Published online Nov 6, 2022. doi: 10.12998/wjcc.v10.i31.11607
Peer-review started: July 19, 2022
First decision: August 19, 2022
Revised: August 30, 2022
Accepted: September 27, 2022
Article in press: September 27, 2022
Published online: November 6, 2022
Processing time: 99 Days and 23.5 Hours
Gastric linitis plastica (GLP) is a subset of gastric cancer with a poor prognosis. It is difficult to obtain a definitive diagnosis by endoscopic mucosal biopsies, and the usefulness of an endoscopic ultrasonography-guided fine-needle biopsy (EUS-FNB) for GLP has been recently reported. Meanwhile, autoimmune diseases are occasionally known to coexist with malignant tumors as paraneoplastic syn
An 81-year-old man was admitted to our hospital for a 1-mo history of epigastric pain that increased after eating. His laboratory data revealed high levels of serum carbohydrate antigen 19-9 and immunoglobulin-G4. Endoscopic examinations showed giant gastric folds and reddish mucosa; however, no epithelial changes were observed. The gastric lumen was not distensible by air inflation, suggesting GLP. Computed tomography showed the thickened gastric wall, the diffuse enlargement of the pancreas, and the peripancreatic rim, which suggested autoimmune pancreatitis (AIP) coexisting with GLP. Because the pathological findings of the endoscopic biopsy showed no malignancy, he underwent an EUS-FNB and was diagnosed with GLP. He received chemotherapy for unresectable gastric cancer due to peritoneal metastasis, after which both the gastric wall thickening and diffuse enlargement of the pancreas were improved.
An EUS-FNB for GLP with a negative endoscopic biopsy is useful, and AIP may develop as a paraneoplastic syndrome.
Core Tip: Gastric linitis plastica (GLP) is a form of gastric cancer that is difficult to diagnose by an endoscopic biopsy. An ultrasonography-guided fine-needle biopsy is useful for diagnosing GLP with negative endoscopic biopsy findings. Meanwhile, autoimmune pancreatitis (AIP) is an immunoglobulin-G4 related disease (IgG4-RD) that occasionally coexists with gastric cancer. Some cases of IgG4-RD have been reported to be improved by the treatment of malignant tumors, suggesting that IgG4-RD may develop as a paraneoplastic syndrome. From the clinical course and image findings, we experienced a suspected case of AIP developed as paraneoplastic syndrome coexisting with GLP.
- Citation: Sato R, Matsumoto K, Kanzaki H, Matsumi A, Miyamoto K, Morimoto K, Terasawa H, Fujii Y, Yamazaki T, Uchida D, Tsutsumi K, Horiguchi S, Kato H. Gastric linitis plastica with autoimmune pancreatitis diagnosed by an endoscopic ultrasonography-guided fine-needle biopsy: A case report. World J Clin Cases 2022; 10(31): 11607-11616
- URL: https://www.wjgnet.com/2307-8960/full/v10/i31/11607.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i31.11607
Gastric linitis plastica (GLP) is a subset of gastric cancer with a poor prognosis, showing a frequency of 8%-17% among overall gastric cancers[1]. The submucosa and muscularis propria are invaded by poorly differentiated adenocarcinoma cells or signet-ring cells, resulting in a thickened gastric wall. Characteristic endoscopic findings include giant folds and poor distension by air inflation; however, no malignant cells are often obtained by an endoscopic mucosal biopsy due to the lack of epithelial changes of the mucosa[1]. Recently, the usefulness of an endoscopic ultrasonography-guided fine-needle biopsy (EUS-FNB) for GLP with a negative endoscopic biopsy has been reported[2].
Immunoglobulin G4-related disease (IgG4-RD) is characterized by the infiltration of lymph
However, IgG4-RD is also known to be associated with malignant tumors, and some have reported that chronic inflammatory stimulation was involved, although the exact mechanism has not been elucidated[5]. One pathway, involving interleukin-33, which plays a role in the pathogenesis of IgG4-RD, has also been reported to be associated with the development of malignancy[6,7]. In particular, autoimmune pancreatitis (AIP) is an IgG4-RD that occasionally coexists with gastric cancer. Some cases of IgG4-RD, including AIP, have been reported to be improved by the treatment of malignant tumors, suggesting that IgG4-RD may develop as a paraneoplastic syndrome[8].
We herein report a case of GLP diagnosed by a EUS-FNB with AIP that improved after starting chemotherapy for GLP. These findings suggest the usefulness of an EUS-FNB for GLP with a negative endoscopic biopsy and the possibility that AIP may develop as a paraneoplastic syndrome.
An 81-year-old man presented with epigastric pain lasting for a month.
His pain increased after eating. He had undergone esophagogastroduodenoscopy (EGD) at the previous hospital, which showed findings suggesting GLP. He was therefore admitted to our hospital for further examinations.
The patient had a history of postoperative benign prostatic hyperplasia and eradication of Helicobacter pylori.
He had no specific personal and family history.
His vital signs were normal, and the abdomen examination revealed mild epigastric tenderness and no guarding or rebound tenderness. He had no swollen Virchow's lymph nodes or parotid or lacrimal glands.
