Published online Dec 6, 2022. doi: 10.12998/wjcc.v10.i34.12605
Peer-review started: August 15, 2022
First decision: October 12, 2022
Revised: October 20, 2022
Accepted: November 4, 2022
Article in press: November 4, 2022
Published online: December 6, 2022
Processing time: 108 Days and 23 Hours
Cytomegalovirus (CMV) infection is usually subclinical and asymptomatic in the healthy population, whereas severe complications occur in immunocompromised patients.
In this case report, we described a rare case of acute CMV hepatitis in a 35-year-old male immunocompetent patient who presented with a history of week-long intermittent fever with nonspecific constitutional symptoms. Acute hepatitis was suspected according to the initial serological tests. After ruling out other etiologies, including viral hepatitis A, B, C, drug, alcohol, autoimmune, and Wilson disease, acute CMV hepatitis was diagnosed based on positive CMV IgM and DNA quantitative tests. Because there was no any local acute hepatitis E reported in Taiwan, so hepatitis E was not checked. The patient recovered both clinically and serologically with symptomatic management and without antiviral therapy within 12 days from the onset of symptom.
In conclusion, a diagnosis of CMV infection should be considered when nonspecific prodromal symptoms occur in acute hepatitis with an uncertain etiology. Antiviral therapy should not be used in immunocompetent patient who had no decompensation of the liver, such as this patient. Widely available non
Core Tip: Cytomegalovirus (CMV) infection is usually subclinical and asymptomatic in the healthy population, whereas severe complications occur in immunocompromised patients. In this case report, we described a rare case of acute CMV hepatitis in a 35-year-old male immunocompetent patient who presented with a history of week-long intermittent fever with nonspecific constitutional symptoms. Acute hepatitis was suspected according to the initial serological tests. After ruling out other etiologies, acute CMV hepatitis was diagnosed based on positive CMV IgM and DNA quantitative tests. The patient recovered both clinically and serologically with symptomatic management and without antiviral therapy within 12 d from the onset of symptom.
- Citation: Wang JP, Lin BZ, Lin CL, Chen KY, Lin TJ. Acute cytomegalovirus hepatitis in an immunocompetent patient: A case report. World J Clin Cases 2022; 10(34): 12605-12609
- URL: https://www.wjgnet.com/2307-8960/full/v10/i34/12605.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i34.12605
Cytomegalovirus (CMV), a member of the Herpesviridae family, is a double-stranded DNA virus[1]. Adult infection is transmitted through multiple routes, including oropharyngeal secretions, genital fluids, urine, blood, breast milk, perinatal and occupational exposure[2,3]. While CMV infection is associated with both significant morbidity and mortality in immunocompromised hosts, usually, it causes a mild subclinical infection in most nonimmunosuppressed patients[2,3]. CMV disease is defined as evidence of CMV infection with attributable symptoms, categorized as a viral syndrome with fever, malaise, leukopenia and thrombocytopenia or as a tissue-invasive disease[2]. However, CMV infection in immunocompetent patients can seldom lead to acute hepatitis, acute liver failure requiring liver transplantation, acute pancreatitis, portal vein thrombosis, or splenic infarction[2]. Here, we report the case of a young healthy male who presented with acute hepatitis due to CMV infection.
Intermittent fever with constitutional symptoms for 1 wk.
A 35-year-old male without any pre-existing comorbidity presented with a week-long history of intermittent fever with associated symptoms including chills, malaise, myalgia, headache, mild sore throat, and diarrhea. No features of altered consciousness, poor appetite, cough, dyspnea, abdominal pain, nausea or vomiting, dysuria, or urinary frequency were reported. The patient lived with his wife in a monogamous relationship and without any pets.
He denied any past illness.
He denied any sick contacts at work or home and a traveling history outside of Taipei city. Additionally, he did not have a history of smoking, alcohol, drug, and Chinese herbal medicine use.
On admission, his vitals were stable – temperature: 37.8 °C, pulse rate: 90 beats per minute, respiratory rate: 20 breaths per min, and normal blood pressure.
Initial complete blood count (CBC) showed a leukocyte count: 6590/µL [lymphocytes: 63.7% (21.2%-51.0%); monocytes: 18.2% (3.1%-8.0%); neutrophils: 12.7% (41.2%-74.7%)], normal hemoglobin, and platelet count. The liver function tests revealed normal bilirubin and prothrombin time levels; however, the serum levels of aspartate aminotransferase: 396 U/L (10-42 U/L), alanine aminotransferase: 505 U/L (7-42 U/L), alkaline phosphatase: 204 U/L (35-129 U/L), and gamma-glutamyl transferase: 174 U/L (5-61 U/L), were significantly raised. The urinalysis was negative for glucose, protein, blood, nitrite, and leukocytes.
