Published online Dec 6, 2022. doi: 10.12998/wjcc.v10.i34.12671
Peer-review started: July 12, 2022
First decision: August 21, 2022
Revised: August 29, 2022
Accepted: October 12, 2022
Article in press: October 12, 2022
Published online: December 6, 2022
Processing time: 143 Days and 0.6 Hours
Bronchogenic cysts are cystic masses caused by congenital abnormal development of the respiratory system, and usually occur in the pulmonary parenchyma or mediastinum.
A rare case of a bronchogenic cyst discovered in the abdominal cavity of a 35-year-old man is reported. Physical examination found a space-occupying lesion in the patient’s abdomen for 4 d. Laparoscopic exploration found the cyst tightly adhered to the stomach and its peripheral blood vessels; therefore, intraoperative laparotomy was performed. The cystic mass was resected en bloc with an Endo-GIA stapler. The final postoperative pathological diagnosis confirmed an abdo
This is a rare case of a bronchogenic cyst that was discovered within the abdominal cavity of a male patient. The cyst is easily confused with or misdiagnosed as other lesions. Therefore, it is necessary to distinguish abdominal bronchogenic cyst from gastrointestinal stromal tumor, Meckel’s diverticulum, enteric duplication cyst, or lymphangioma. Although computer tomography and magnetic resonance imaging were the primary diagnostic approaches, endoscopic ultrasound-guided fine-needle aspiration could assist with clarification of the cytological or histopathological diagnosis before surgery.
Core tip: This case report proposes endoscopic ultrasound-guided fine-needle aspiration as an applicable and more reliable method for preoperative diagnosis of abdominal bronchogenic cyst. This method is important preoperatively for surgeons because it helps to confirm the source of the tumor tissue and identifies whether the tumor is benign or malignant. More importantly, it provides surgeons with a better option for a more secure and feasible surgical treatment.
- Citation: Li C, Zhang XW, Zhao CA, Liu M. Abdominal bronchogenic cyst: A rare case report. World J Clin Cases 2022; 10(34): 12671-12677
