Published online Dec 6, 2022. doi: 10.12998/wjcc.v10.i34.12734
Peer-review started: September 5, 2022
First decision: September 26, 2022
Revised: October 10, 2022
Accepted: October 31, 2022
Article in press: October 31, 2022
Published online: December 6, 2022
Processing time: 88 Days and 5.4 Hours
Pitt-Hopkins syndrome (PTHS; MIM #610954) is a rare genetic neurological disorder. Myopia and strabismus have been reported in approximately 50% of PTHS patients. No studies have reported details about the required surgery for PTHS with strabismus and early-onset myopia. Here, we retrospectively reviewed the surgical management of two patients with PTHS combined with strabismus and/or early-onset myopia.
A 5-year-old girl presented with congenital esotropia and left eye myopia, and the second girl was a 5-year-old girl who presented with intermittent exotropia. Genetic testing performed on both patients showed a mutation in transcription factor 4, which is a diagnostic marker of PTHS. The first girl underwent bilateral medial rectus recession combined with posterior scleral reinforcement (PSR) in the left eye and the second patient underwent bilateral lateral rectus recession strabismus surgery. We made key innovations in surgical timing and strategy, and the results were satisfactory. The combination of strabismus and PSR surgery is an innovative strategy for patients with both strabismus and early-onset myopia.
Early treatment of strabismus and myopia positively influence motor develop
Core Tip: Ophthalmic conditions and surgical outcomes were observed in two children with Pitt-Hopkins syndrome (PTHS). We made key innovations in surgical timing and strategy, and the results were satisfactory. The combination of strabismus and posterior scleral reinforcement surgery is an innovative strategy for patients with both strabismus and early-onset myopia. Furthermore, early diagnosis and treatment of strabismus and myopia positively influence motor development and have, therefore, been included in rehabilitation programs for patients with PTHS. Overall, this study laid the groundwork for future surgical treatment of PTHS associated with strabismus and early-onset myopia.
- Citation: Huang Y, Di Y, Zhang XX, Li XY, Fang WY, Qiao T. Surgical treatment of Pitt-Hopkins syndrome associated with strabismus and early-onset myopia: Two case reports. World J Clin Cases 2022; 10(34): 12734-12741
- URL: https://www.wjgnet.com/2307-8960/full/v10/i34/12734.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i34.12734
Pitt-Hopkins syndrome (PTHS; MIM #610954) is a rare, genetic, neurological disorder first described by Australian physicians Pitt and Hopkins[1] in 1978 and Zollino et al[2] in 2019. Prevalence is estimated to be 1:225000–1:300000[2]. The phenotypic presentations most frequently observed in PTHS are developmental delay, intellectual disability, distinctive facial features, breathing pattern abnormalities, and high rates of autism spectrum disorder[3].
PTHS is caused by a mutation in the transcription factor 4 (TCF4) gene (MIM #602272), a causative gene discovered in 2008[4]. The human TCF4 gene encodes a type I basic helix-loophelix which binds to E-box DNA sequences. It is located in the 18q21.2 region, containing 41 exons and spanning 437 kb[5]. TCF4 mutations play an essential role in the nervous system and have been linked to autism, schizophrenia, and PTHS in humans[6]. PTHS mutations are spontaneous and do not run in families[7,8].
Myopia and strabismus have been reported in approximately 50% of patients[9]. Myopia is typically severe and usually occurs before 2 years of age[10]. Early-onset myopia is a relevant factor related to later development of high myopia. Hence, once diagnosis of PTHS has been confirmed, children should be referred to an ophthalmologist[2]. Posterior scleral reinforcement (PSR) is recognized as an effective strategy to slow axial length (AL) elongation and prevent high myopic complications[11]. Strabismus surgery is generally performed when conservative treatments are unsuccessful in aligning the eyes[12]. Surgical treatment of strabismus and/or early-onset myopia in patients with PTHS has not been previously described. This report documents our experience in managing two PTHS cases of PTHS with ocular abnormalities.
Case 1: Esodeviation for 5 years and left eye myopia for 2 years.
Case 2: Exodeviation for 1 year.