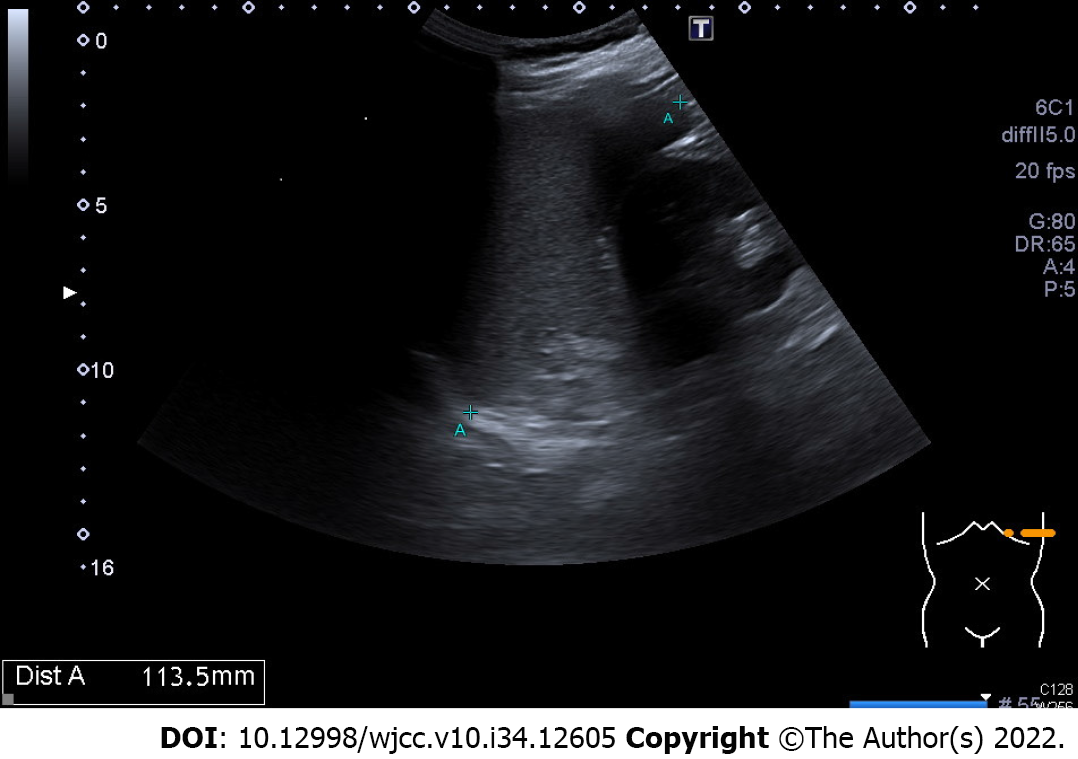
The abdominal ultrasonography showed the length of splenic long –axis was 11.4 cm, so splenomegaly was impressed (Figure 1).
The patient was admitted to our ward with a probable diagnosis of acute viral hepatitis. The reason of admission is not only intractable symptoms but also the risk of acute hepatitis in progress[t1]. Serological tests for viral hepatitis A, B, C, herpes simplex, and Epstein–Barr virus (EBV) were all negative; however, CMV serology was positive with an IgM titer of 2.42 index (< 1.0) but the level of IgG was not checked[t2]. Antinuclear antibody, antismooth muscle antibody, and antimitochondrial antibody levels were negative, and IgG and ceruloplasmin levels were unremarkable. CMV–DNA polymerase chain reaction (PCR) revealed 486 copies/mL (1 copy = 0.91 IU), and the antihuman immunodeficiency virus (HIV) rapid test was also negative. Follow-up (on day 4) CBC revealed a leukocyte count of 14120/µL (lymphocytes: 70.8%, monocytes: 8.5%, neutrophils: 10.4%, and atypical lymphocyte: 0.9%), normal hemoglobin, and platelet counts. Accordingly, a diagnosis of acute CMV hepatitis was made. However, a decision to not go for antiviral therapy was made due to its associated toxicity and no hepatic decompensation.