- URL: https://www.wjgnet.com/2307-8960/full/v10/i34/12671.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i34.12671
Bronchogenic cysts are cystic masses caused by the congenital abnormal development of the respiratory system, and usually occur in the pulmonary parenchyma or mediastinum. Abdominal bronchogenic cysts are rarely documented. We report a rare case of an ectopic bronchogenic cyst within the abdominal cavity of a 35-year-old male patient.
Physical examination revealed an abdominal space-occupying lesion in a 35-year-old male patient for 4 d.
Four days ago, the patient presented to our hospital for physical examination that discovered a space-occupying lesion in his abdomen.
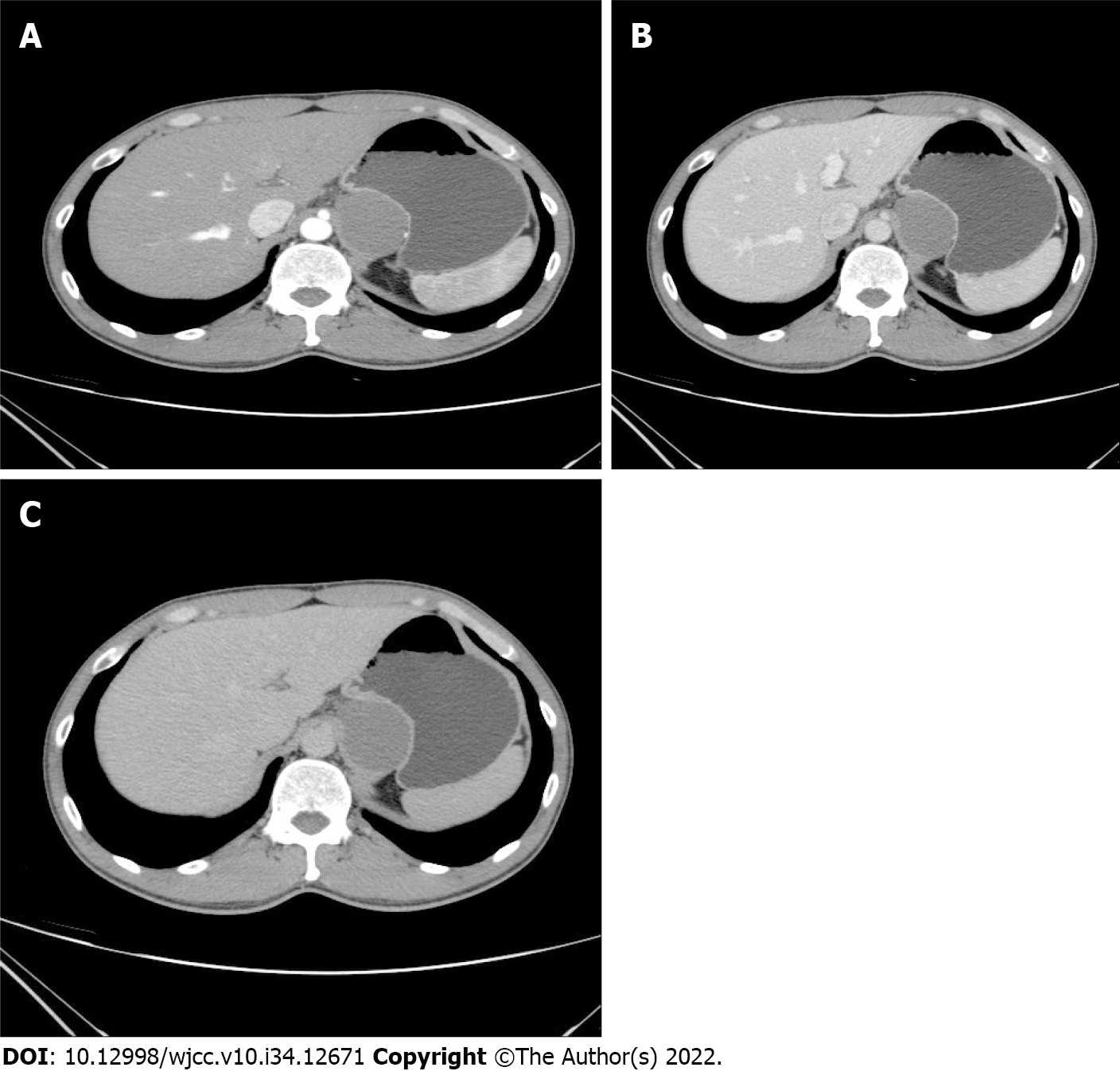
The patient was admitted to our department for surgical treatment. Abdominal computed tomography (CT) and enhanced CT revealed a hepatogastric space-occupying lesion. Abdomystic lymphangioma was initially suspected. There were no complaints of abdominal pain, fever, nausea, or vomiting after admission.
The patient previously underwent laparoscopic argon knife surgery for gastric, cardia, and colon polyps. Past medical history showed that the patient was a carrier of hepatitis B surface antigen (HBsAg). Family history was denied, as well as infectious and genetic diseases.
The patient’s vital signs were: body temperature, 36.5C; heart rate, 69 beats/min; respiratory rate, 16 breaths/min; and blood pressure, 110/70 mmHg. Cardiopulmonary examination was normal. The whole abdomen was soft to the touch, without tenderness and rebound tenderness. Examination of the liver, gallbladder, spleen and kidneys revealed no abnormalities. Bowel sounds 4 times per min.
No abnormality was found in routine blood, urine and feces analyses, hepatonephric function, and blood coagulation tests. Blood transfusion-related tests were positive for HBsAg+, hepatitis B core antibody, and hepatitis B e antibody, but negative for hepatitis C, and HIV antibodies.
Chest CT scan and B-ultrasound of the liver, gallbladder, pancreas, spleen and kidneys revealed no abnormality. Abdominal CT and enhanced CT revealed a hepatogastric space-occupying lesion, which was considered to be abdominal cystic lymphangioma (Figure 1).