A blood examination showed that inflammatory markers, serum pancreatic enzymes, and total and direct bilirubin levels were normal (Table 1). The levels of serum carbohydrate antigen 19-9 (CA19-9) and IgG4 were elevated (2556 U/mL and 280.5 mg/dL, respectively).
| Laboratory data | (Reference range, units) | |
| Hematology | ||
| WBC | 5290 | (3300-8600, /μL) |
| Neu | 61.5 | (40.0-70.0, %) |
| Ly | 29.1 | (16.5-49.5, %) |
| Mono | 6.0 | (2.0-10.0, %) |
| Eos | 2.4 | (0.0-8.5, %) |
| Bas | 0.9 | (0.0-2.5, %) |
| RBC | 449 | (435-555, × 104/μL) |
| Hb | 14.1 | (13.7-16.8, g/dL) |
| Ht | 43.0 | (40.7-50.1, %) |
| PLT | 14.8 | (15.8-34.8, × 104/μL) |
| Coagulation | ||
| APTT | 32.1 | (26.9-38.1, sec) |
| PT | 82.0 | (73-118, %) |
| PT-INR | 1.1 | (< 2.99) |
| D-dimer | 2.2 | (0.0-0.9, μg/mL) |
| Biochemistry | ||
| TP | 7.4 | (6.6-8.1, g/dL) |
| Alb | 3.9 | (4.1-5.1, g/dL) |
| T.Bil | 0.67 | (0.40-1.50, mg/dL) |
| AST | 22 | (13-30, U/L) |
| ALT | 19 | (10-42, U/L) |
| γ-GTP | 15 | (38-75, U/L) |
| ALP | 91 | (38-113, U/L) |
| AMY | 95 | (44-132, U/L) |
| LIPA | 39 | (13-55, U/L) |
| BUN | 14.1 | (8.0-20.0, mg/dL) |
| CRE | 1.06 | (0.65-1.07, mg/dL) |
| Na | 137 | (138-145, mmol/L) |
| K | 4.4 | (3.6-4.8, mmol/L) |
| Cl | 105 | (101-108, mmol/L) |
| Ca | 9.4 | (8.8-10.1, mg/dL) |
| CRP | 0.05 | (< 0.15, mg/dL) |
| Tumor marker | ||