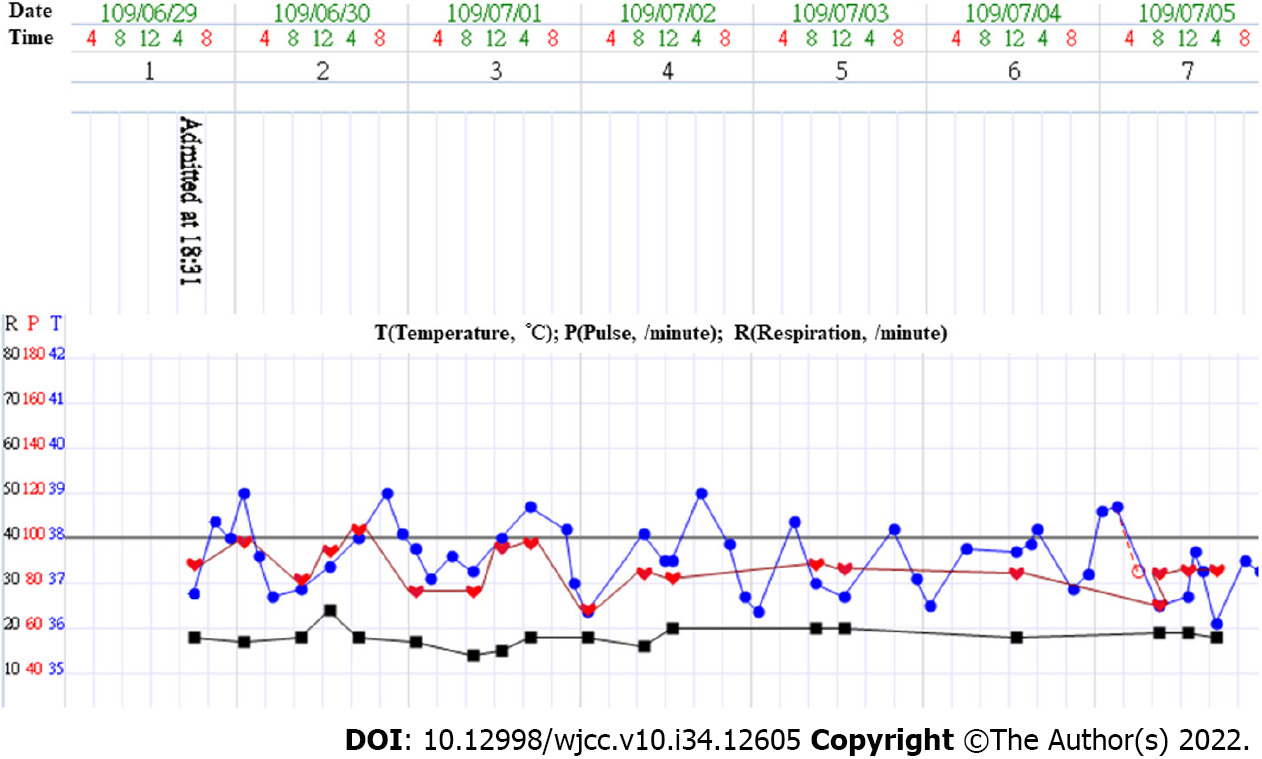
Following admission, he continued to have temperature spikes up to 39 °C in the first three days (Figure 2). Antibiotics—piperacillin/tazobactam—were given since a superimposed bacterial infection could not be ruled out. Additionally, he was managed with an antipyretic, intravenous fluid supply, and monitoring the fever pattern and liver function. The blood culture turned out to be negative for any microbial growth.
The fever subsided by day 7; the liver enzyme profiles also trended down gradually. With symptomatic and clinical recovery, the patient was discharged after 12 d of hospitalization. He was then followed up at our outpatient department until his liver enzyme profiles had resolved to within the normal limits 1 mo later.
Notably, only about 10% of CMV infections lead to clinical symptoms in immunocompetent patients[4], which have a mononucleosis-like presentation, consisting of prolonged fever, malaise, myalgia, and headache[2,5]. The CBC may show leukopenia, neutropenia, thrombocytopenia, or atypical lymphocytes. Furthermore, the liver is frequently involved and hepatosplenomegaly is seen in 10%-38% of patients[3]. About 70% of acute CMV infections result in abnormalities in hepatic enzyme tests in both immunocompetent and immunosuppressed patients[6]; however, these levels are rarely elevated by more than five times the upper limit[3]. Our patient’s clinical profile mimicked a mononucleosis-like syndrome, including significantly constitutional symptoms, monocytosis, lymphocytosis, atypical lymphocytes, and splenomegaly. Therefore, acute viral hepatitis was primarily suspected on the first day of admission. EBV infection had been excluded, and a simultaneously positive anti-CMV IgM test, coupled with detectable CMV-DNA levels and a negative anti-HIV rapid test confirmed our diagnosis of acute CMV hepatitis in an immunocompetent patient. Other differential diagnoses – acute drug hepatitis, alcoholic hepatitis, autoimmune hepatitis, Wilson’s disease, viral hepatitis A, B, C, and herpes simplex, were excluded based on his medical history and serological tests. No any local acute hepatitis E had been reported in Taiwan in the past, so hepatitis E was not checked. Therefore, a liver biopsy was not needed for diagnosis in this patient.