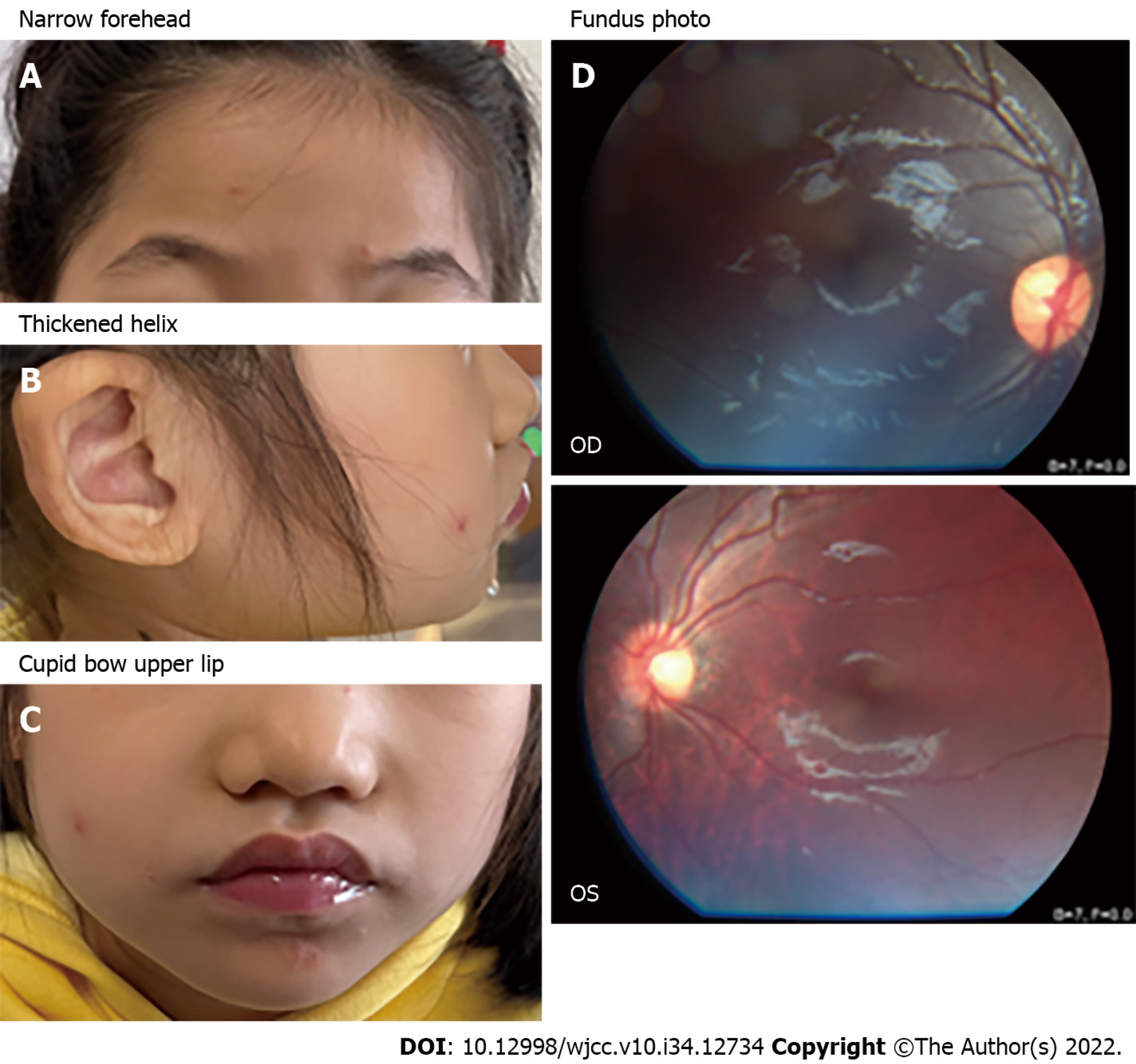
Case 1: The patient presented with distinctive facial features (Figure 1A-C), drooling, stereotypic movements such as hand clapping and hand shaking, and episodic hyperventilation.
Case 2: The patient presented with distinctive facial features, breath-holding while awake, and severe constipation. The patient had intellectual disabilities which were confirmed by a child neurologist.
Case 1: She was born at full term with a birth weight of 3150 g and a length of 49 cm. Apgar score was 10 at one and five minutes. No fetal complications were observed during pregnancy. She was unable to sit until she was 1-year-old. Walking ability was assessed at 4 years of age. Speech development was absent.
Case 2: The child was born at the 38th week (eutopic labor), with a birth weight of 3300 g and a length of 50 cm. Apgar score was 10 at one and five minutes. No fetal complications were observed during pregnancy.