| CEA | 3.2 | (< 5.00, ng/mL) |
| CA19-9 | 2556 | (0.0-35.4, U/mL) |
| Immunoglobulin | ||
| IgG4 | 280.5 | (4.5-117.0, mg/dL) |
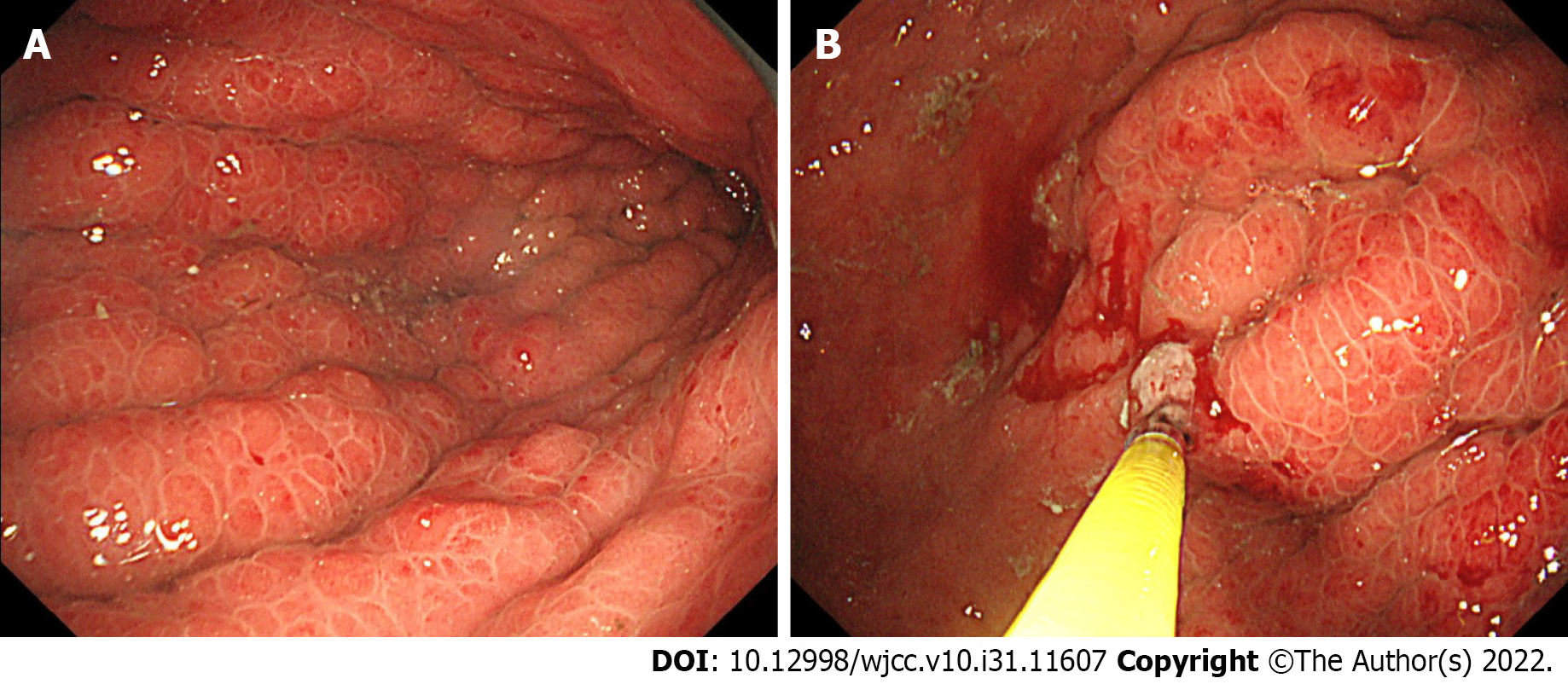
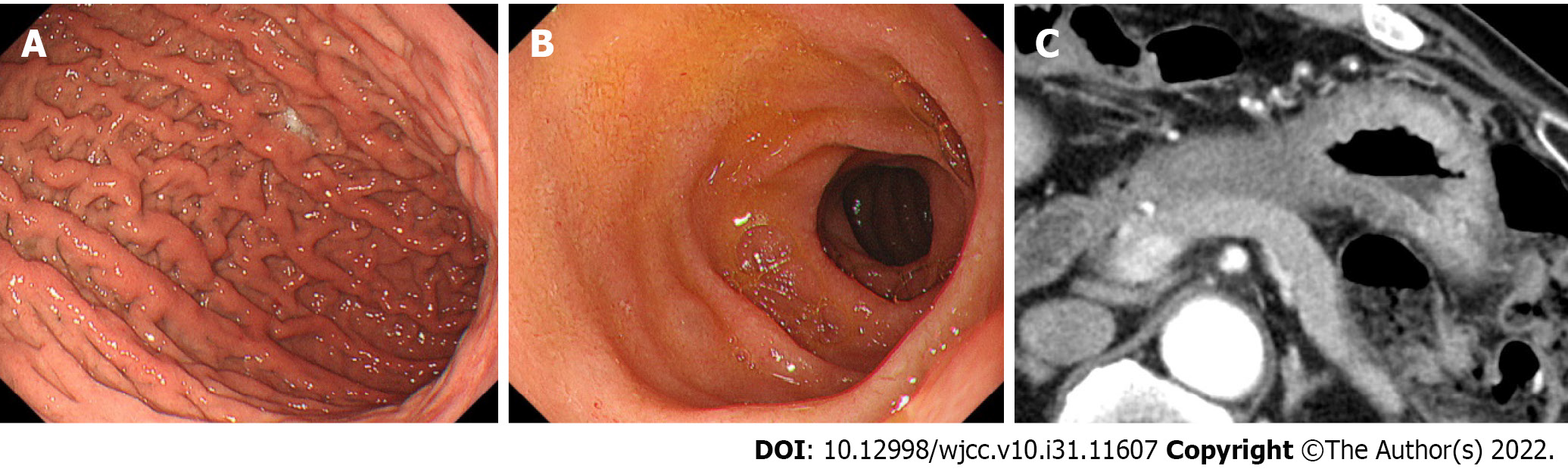
Endoscopy: EGD showed giant gastric folds and reddish mucosa; however, no epithelial changes were observed. The gastric lumen was not distensible by air inflation, making duodenoscopy impossible to perform (Figure 1A). We suspected GLP, so seven specimens were obtained by a gastric mucosal biopsy, none of which showed malignancy (Figure 1B).
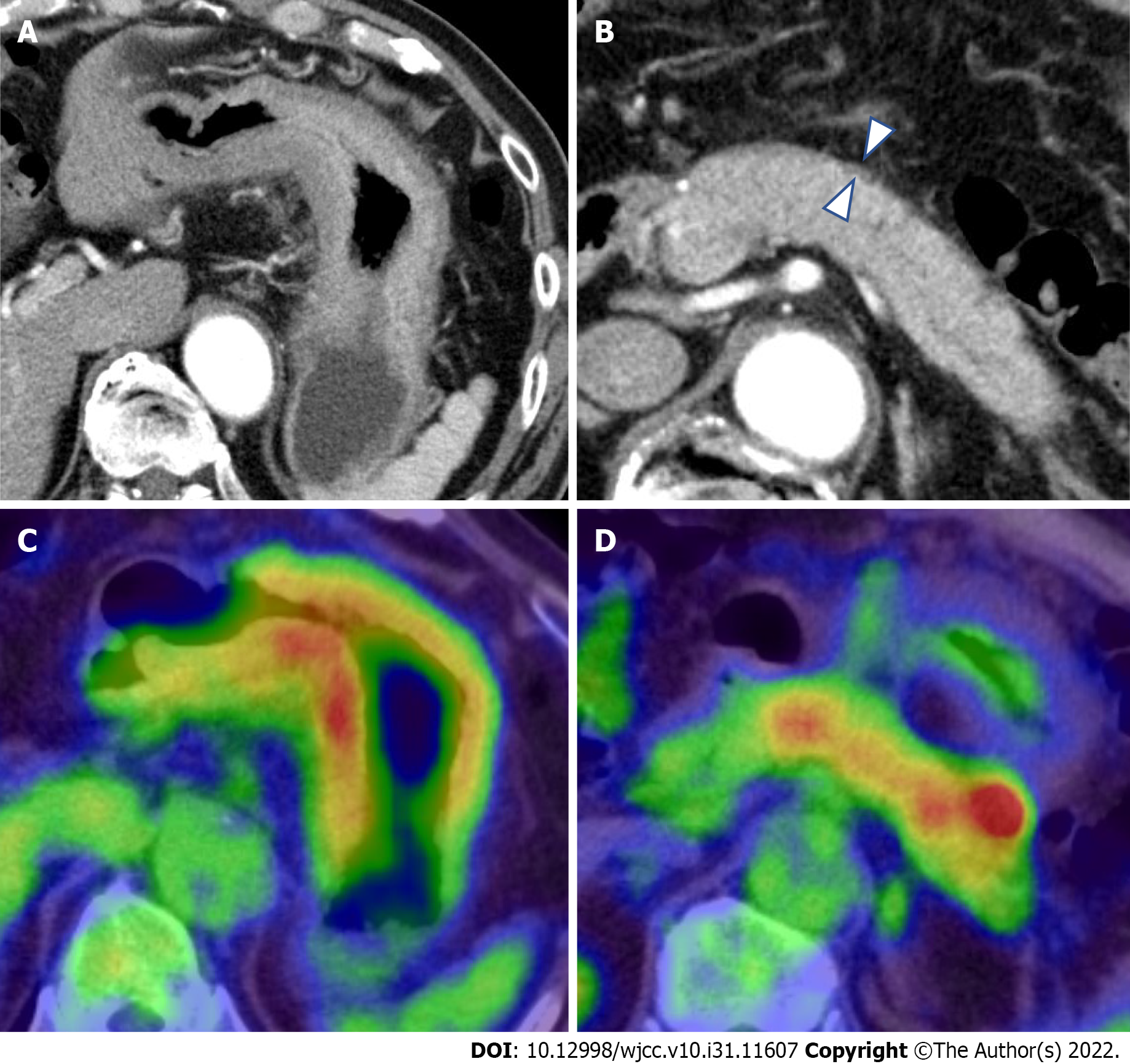
Computed tomography and fluorodeoxyglucose-positron emission tomography/computed tomography: Contrast-enhanced computed tomography (CT) showed thickening of the wall of the gastric body (Figure 2A). Incidentally, CT showed diffuse enlargement of the pancreas and peripancreatic rim, suggesting the coexistence of AIP (Figure 2B). No evidence of bile duct obstruction or dilation was observed. After fluorodeoxyglucose-positron emission tomography/CT (FDG-PET/CT), the accumulation of FDG was found in both the gastric wall [maximum standardized uptake value (SUVmax: 19.2) and pancreas (SUVmax: 4.9)] (Figure 2C and D). No other organ involvement complicating AIP and no obvious metastasis of GLP nor swollen nodules in which FDG had accumulated were observed.