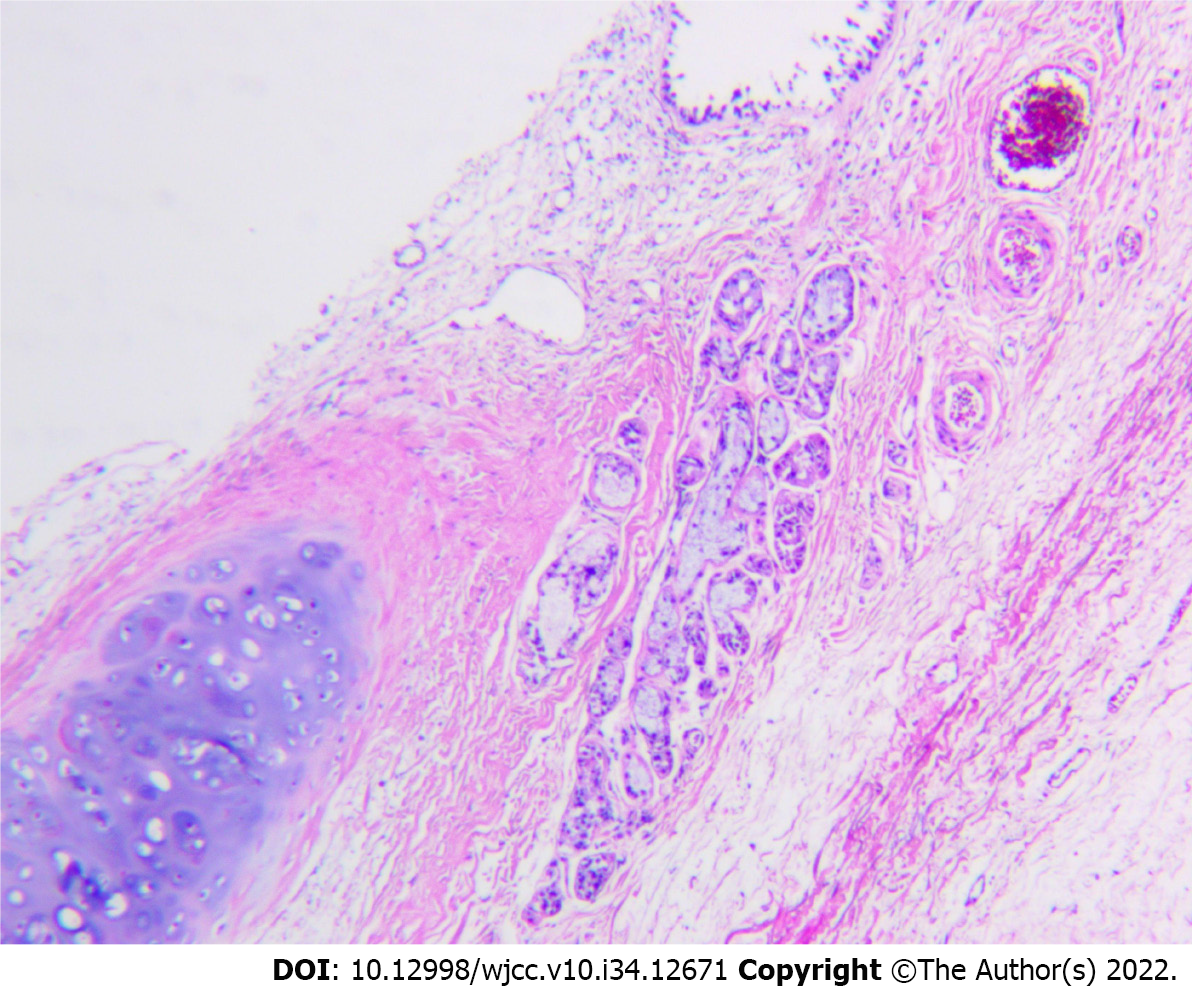
The final diagnosis was ectopic bronchogenic cyst within the abdominal cavity.