There was no remarkable personal or family history.
Case 1: The patient did not cooperate in visual acuity or stereoacuity examinations. Cycloplegic refraction was +1.75 sph/-1.00 cyl/5° in the right eye and -3.75 sph/-2.25 cyl/5° in the left eye. AL measured with Intraocular Len Master was 23.13 and 25.70 mm in the right and left eyes, respectively. The primary alignment was +40 prism diopter (PD) esotropia with correction. The anterior segments were normal with clear lenses.
Case 2: Visual acuity was 20/40 in both eyes, and near-and distance-stereoacuity was lost. The cycloplegic refraction was +0.25 sph/-1.75 cyl/170° in the right eye and +0.25 sph/-1.75 cyl/15° in the left eye. The AL was 23.42 and 23.39 mm in the right and left eye, respectively. The primary position alignment was -50 PD of exotropia. The intraocular pressure was 17 mmHg in both eyes. The anterior segments were normal with clear lenses.
Case 1: Genetic testing revealed a heterozygous variant (c.1146+3A>G/p.?) in the TCF4 gene. The patient did not cooperate in visual acuity or stereoacuity examinations.
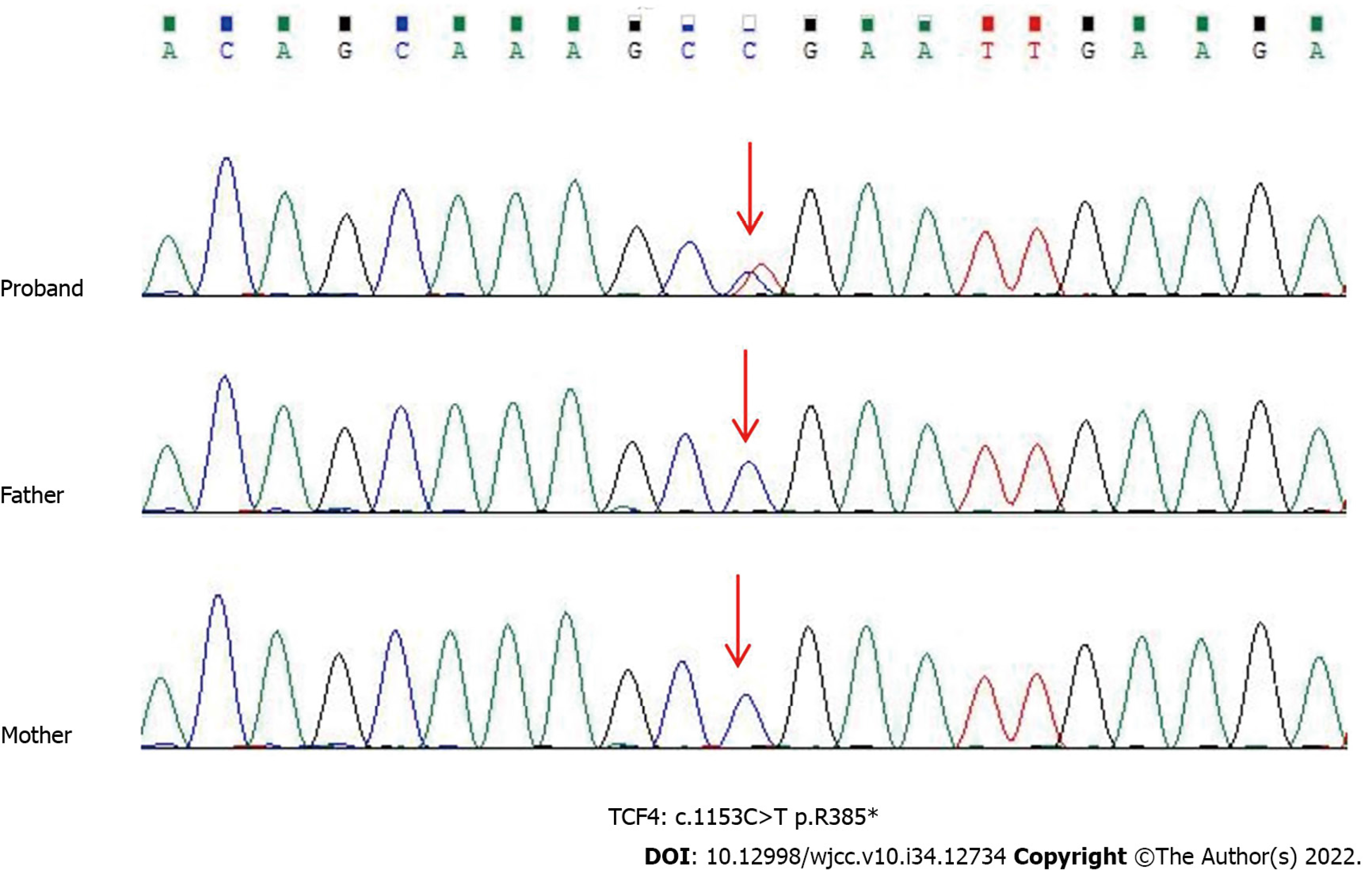
Case 2: Sequencing results indicated that c.1153C>T of TCF4 had a nonsense mutation, leading to early termination of protein translation (p. Arg385*), which was confirmed by Sanger sequencing (Figure 2).