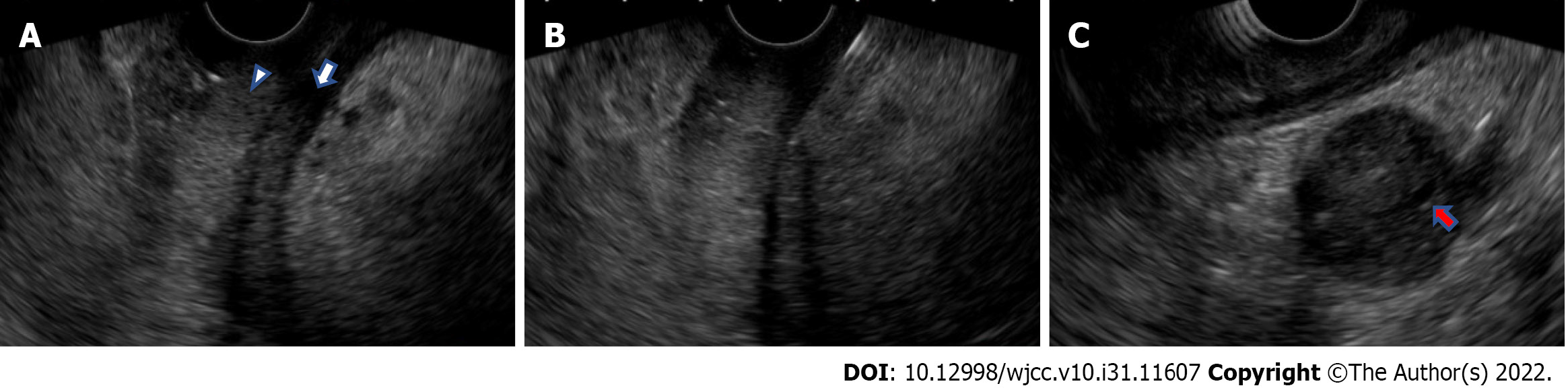
EUS and EUS-FNB findings: An EUS-FNB was performed for the histopathological diagnosis. With a linear array echoendoscope and a universal ultrasonography processor (EG-580UT and SU-1; Fujifilm, Tokyo, Japan), the thickened third layer of the gastric wall (representing the submucosa) and the fourth layer (representing the muscularis propria) were observed (Figure 3A), which was consistent with the findings of GLP. The thickness of the gastric wall as measured by EUS was up to 18.5 mm. The thickened fourth layer of the gastric wall was punctured a total of three times using a 19-gauge needle (SharkCore; Medtronics, Minneapolis, MN, United States) (Figure 3B). EUS also revealed hyperechoic spots in the diffuse hypoechoic pancreatic parenchymal and duct-penetrating sign (Figure 3C). These findings were consistent with AIP, and no obvious pancreatic tumor was observed. Puncturing the pancreas to obtain pancreatic tissue seemed undesirable because a transgastric puncture might cause seeding of cancer, and transduodenal puncture was impossible due to difficulty reaching the duodenum with the scope. No adverse event related to an EUS-FNB occurred.
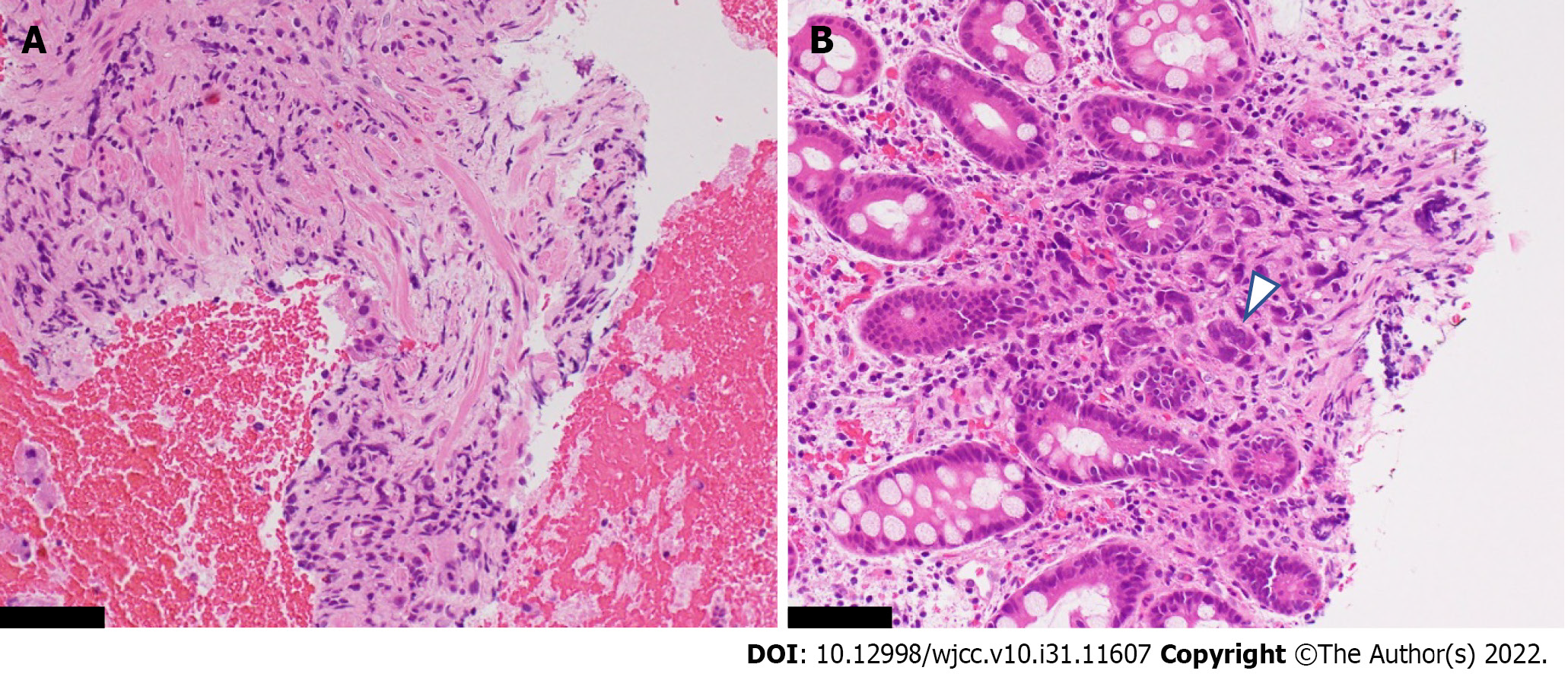
Histopathology: The histopathological findings of the gastric wall showed poorly differentiated adenocarcinoma within the muscularis propria and the deeper site of the mucosa (Figure 4A and B). No cancer cells were found in the shallow site of the mucosa. In the muscularis mucosae, fibroblasts had proliferated and were considered to be the cause of gastric wall thickening.