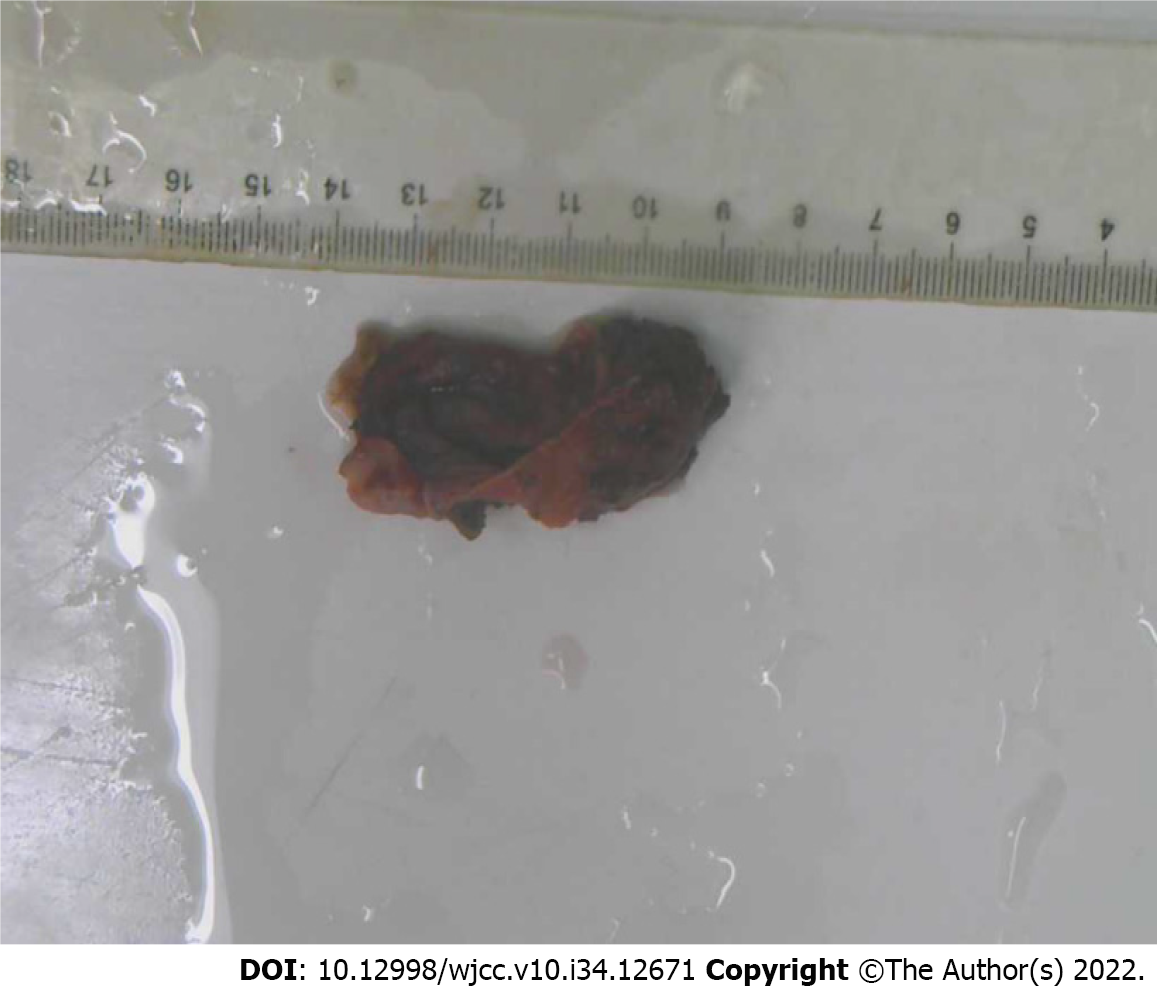
The patient underwent laparoscopic exploration under general anesthesia with endotracheal intubation. Abdominoscope discovered that the cyst was located between the lower left diaphragm, aorta abdominalis, left gastric vessel, splenic artery, and pancreas. Laparoscopic exploration found that the cyst tightly adhered to the stomach and its peripheral blood vessels, so the surgery was converted to laparotomy, and the cystic mass was resected en bloc by Endo-GIA stapler (Figure 2). The surgery went smoothly. The final postoperative pathological diagnosis confirmed abdominal bronchogenic cyst (Figure 3).