Case 1: Fundoscopy revealed slight tesselation in the left eye (Figure 1D).
Case 2: There were no abnormalities on fundus examination.
Case 1: PTHS with congenital esotropia and left eye myopia.
Case 2: PTHS with intermittent exotropia.
Case 1: To repair the strabismus and slow AL elongation to prevent high myopic complications, the patient underwent 5 mm of bilateral medial rectus recession (BMR) combined with PSR in the left eye.
Case 2: The patient underwent 8 mm of bilateral lateral rectus recession (BLR) strabismus surgery to recover visual function and improve her appearance.
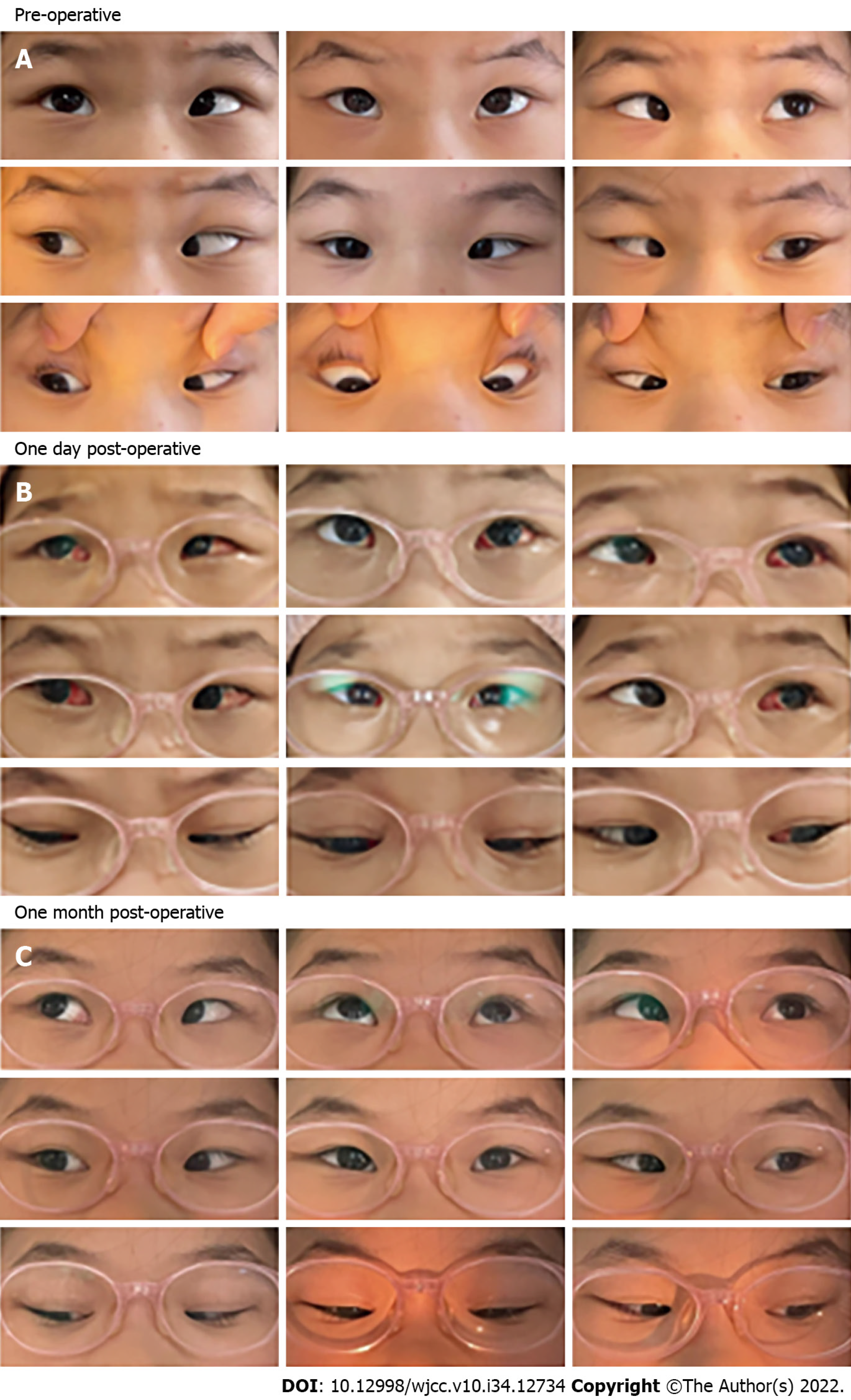
Case 1: Esotropia was significantly relieved on the day after surgery. Conjunctival congestion and edema were observed at an early postoperative stage, although both conditions were completely alleviated after 1 mo (Figure 3). No postoperative infection was observed during slit-lamp examination. No postoperative pathological signs were found in either eye during fundus examination performed at the 1 year postoperative follow-up.
Case 2: The patient’s exotropia was significantly relieved on the first postoperative day (Figure 4). At the 3 mo postoperative follow-up, her parents noticed that she presented with an improvement in walking and fine motor tasks; she also reported a significant improvement in stereoacuity.
Variants of TCF4 in PTHS usually occur de novo, and empirical recurrence risk can be as high as 2%[2]. Myopia is a supportive feature of the diagnostic criteria for PTHS. In a previous study[2], myopia occurred in 52% of patients with PTHS, and strabismus was present in 44% of patients. One patient (case 1) had early-onset myopia. Consistent with previous literature, which states that strabismus occurs at a very high frequency, case 1 presented with strabismus (congenital esotropia type, unlike case 2, which presented with intermittent exotropia). Early-onset myopia may be related to the hapoinsufficiency of TCF4, causing increased AL, and resulting in myopia combined with rough and partially unpigmented eyes, as demonstrated in zebrafish and drosophila models[13,14]. Interestingly, although refractive errors and strabismus are ubiquitous in PTHS patients, other structural ocular abnormalities, including enophthalmia, small optic nerves, and macular degeneration are uncommon[2].
