun Y, Zheng Y, Wang C, Liu Y. Glutathione depletion induces ferroptosis, autophagy, and premature cell senescence in retinal pigment epithelial cells. Cell Death Dis. 2018;9:753. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 195] [Cited by in RCA: 443] [Article Influence: 55.4] [Reference Citation Analysis (0)] |
| 87. | Wu Z, Geng Y, Lu X, Shi Y, Wu G, Zhang M, Shan B, Pan H, Yuan J. Chaperone-mediated autophagy is involved in the execution of ferroptosis. Proc Natl Acad Sci U S A. 2019;116:2996-3005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 469] [Article Influence: 67.0] [Reference Citation Analysis (0)] |
| 88. | Du J, Wang T, Li Y, Zhou Y, Wang X, Yu X, Ren X, An Y, Wu Y, Sun W, Fan W, Zhu Q, Wang Y, Tong X. DHA inhibits proliferation and induces ferroptosis of leukemia cells through autophagy dependent degradation of ferritin. Free Radic Biol Med. 2019;131:356-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 357] [Article Influence: 51.0] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ali FE, Egypt; Mogahed EA, Egypt S-Editor: Liu JH L-Editor: A P-Editor: Liu JH