The patient recovered well upon follow-up 3 mo after surgery.
CT and MRI are the major diagnostic methods for abdominal masses. Most of the bronchogenic lesions are reported to be cystic and only a few are solid. Therefore, it is valuable to distinguish them from gastrointestinal stromal tumors, Meckel’s diverticulum, cystic intestinal duplication and lymphangioma. CT and MRI can help to identify the imaging features of the lesions, and endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) can assist surgeons to identify the tissue source of the lesions and confirm the cyst as benign or malignant.
The exact pathogenesis of bronchogenic cysts remains unknown. It is possibly related to embryonic development. During embryonic development, the respiratory tract epithelium is separated from the tracheobronchial tree, migrates from the previously developed site to distal sites, and gradually expands. Its increased internal mucus secretion, which cannot be discharged, results in a mucus-containing cyst consisting of bronchial tissue comprising its walls. The disease is categorized into three types according to the pathogenic location: mediastinal, intrapulmonary, and ectopic. The mediastinal type is more common, the intrapulmonary type is less common, while the ectopic type is extremely rare, occurring in intracranial cavities, sublingual cavities, diaphragm, and the abdominal cavity[1]. Abdominal bronchogenic cysts, including abdominal and retroperitoneal cysts, are extremely rare. There are few documented cases of bronchial cysts within the abdominal cavity, most of which, are in the retroperitoneal region, while cases of lesions attached to the stomach are extremely unusual[2]. No specific clinical symptoms are present when the cyst is small, so abdominal bronchogenic cysts are difficult to diagnose preoperatively and are usually discovered through physical examinations, so they are more easily misdiagnosed as an abdominal space-occupying lesion. Imaging of abdominal bronchogenic cysts reveals circular or elliptical cystic spaces occupying primarily single cavities with well-defined margins. The long axis of the cyst in the abdominal tissue space is consistent with the long axis of the tissues, which may compress the adjacent tissues. No enhancement is found on contrast-enhanced CT, except that the thickened cystic wall shows annular enhancement. CT and MRI can provide more valuable imaging characteristics of the lesion, while EUS-FNA is an effective technique for obtaining tissue samples[3-6], which could cause minimal injury to the patients. This technique can assist surgeons to clarify the tissue source of the tumor and determine the benign or malignant nature of the tumor. This technique is adaptable to some abdominal, retroperitoneal and mediastinal lesions, which helps to make a definite cytological or histopathological diagnosis before surgery[7]. However, if tissue necrosis or hemorrhage occurs, the pathological results might be affected. Differential diagnosis of the abdominal bronchogenic cysts includes gastrointestinal stromal tumor, Meckel’s diverticulum, intestinal duplication cyst, and lymphangioma[8]. We consider that preoperative imaging is an important approach for differentiation of abdominal bronchogenic cysts from gastrointestinal stromal tumor, Meckel’s diverticulum, intestinal duplication cyst and lymphangioma. Low-grade malignant gastrointestinal stromal tumors are usually < 5 cm in diameter, with a regular well-circumscribed shape, and minimal compression of the adjacent tissues. Malignant tumors are > 5 cm, with undefined margins, uneven density, and are normally accompanied by necrosis, bleeding, calcification, cystic change, and invasion into the adjacent tissues. Severe necrosis can communicate with the intestinal cavity to form a gas–liquid surface, indicating symptoms of a pseudointestinal cavity. However, there are few signs of intestinal obstruction[9]. The CT value of malignant gastrointestinal stromal tumors increases significantly, surrounded by garland-shaped or irregular patchy enhancements. The blood supply in most gastrointestinal stromal tumors is significantly enhanced, and CT values of the venous phase are higher than those of the arterial phase. High expression of CD117 and DOG-1 can be a sensitive marker for small intestinal or gastrointestinal stromal tumor. Tumor diameter ≥ 5.3 cm and nuclear division number > 5/50 can be independent risk factors for prediction of postoperative adverse outcome for small intestinal stromal tumor[10].