In this report, case 1 was diagnosed with congenital esotropia and left-eye myopia. Transient visual disturbances during the critical developmental period may be sufficient to impair normal binocular function. Restoring proper ocular alignment as soon as possible can help to improve fine motor development and other visual tasks. Patients with esotropia > 40 PD and a stable or increasing angle on at least two examinations, 2 or more weeks apart following full spectacle correction, are candidates for early surgery[12]. Additionally, case 1 was diagnosed with myopia in the left eye, given that early-onset severe myopia is a frequent clinical manifestation of PTHS, the AL of the patient’s left eye was 25.70 mm and much longer than the right, and the left fundoscopy revealed slight visibility of the large choroidal vessels. PSR was considered to slow AL elongation and prevent highly myopic complications.
Both strabismus and PSR surgeries require conjunctival incision and extraocular muscle exposure, and they require a similar surgical approach. The surgeon covered the scleral buckle to the posterior pole, which corresponds to the peripheral macula, ensuring the therapeutic effect of the procedure and not affecting the recession or transposition of the extraocular muscle. In case 1, BMR combined with PSR was preferred because it avoided the need for a second surgery for postoperative adhesions, thereby reducing the patient’s expenses. It should be noted that acquired esotropia fixus is associated with high myopia[15]; therefore, the amount of recession would be 5–10 PD less than standard surgical numbers. For this child, we performed a 5 mm BMR for a target angle of +40 PD. Intraoperatively, the extraocular muscles were sufficiently exposed and the implanted material covered the posterior pole, which corresponded to the peripheral macula. This patient showed orthotropia in the primary position after combined surgery; therefore, presurgical planning was proven to be correct.