Ideally, a diagnosis of acute CMV hepatitis is based on clinical suspicion, a positive anti-CMV IgM or elevated IgG titers, or a qualitative or quantitative CMV–DNA PCR assay[7,8]. Quantitative PCR for CMV-DNA is the standard method for the early detection of CMV infections[7], so CMV antigen pp65 and IgG levels were not checked in our patient[t1]. Although a liver biopsy is not mandatory, it may be used in cases when the diagnosis is uncertain[2,5]. Histological findings of the specimen include sinusoidal infiltration of predominantly mononuclear cells and mild hepatocellular necrosis[5]. Although nonspecific, the most important pathological finding is the intranuclear inclusion of body formations in the infected liver cells. Immunohistochemical staining of the CMV antigen can also help confirm the diagnosis of CMV hepatitis[2].
Currently, there are four licensed antiviral drugs for the treatment of CMV infections – ganciclovir, valganciclovir, foscarnet, and cidofovir[9]. Mostly, acute CMV hepatitis in immunocompetent patients is self-limiting, so antiviral therapy is not necessary. Because of their potential side effects, including myelosuppression, hepatotoxicity, teratogenicity, and infertility, they are reserved for severe infection or immunocompromised patients[10]. Therefore, antiviral therapy should not be used in immunocompetent patient who had no decompensation of the liver, such as this patient.
In conclusion, a diagnosis of CMV infection should be considered when nonspecific prodromal symptoms occur in acute hepatitis with an uncertain etiology. Widely available noninvasive tests for CMV can facilitate early diagnosis if used appropriately. However, harm–benefit analysis is essential before using antiviral therapy in immunocompetent patients.
| 1. | Crough T, Khanna R. Immunobiology of human cytomegalovirus: from bench to bedside. Clin Microbiol Rev. 2009;22:76-98, Table of Contents. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 438] [Cited by in RCA: 498] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 2. | Bunchorntavakul C, Reddy KR. Epstein-Barr Virus and Cytomegalovirus Infections of the Liver. Gastroenterol Clin North Am. 2020;49:331-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 3. | Fakhreddine AY, Frenette CT, Konijeti GG. A Practical Review of Cytomegalovirus in Gastroenterology and Hepatology. Gastroenterol Res Pract. 2019;2019:6156581. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 59] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 4. | Eddleston M, Peacock S, Juniper M, Warrell DA. Severe cytomegalovirus infection in immunocompetent patients. Clin Infect Dis. 1997;24:52-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 169] [Article Influence: 5.8] [Reference Citation Analysis (1)] |
| 5. | Noor A, Panwala A, Forouhar F, Wu GY. Hepatitis caused by herpes viruses: A review. J Dig Dis. 2018;19:446-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 6. | Leonardsson H, Hreinsson JP, Löve A, Björnsson ES. Hepatitis due to Epstein-Barr virus and cytomegalovirus: clinical features and outcomes. Scand J Gastroenterol. 2017;52:893-897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 7. | Kotton CN, Kumar D, Caliendo AM, Huprikar S, Chou S, Danziger-Isakov L, Humar A; The Transplantation Society International CMV Consensus Group. The Third International Consensus Guidelines on the Management of Cytomegalovirus in Solid-organ Transplantation. Transplantation. 2018;102:900-931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 509] [Cited by in RCA: 876] [Article Influence: 125.1] [Reference Citation Analysis (1)] |
| 8. | Ross SA, Novak Z, Pati S, Boppana SB. Overview of the diagnosis of cytomegalovirus infection. Infect Disord Drug Targets. 2011;11:466-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 146] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 9. | Schleiss MR. Antiviral therapy of congenital cytomegalovirus infection. Semin Pediatr Infect Dis. 2005;16:50-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 29] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 10. | Rafailidis PI, Mourtzoukou EG, Varbobitis IC, Falagas ME. Severe cytomegalovirus infection in apparently immunocompetent patients: a systematic review. Virol J. 2008;5:47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 332] [Cited by in RCA: 388] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Taiwan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): E
P-Reviewer: Bessone F, Argentina; Inoue K, Japan; Kurtcehajic A, Bosnia and Herzegovina S-Editor: Liu JH L-Editor: A P-Editor: Liu JH