Meckel’s diverticulum is a distal ileal diverticulum formed by an incomplete vitelline canal, which communicates with the ileum during embryonic development. The most usual complications of Meckel’s diverticulum include bleeding and obstruction. Bleeding is a frequent childhood life-threatening complication, while intestinal obstruction is a common complication in adulthood[11]. Routine barium enema retrograde ileography is performed, indicating that a pleated sac filled with contrast medium has adhered to the mesenteric margin of the small intestine. New diagnostic methods such as capsule endoscopy, double-balloon enteroscopy and MR enterography have emerged in recent years[12]. Intestinal duplication cysts (enteric duplication cysts) are rare congenital gastrointestinal malformations. According to medical reports, the incidence of enteric duplication cysts is 1:4500 births[13], but their etiology remains unclear. Their clinical presentation varies because of the different sizes, locations, types, and mucosal patterns of the cysts[13]. Intestinal duplication cysts occur more commonly in infants and children under 2 years of age and often results in abdominal pain, intestinal obstruction, hematochezia, and peritonitis. Intestinal duplication cysts in adulthood are the potential risks for malignant transformation[13]. Abdominal ultrasonography is the most commonly used diagnostic approach because of its more frequent occurrence in young children. Ultrasound imaging shows that intestinal duplication cysts consist of five layers: mucosal, submucosa, mucosal muscularis, muscularis propria and serosal[14-17]. For imaging diagnosis, enteric duplication cysts can be confirmed via mucosal patterns and smooth muscle layers, which must adhere to the intestinal fistula[18,19]. Other cystic lesions in the abdomen, such as omental or mesenteric cysts, do not demonstrate this characteristic multilayered wall[17]. Abdominal cystic lymphangioma, usually congenital, is more common in children and rare in adults. It often develops along the abdominal space. When the lesion is small, there is no apparent compression on the adjacent tissues. As the lesion increases in size, it is often distributed along the omentum, retroperitoneal cavity, and mesentery, demonstrating the characteristics of crawling growth in the crevices. CT shows a well-defined thin-walled cystic lesion with multilocular capsules and visible partitions. The partitions and wall can be strengthened without wall nodules. Laparoscopic surgery is the most used treatment for abdominal bronchogenic cysts. If surgery is not performed, infection, bleeding, and malignant transformation may occur[19].
As previously reported, it is necessary to establish the patient’s age at onset, common symptoms, and clinical presentations of abdominal bronchogenic cyst. As for the abdominal space-occupying lesions that have not been clearly diagnosed, apart from preoperative abdominal CT and MRI, EUS-FNA is recommended as a viable diagnostic procedure. However, EUS-FNA has not been widely used as a useful diagnostic technique in most hospitals. CT and MRI are helpful to better perceive the imaging characteristics of the mass, while EUS-FNA may help the surgeons to confirm the source of the tissues and define the nature of the cyst. Intraoperatively, if the cyst is found closely adhered to the adjacent organs, tissues, and blood vessels, damage to the capsule wall should be avoided to ensure complete surgical resection to reduce recurrence. Postoperative follow-up is recommended. Routine postoperative histopathological examination is required to clarify the tissue source and to determine whether the cyst is benign or malignant. Resections should be performed if the cyst is confirmed as malignant.
The authors would like to express special gratitude to Professor Liang Zhou, surgeons Wei-Hua Huang and Wei-Wei Fan of Department of General Surgery, Xi’an Gaoxin Hospital for their support to publish this article.