Case 2 was a patient with congenital exotropia, a rare condition that occurs in the first few weeks of life and frequently in stunted children. Typically, stereoscopic vision is preserved when exotropia is well-controlled, and the timing of surgical procedures is based on stereopsis and frequency of exotropia. Nonetheless, for patients with PTHS, we believe that stereopsis and exotropia frequency may not be the most reliable indicators of surgery. Owing to the common comorbidity of severe intellectual disability and speech impairment in children with PTHS, examinations can prove challenging. In these cases, we suggest early surgical interventions after confirming the type of strabismus and the angle of deviation to promote motor coordination ability and balance function recovery.
The two main options for surgical correction include BLR, unilateral lateral rectus recession, and medial rectus resection [16]. However, there is no perfect preoperative plan. Although both PTHS and Angelman syndrome are muscle tone abnormality disorders, no reports are available on exotropia surgery for PTHS. We selected lateral rectus weakening surgery as the major strategy, and BLR with surgical doses similar to standard exotropia surgery, as in our previous study on Angelman syndrome[17]. Eventually, the patient underwent an 8 mm BLR surgery and achieved stable ocular alignment. A limitation of our study was the small sample size owing to the low incidence rate of PTHS. Another important limitation was the need for further long-term follow-ups of outcomes.
Ophthalmic conditions and surgical outcomes were observed in two children with PTHS. To the best of our knowledge, this is the first report of strabismus and PSR surgery in patients with PTHS. We made key innovations in surgical timing and strategy, and the results were satisfactory. The combination of strabismus and PSR surgery is an innovative strategy for patients with both strabismus and early-onset myopia. Furthermore, early diagnosis and treatment of strabismus and myopia positively influence motor development and have, therefore, been included in rehabilitation programs for patients with PTHS. Overall, this study laid the groundwork for future surgical treatment of PTHS associated with strabismus and early-onset myopia.
| 1. | Pitt D, Hopkins I. A syndrome of mental retardation, wide mouth and intermittent overbreathing. Aust Paediatr J. 1978;14:182-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 46] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 2. | Zollino M, Zweier C, Van Balkom ID, Sweetser DA, Alaimo J, Bijlsma EK, Cody J, Elsea SH, Giurgea I, Macchiaiolo M, Smigiel R, Thibert RL, Benoist I, Clayton-Smith J, De Winter CF, Deckers S, Gandhi A, Huisman S, Kempink D, Kruisinga F, Lamacchia V, Marangi G, Menke L, Mulder P, Nordgren A, Renieri A, Routledge S, Saunders CJ, Stembalska A, Van Balkom H, Whalen S, Hennekam RC. Diagnosis and management in Pitt-Hopkins syndrome: First international consensus statement. Clin Genet. 2019;95:462-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 69] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 3. | Watkins A, Bissell S, Moss J, Oliver C, Clayton-Smith J, Haye L, Heald M, Welham A. Behavioural and psychological characteristics in Pitt-Hopkins syndrome: a comparison with Angelman and Cornelia de Lange syndromes. J Neurodev Disord. 2019;11:24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 4. | Zweier C, Sticht H, Bijlsma EK, Clayton-Smith J, Boonen SE, Fryer A, Greally MT, Hoffmann L, den Hollander NS, Jongmans M, Kant SG, King MD, Lynch SA, McKee S, Midro AT, Park SM, Ricotti V, Tarantino E, Wessels M, Peippo M, Rauch A. Further delineation of Pitt-Hopkins syndrome: phenotypic and genotypic description of 16 novel patients. J Med Genet. 