An EUS-FNB and the histopathological findings led to the definite diagnosis of GLP. Furthermore, based on the diffuse pancreatic enlargement and high serum levels of IgG4, a diagnosis of definite type 1 AIP was also made according to the Japanese Clinical Diagnostic Criteria for AIP[9]. Although we were unable to perform an EUS-FNB of the enlarged pancreas, AIP was deemed the most likely culprit based on the characteristic findings of imaging modalities, including CT and EUS, and the high levels of serum IgG4.
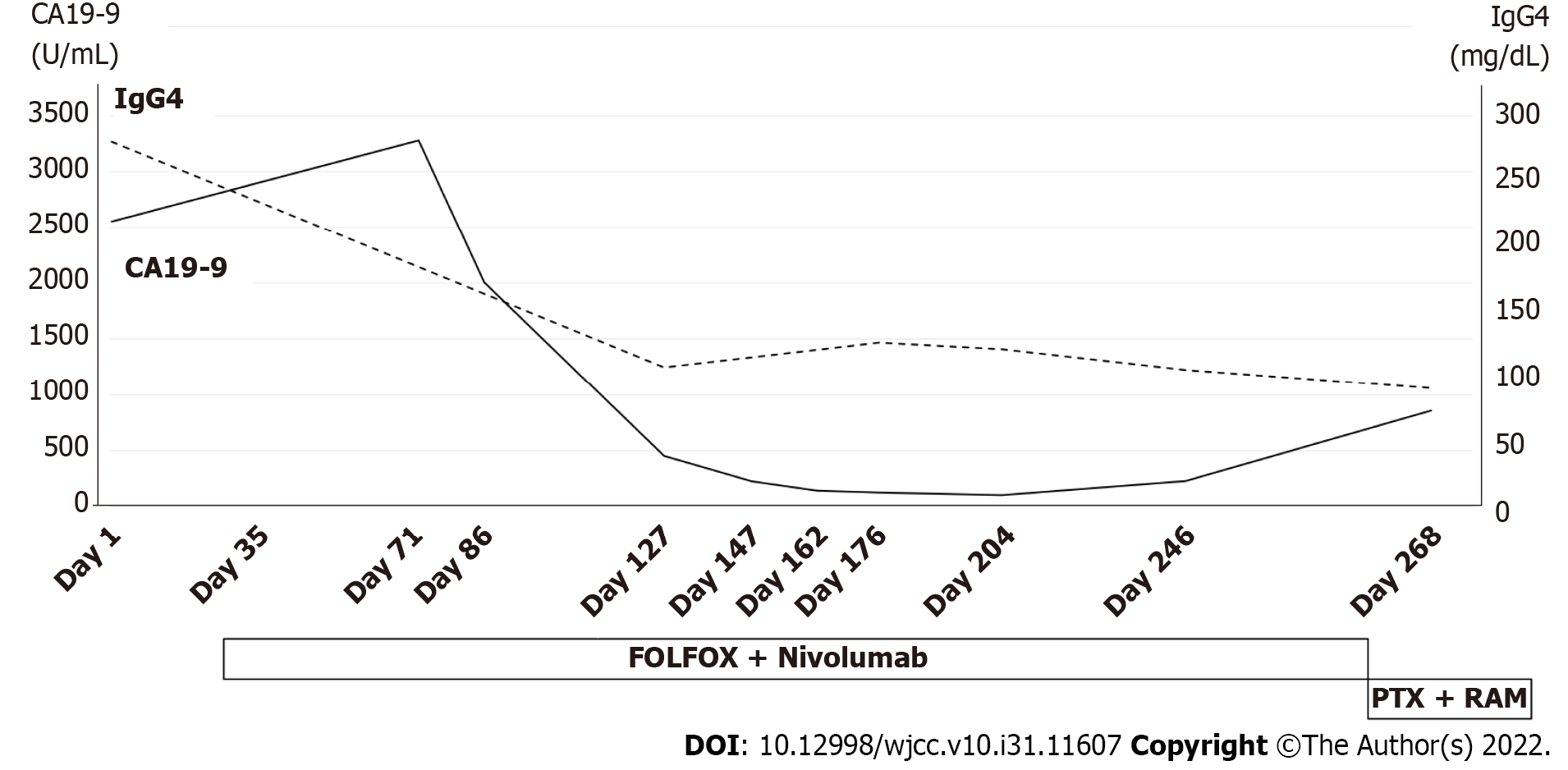
CT and FDG-PET/CT showed no obvious metastasis; thus, we planned total gastrectomy. There were no intraoperative findings of GLP direct invasion of the pancreas; however, peritoneal metastasis was observed. Thus, jejunostomy was performed to prevent gastrointestinal obstruction. The patient then received first-line chemotherapy with a postoperative FOLFOX plus nivolumab regimen (5-fluorouracil, leucovorin, and oxaliplatin: 5-fluorouracil 400 mg/m2, day 1 and 1200 mg/m2, days 1-2, leucovorin 400 mg/m2, day 1, oxaliplatin 85 mg/m2, day 1; and nivolumab 240 mg, day 1, every 2 wk). There were no symptoms of AIP, such as abdominal pain or jaundice, and there was no evidence of involvement of other organs. Therefore, treatment for AIP was deemed unnecessary.