| 1. | Liou CH, Hsu HH, Hsueh CJ, Juan CJ, Chen CY. Imaging findings of intradiaphragmatic bronchogenic cyst: a case report. J Formos Med Assoc. 2001;100:712-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 60] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 2. | Ubukata H, Satani T, Motohashi G, Konishi S, Goto Y, Watanabe Y, Nakada I, Tabuchi T. Intra-abdominal bronchogenic cyst with gastric attachment: report of a case. Surg Today. 2011;41:1095-1100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 3. | Akahoshi K, Sumida Y, Matsui N, Oya M, Akinaga R, Kubokawa M, Motomura Y, Honda K, Watanabe M, Nagaie T. Preoperative diagnosis of gastrointestinal stromal tumor by endoscopic ultrasound-guided fine needle aspiration. World J Gastroenterol. 2007;13:2077-2082. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 159] [Cited by in RCA: 185] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 4. | Okubo K, Yamao K, Nakamura T, Tajika M, Sawaki A, Hara K, Kawai H, Yamamura Y, Mochizuki Y, Koshikawa T, Inada K. Endoscopic ultrasound-guided fine-needle aspiration biopsy for the diagnosis of gastrointestinal stromal tumors in the stomach. J Gastroenterol. 2004;39:747-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 74] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 5. | Chatzipantelis P, Salla C, Karoumpalis I, Apessou D, Sakellariou S, Doumani I, Papaliodi E, Konstantinou P. Endoscopic ultrasound-guided fine needle aspiration biopsy in the diagnosis of gastrointestinal stromal tumors of the stomach. A study of 17 cases. J Gastrointestin Liver Dis. 2008;17:15-20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 45] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 6. | Wang M, Qiu X, He X, Tian C. Characteristic of extra luminal gastric stromal tumor arising from the lesser curvature of the stomach: A case report. Medicine (Baltimore). 2020;99:e19885. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | Chinese Ultrasound Doctors Association. [Guidelines for the Clinical Application of Interventional Ultrasound in China]. Beijing: People’s Medical Publishing House, Co., Ltd., 2017: 78. |
| 8. | Chen HY, Fu LY, Wang ZJ. Ileal bronchogenic cyst: A case report and review of literature. World J Clin Cases. 2018;6:807-810. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 8] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 9. | Meng QX, Liu C, Tian J. [Practical diagnostics of CT]. Beijing: Scientific and Technical Documentation Press, 2009: 547-548. |
| 10. | Zhao L, Zhao Z, Wang W, Zhao W, Tuo S, Shi Y, Zhang W, Chen L, Hong L, Yang J, Lu W, Wu Q, Wang J, Wu K. Current characteristics on small intestinal stromal tumor-a case control study. Ann Palliat Med. 2020;9:98-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 11. | Kuru S, Kismet K. Meckel's diverticulum: clinical features, diagnosis and management. Rev Esp Enferm Dig. 2018;110:726-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 12. | Liu R, Adler DG. Duplication cysts: Diagnosis, management, and the role of endoscopic ultrasound. Endosc Ultrasound. 2014;3:152-160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 110] [Cited by in RCA: 141] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 13. | Sangüesa Nebot C, Llorens Salvador R, Carazo Palacios E, Picó Aliaga S, Ibañez Pradas V. Enteric duplication cysts in children: varied presentations, varied imaging findings. Insights Imaging. 2018;9:1097-1106. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 83] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 14. | Islah MA, Hafizan T. Perforated ileal duplication cyst presenting with right iliac fossa pain mimicking perforated appendicitis. Med J Malaysia. 2008;63:63-64. [PubMed] |
| 15. | Di Serafino M, Mercogliano C, Vallone G. Ultrasound evaluation of the enteric duplication cyst: the gut signature. J Ultrasound. 2016;19:131-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 16. | Krishna Kumar G. Gastric duplication cyst in an infant presenting with non-bilious vomiting. Malays J Med Sci. 2012;19:76-78. [PubMed] |
| 17. | Ho CK. Obstructed jejunal duplication cyst in an infant. Med J Malaysia. 2020;75:167-168. [PubMed] |
| 18. | Fan RY, Li N, Yang GZ, Sheng JQ. Bronchogenic cyst in the omental bursa: A case report. J Dig Dis. 2016;17:52-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 19. | Xiao J, Zhang R, Chen W, Wu B. Ectopic bronchogenic cyst of the gastric cardia considered to be a gastrointestinal stromal tumor before surgery: a case report. BMC Surg. 2020;20:42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Respiratory system
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Thompson LDR, United States; Yui K, Japan S-Editor: Wang JL L-Editor: Kerr C P-Editor: Wang JL