2008;45:738-744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 81] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 5. | Sepp M, Kannike K, Eesmaa A, Urb M, Timmusk T. Functional diversity of human basic helix-loop-helix transcription factor TCF4 isoforms generated by alternative 5' exon usage and splicing. PLoS One. 2011;6:e22138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 109] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 6. | De Rubeis S, He X, Goldberg AP, Poultney CS, Samocha K, Cicek AE, Kou Y, Liu L, Fromer M, Walker S, Singh T, Klei L, Kosmicki J, Shih-Chen F, Aleksic B, Biscaldi M, Bolton PF, Brownfeld JM, Cai J, Campbell NG, Carracedo A, Chahrour MH, Chiocchetti AG, Coon H, Crawford EL, Curran SR, Dawson G, Duketis E, Fernandez BA, Gallagher L, Geller E, Guter SJ, Hill RS, Ionita-Laza J, Jimenz Gonzalez P, Kilpinen H, Klauck SM, Kolevzon A, Lee I, Lei I, Lei J, Lehtimäki T, Lin CF, Ma'ayan A, Marshall CR, McInnes AL, Neale B, Owen MJ, Ozaki N, Parellada M, Parr JR, Purcell S, Puura K, Rajagopalan D, Rehnström K, Reichenberg A, Sabo A, Sachse M, Sanders SJ, Schafer C, Schulte-Rüther M, Skuse D, Stevens C, Szatmari P, Tammimies K, Valladares O, Voran A, Li-San W, Weiss LA, Willsey AJ, Yu TW, Yuen RK; DDD Study; Homozygosity Mapping Collaborative for Autism; UK10K Consortium, Cook EH, Freitag CM, Gill M, Hultman CM, Lehner T, Palotie A, Schellenberg GD, Sklar P, State MW, Sutcliffe JS, Walsh CA, Scherer SW, Zwick ME, Barett JC, Cutler DJ, Roeder K, Devlin B, Daly MJ, Buxbaum JD. Synaptic, transcriptional and chromatin genes disrupted in autism. Nature. 2014;515:209-215. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1776] [Cited by in RCA: 2072] [Article Influence: 172.7] [Reference Citation Analysis (0)] |
| 7. | Kousoulidou L, Tanteles G, Moutafi M, Sismani C, Patsalis PC, Anastasiadou V. 263.4 kb deletion within the TCF4 gene consistent with Pitt-Hopkins syndrome, inherited from a mosaic parent with normal phenotype. Eur J Med Genet. 2013;56:314-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 8. | Steinbusch CV, van Roozendaal KE, Tserpelis D, Smeets EE, Kranenburg-de Koning TJ, de Waal KH, Zweier C, Rauch A, Hennekam RC, Blok MJ, Schrander-Stumpel CT. Somatic mosaicism in a mother of two children with Pitt-Hopkins syndrome. Clin Genet. 2013;83:73-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 9. | de Winter CF, Baas M, Bijlsma EK, van Heukelingen J, Routledge S, Hennekam RC. Phenotype and natural history in 101 individuals with Pitt-Hopkins syndrome through an internet questionnaire system. Orphanet J Rare Dis. 2016;11:37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 10. | Giurgea I, Missirian C, Cacciagli P, Whalen S, Fredriksen T, Gaillon T, Rankin J, Mathieu-Dramard M, Morin G, Martin-Coignard D, Dubourg C, Chabrol B, Arfi J, Giuliano F, Claude Lambert J, Philip N, Sarda P, Villard L, Goossens M, Moncla A. TCF4 deletions in Pitt-Hopkins Syndrome. Hum Mutat. 2008;29:E242-E251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 52] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 11. | Miao Z, Li L, Meng X, Guo L, Cao D, Jia Y, He D, Huang L, Wang L. Modified Posterior Scleral Reinforcement as a Treatment for High Myopia in Children and Its Therapeutic Effect. Biomed Res Int. 2019;2019:5185780. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 12. | Kanukollu VM, Sood G. Strabismus. 2022 Feb 17. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. [PubMed] |
| 13. | Brockschmidt A, Filippi A, Charbel Issa P, Nelles M, Urbach H, Eter N, Driever W, Weber RG. Neurologic and ocular phenotype in Pitt-Hopkins syndrome and a zebrafish model. Hum Genet. 2011;130:645-655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 14. | Tamberg L, Sepp M, Timmusk T, Palgi M. Introducing Pitt-Hopkins syndrome-associated mutations of TCF4 to Drosophila daughterless. Biol Open. 2015;4:1762-1771. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 15. | Ho TH, Lin MC, Sheu SJ. Surgical treatment of acquired esotropia in patients with high myopia. J Chin Med Assoc. 2012;75:416-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 16. | Chougule P, Kekunnaya R. Surgical management of intermittent exotropia: do we have an answer for all? BMJ Open Ophthalmol. 2019;4:e000243. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 17. | Ye H, Lan X, Liu Q, Zhang Y, Wang S, Zheng C, Di Y, Qiao T. Ocular findings and strabismus surgery outcomes in Chinese children with Angelman syndrome: Three case reports. Medicine (Baltimore). 2019;98:e18077. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Atanasova EG, Bulgaria; Tangsuwanaruk T, Thailand S-Editor: Wei ZH L-Editor: A P-Editor: Wei ZH