After the start of chemotherapy, the endoscopic findings, such as the giant folds, were improved, and the gastric lumen became distensible, which allowed for duodenoscopy (Figure 5A and B). The CT findings of the thickened gastric wall and diffuse enlargement of the pancreas were also improved (Figure 5C). The serum levels of IgG4 improved along with CA19-9 during chemotherapy (Figure 6). These facts indicated that his AIP had improved along with his GLP. He is still receiving chemotherapy with regimen modification.
GLP is suspected in cases with endoscopic findings showing giant folds and a lack of distension by air inflation[1]. Malignant cells invade the submucosa and muscularis propria without epithelial changes in the mucosa, which results in a negative endoscopic biopsy in about 55.9% of cases of Borrmann type IV gastric cancer[10]. GLP presents with a diffuse thickened gastric wall on CT. EUS shows the thickened fourth layer which represents the muscularis propria, or the disappearance of the layer structure[11].
Although GLP was the most strongly suspected disease in this case, we had to consider the possibility of other diseases associated with thickening of the submucosa or muscularis propria, including Menetrie's gastritis, lymphoid hyperplasia, amyloidosis, and malignant lymphoma[12]. Adequate specimens to evaluate malignancy are difficult to obtain from submucosa lesions, including GLP. As deep endoscopic biopsy techniques, endoscopic mucosal resection (EMR), bite-on-bite techniques, mucosal incision-assisted biopsies, and EUS-FNBs are used[13]. Although the European Society of Gastrointestinal Endoscopy recommends a MIAB or EUS-FNB for the tissue diagnosis of subepithelial lesions, including gastrointestinal stromal tumor (GIST)[14], there is no standard method for making a tissue diagnosis of GLP. In the previous articles, an EUS-FNB for GLP cases with a negative endoscopic biopsy was reported to be useful in that EUS could directly visualize whether or not the target had been punctured properly, and the positive rates were high with few adverse events (Table 2)[13,15-17]. However, Zhou et al[18] conversely reported that the diagnostic yield of EMR with the bite-on-bite technique for gastric-infiltrating tumors, including GLP, was 82.2% (23/28). No severe hemorrhaging has occurred in any cases, but minor oozing of blood managed with argon plasma coagulation or epinephrine was observed in seven cases[18], whereas no bleeding was reported with an EUS-FNB of the gastric wall. In our case, we were able to directly observe the thickened third and fourth layer of the gastric wall under EUS and puncture each layer over a few sessions without any adverse events.
| Ref. | Country | Number of patients | Gastric wall thickness per mm, median (range) | Type of FNB needle, gauge | Number of needle passes | Positive rates, n (%) | Adverse event with FNB |
| Ye et al[15], 2018 | China | 24 | 15.7 (7.4-22.0) | 19 | 1-3 | 13 (54.2) | None |
| Liu et al[16], 2019 | China | 9 | 12.9 (8.30-22.70) | 19, 22, 25 | 4-8 | 6 (66.7) | None |
| Takada et al[17], 2021 | Japan | 13 | 20 (15.0-25.0) | 22 | 2 | 10 (76.9) | None |
| Takahashi et al[13], 2021 | Japan | 2 | 15.7 (9.3-22.0) | 22 | 3-5 | 2 (100.0) | None |
In the present case, findings suggestive of AIP, an IgG4-RD, were observed along with GLP. The specificity of serum IgG4 for distinguishing AIP from pancreatic cancer was reported to be 93%[19], and that for IgG4 > 280 mg/dL was 99%[20]. Diffuse pancreatic enlargement, which must be differentiated from AIP, can be definitive of pancreatic cancer or a metastatic pancreatic tumor[21,22]. However, the EUS findings in this case were characteristic of AIP. Although we did not perform a EUS-FNB from the pancreas, a definitive diagnosis was obtained based on the serum levels of IgG4 and typical imaging findings.
Recently, the concept of IgG4-related gastrointestinal disease (GID) in the setting of IgG4-RD has arisen. For example, ulceration, polyps, and thickening walls were reported as the characteristic features of IgG4-GID[23]. A striated inflammatory lesion in the muscularis propria causing the thickening of muscularis propria was identified as a unique histologic pattern[24]. This finding was consistent with those on CT and EUS in our case. Therefore, IgG4-GID was considered a differential disease in this case, and a histopathological examination was deemed essential in determining the treatment plan. When patients with IgG4-RD have gastric wall thickening, IgG4-GID should thus be considered in addition to GLP. A EUS-FNB may be useful in differentiating IgG4-GID from IgG4-RD.
The correlation between GLP as cancer and AIP as an autoimmune disease remains unclear; however, IgG4-RD is known to be associated with malignant tumors. One possibility is carcinogenesis due to chronic inflammation. In patients with IgG4-RD, it has been suggested that stimulation from chronic inflammation may trigger carcinogenesis, and one such pathway involves interleukin-33, which plays a role in the pathogenesis of IgG4-RD and is also associated with malignancy[5-7]. Tumor secretion of hormones, peptides, and cytokines or immune cross-reactivity promote the onset of autoimmune disease, widely known as paraneoplastic syndrome[25], which is separate from the carcinogenic pathway due to chronic inflammation. Shiokawa et al[26] reported that 15 of 108 AIP patients had malignancy, with a standardized incidence rate (95% confidence interval (CI) of cancer within 1 year of the AIP diagnosis of 6.1 (95%CI 2.3-9.9) and 1.5 (95%CI 0.3-2.8) beyond 1 year after the AIP diagnosis, indicating a higher risk of cancer within 1 year of the AIP diagnosis than beyond it. In addition, serum IgG4 Levels were significantly higher in AIP patients with cancer than in those without cancer. Furthermore, only 1 of the 8 AIP patients with cancer, whose cancer was resected prior to steroid therapy for AIP had a relapse of AIP, whereas 16 of the 93 AIP patients without cancer had a relapse[26]. In short, some patients with AIP had a high risk of malignancy within one year of the diagnosis of autoimmune disease, and the clinical course differed between cases with and without malignancy, with treatment for malignancy reducing the relapse rate, which was consistent with the features of paraneoplastic syndrome. Therefore, some AIP may be caused by the same mechanism. In the present case, AIP was diagnosed at the same time as GLP, and the serum IgG4 Level was as high as 280 mg/dL. After the start of chemotherapy for GLP, both the CT findings of the pancreas and the serum IgG4 Level improved along with GLP, and the patient was free from relapse of AIP. These results suggested that the AIP in the present case developed as paraneoplastic syndrome.
Nevertheless, a pancreatic tissue biopsy by an EUS-FNB is preferred for the diagnosis of AIP. A pancreatic tissue biopsy should be considered in similar cases in the future. In addition, Shiokawa et al[26] reported a reduced AIP relapse rate after resection of malignancy as a feature of AIP as paraneoplastic syndrome. However, the present case differed in that the tumor was not cured by resection but rather shrunken by chemotherapy. The accumulation of similar cases and further studies on autoimmune disease as paraneoplastic syndrome are also needed.
A EUS-FNB was useful for the histopathological diagnosis of patients with GLP for whom a definitive diagnosis could not be obtained with a gastric mucosa biopsy. Furthermore, autoimmune disease that develops as a paraneoplastic syndrome should be carefully diagnosed and treated, as they may be able to be improved with a reduced likelihood of relapsing following treatment of the original malignant disease.
| 1. | Agnes A, Estrella JS, Badgwell B. The significance of a nineteenth century definition in the era of genomics: linitis plastica. World J Surg Oncol. 2017;15:123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 47] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 2. | Mastoraki A, Papanikolaou IS, Sakorafas G, Safioleas M. Facing the challenge of managing linitis plastica--review of the literature. Hepatogastroenterology. 2009;56:1773-1778. [PubMed] |
| 3. | Wallace ZS, Deshpande V, Mattoo H, Mahajan VS, Kulikova M, Pillai S, Stone JH. IgG4-Related Disease: Clinical and Laboratory Features in One Hundred Twenty-Five Patients. Arthritis Rheumatol. 2015;67:2466-2475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 355] [Cited by in RCA: 485] [Article Influence: 44.1] [Reference Citation Analysis (0)] |
| 4. | Umehara H, Okazaki K, Kawa S, Takahashi H, Goto H, Matsui S, Ishizaka N, Akamizu T, Sato Y, Kawano M; Research Program for Intractable Disease by the Ministry of Health, Labor and Welfare (MHLW) Japan. The 2020 revised comprehensive diagnostic (RCD) criteria for IgG4-RD. Mod Rheumatol. 2021;31:529-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 382] [Article Influence: 76.4] [Reference Citation Analysis (0)] |
| 5. | Yu T, Wu Y, Liu J, Zhuang Y, Jin X, Wang L. The risk of malignancy in patients with IgG4-related disease: a systematic review and meta-analysis. Arthritis Res Ther. 2022;24:14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 56] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 6. | Hong J, Kim S, Lin PC. Interleukin-33 and ST2 Signaling in Tumor Microenvironment. J Interferon Cytokine Res. 2019;39:61-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 7. | Furukawa S, Moriyama M, Miyake K, Nakashima H, Tanaka A, Maehara T, Iizuka-Koga M, Tsuboi H, Hayashida JN, Ishiguro N, Yamauchi M, Sumida T, Nakamura S. Interleukin-33 produced by M2 macrophages and other immune cells contributes to Th2 immune reaction of IgG4-related disease. Sci Rep. 2017;7:42413. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 100] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 8. | Haghbin H, Chuang J, Fatima R, Zakirkhodjaev N, Lee-Smith W, Aziz M. Correlation of Autoimmune Pancreatitis and Malignancy: Systematic Review and Meta-Analysis. Dig Dis Sci. 2022;67:3252-3264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Kawa S, Kamisawa T, Notohara K, Fujinaga Y, Inoue D, Koyama T, Okazaki K. Japanese Clinical Diagnostic Criteria for Autoimmune Pancreatitis, 2018: Revision of Japanese Clinical Diagnostic Criteria for Autoimmune Pancreatitis, 2011. Pancreas. 2020;49:e13-e14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 83] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 10. | Kim JI, Kim YH, Lee KH, Kim SY, Lee YJ, Park YS, Kim N, Lee DH, Kim HH, Park DJ, Lee HS. Type-specific diagnosis and evaluation of longitudinal tumor extent of borrmann type IV gastric cancer: CT versus gastroscopy. Korean J Radiol. 2013;14:597-606. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 11. | Shan GD, Xu GQ, Li YM. Endoscopic ultrasonographic features of gastric linitis plastica in fifty-five Chinese patients. J Zhejiang Univ Sci B. 2013;14:844-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 12. | Oğuz D, Filik L, Parlak E, Dişibeyaz S, Ciçek B, Kaçar S, Aydoğ G, Sahin B. Accuracy of endoscopic ultrasonography in upper gastrointestinal submucosal lesions. Turk J Gastroenterol. 2004;15:82-85. [PubMed] |
| 13. | Takahashi K, Yasuda I, Hanaoka T, Hayashi Y, Araki Y, Motoo I, Kajiura S, Ando T, Fujinami H, Tajiri K, Minemura M, Takahara T. Endoscopic ultrasound-guided fine-needle biopsy for the diagnosis of gastric linitis plastica. DEN open. 2022;2:e38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 14. | Deprez PH, Moons LMG, OʼToole D, Gincul R, Seicean A, Pimentel-Nunes P, Fernández-Esparrach G, Polkowski M, Vieth M, Borbath I, Moreels TG, Nieveen van Dijkum E, Blay JY, van Hooft JE. Endoscopic management of subepithelial lesions including neuroendocrine neoplasms: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2022;54:412-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 308] [Cited by in RCA: 243] [Article Influence: 60.8] [Reference Citation Analysis (1)] |
| 15. | Ye Y, Tan S. Endoscopic ultrasound-guided fine-needle aspiration biopsy for diagnosis of gastric linitis plastica with negative malignant endoscopy biopsies. Oncol Lett. 2018;16:4915-4920. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 16. | Liu Y, Chen K, Yang XJ. Endoscopic ultrasound-guided fine-needle aspiration used in diagnosing gastric linitis plastica: Metastatic lymph nodes can be valuable targets. J Gastroenterol Hepatol. 2019;34:202-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 17. | Takada R, Minaga K, Hara A, Otsuka Y, Omoto S, Kamata K, Yamao K, Takenaka M, Hagiwara S, Honjo H, Matsui S, Chikugo T, Watanabe T, Kudo M. Diagnostic Value of EUS-Guided Fine-Needle Aspiration Biopsy for Gastric Linitis Plastica with Negative Endoscopic Biopsy. J Clin Med. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 18. | Zhou XX, Pan HH, Usman A, Ji F, Jin X, Zhong WX, Chen HT. Endoscopic ultrasound-guided deep and large biopsy for diagnosis of gastric infiltrating tumors with negative malignant endoscopy biopsies. World J Gastroenterol. 2015;21:3607-3613. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 19. | Dai C, Cao Q, Jiang M, Sun MJ. Serum Immunoglobulin G4 in Discriminating Autoimmune Pancreatitis From Pancreatic Cancer: A Diagnostic Meta-analysis. Pancreas. 2018;47:280-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 20. | Ghazale A, Chari ST, Smyrk TC, Levy MJ, Topazian MD, Takahashi N, Clain JE, Pearson RK, Pelaez-Luna M, Petersen BT, Vege SS, Farnell MB. Value of serum IgG4 in the diagnosis of autoimmune pancreatitis and in distinguishing it from pancreatic cancer. Am J Gastroenterol. 2007;102:1646-1653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 434] [Cited by in RCA: 364] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 21. | Miyoshi H, Kano M, Kobayashi S, Ito T, Masuda M, Mitsuyama T, Nakayama S, Ikeura T, Shimatani M, Uchida K, Takaoka M, Okazaki K. Diffuse Pancreatic Cancer Mimicking Autoimmune Pancreatitis. Intern Med. 2019;58:2523-2527. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 22. | Chou YH, Chiou HJ, Hong TM, Tiu CM, Chiou SY, Su CH, Tsay SH. Solitary metastasis from renal cell carcinoma presenting as diffuse pancreatic enlargement. J Clin Ultrasound. 2002;30:499-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 23. | Koizumi S, Kamisawa T, Kuruma S, Tabata T, Chiba K, Iwasaki S, Endo Y, Kuwata G, Koizumi K, Shimosegawa T, Okazaki K, Chiba T. Immunoglobulin G4-related gastrointestinal diseases, are they immunoglobulin G4-related diseases? World J Gastroenterol. 2013;19:5769-5774. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 44] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 24. | Notohara K, Kamisawa T, Uchida K, Zen Y, Kawano M, Kasashima S, Sato Y, Shiokawa M, Uehara T, Yoshifuji H, Hayashi H, Inoue K, Iwasaki K, Kawano H, Matsubayashi H, Moritani Y, Murakawa K, Oka Y, Tateno M, Okazaki K, Chiba T. Gastrointestinal manifestation of immunoglobulin G4-related disease: clarification through a multicenter survey. J Gastroenterol. 2018;53:845-853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 40] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 25. | Pelosof LC, Gerber DE. Paraneoplastic syndromes: an approach to diagnosis and treatment. Mayo Clin Proc. 2010;85:838-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 368] [Cited by in RCA: 451] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 26. | Shiokawa M, Kodama Y, Yoshimura K, Kawanami C, Mimura J, Yamashita Y, Asada M, Kikuyama M, Okabe Y, Inokuma T, Ohana M, Kokuryu H, Takeda K, Tsuji Y, Minami R, Sakuma Y, Kuriyama K, Ota Y, Tanabe W, Maruno T, Kurita A, Sawai Y, Uza N, Watanabe T, Haga H, Chiba T. Risk of cancer in patients with autoimmune pancreatitis. Am J Gastroenterol. 2013;108:610-617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 173] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Al-Ani RM, Iraq; Keikha M, Iran; Pelaez-Luna M, Mexico S-Editor: Chen YL L-Editor: A P-Editor: Chen YL