Published online Nov 6, 2022. doi: 10.12998/wjcc.v10.i31.11508
Peer-review started: May 12, 2022
First decision: June 27, 2022
Revised: July 27, 2022
Accepted: September 21, 2022
Article in press: September 21, 2022
Published online: November 6, 2022
Processing time: 167 Days and 10.2 Hours
Occurring in approximately 30% of hospitalized patients, cardiovascular complications that take place during the course of coronavirus disease 2019 (COVID-19) have been shown to cause morbidity and mortality. This case is the first report of extensive right coronary artery (RCA) thrombosis that was evaluated by intracoronary imaging and intracoronary invasive physiology in a patient with COVID-19.
A 62-year-old woman presented with flu-like symptoms; ten days later, she presented with inferior ST-segment elevations, chest pain, dyspnea, nausea and vomiting. The patient was diagnosed with COVID-19 following a positive test result. Emergency angiography of the RCA and its branches indicated intraluminal filling defects, suggesting a thrombus. Intravascular ultrasound confirmed a subacute thrombus in the RCA, the right posterior descending branch and the right posterior ventricular (RPV) branch. There was also an acute thrombus in the RPV branch and atherosclerosis in the RCA. Dual antiplatelet/ anticoagulation therapy was administered. After 7 d, angiography revealed complete disappearance of the thrombi. Optical coherence tomography confirmed this with the exception of a small thrombus in the RPV branch and atherosclerotic plaque in the RCA. The atherosclerotic RCA was measured using the resting full-cycle ratio, indicating no impairment to coronary physiology. The patient was discharged on the 11th day of hospitalization and remained asymptomatic through the 6-mo follow-up.
This was the first report of RCA thrombosis in a patient with COVID-19. Dual antiplatelet/anticoagulation therapy was successful.
Core Tip: Cardiovascular complications occurring during the course of coronavirus disease 2019 (COVID-19) cause morbidity and mortality. We report the case of a 62-year-old woman with COVID-19 and ST-elevation myocardial infarction. Angiography of the right coronary artery suggested a thrombus, and findings were confirmed via intravascular ultrasound and optimal coherence tomography. Dual antiplatelet therapy and anticoagulation with enoxaparin therapy was administered for 7 d, followed by disappearance of the thrombi. Resting full-cycle ratio was performed without damage to coronary physiology. There is no consensus on the ideal management approach for acute coronary syndrome in this scenario; however, in this case the thrombi disappeared after dual antiplatelet and anticoagulation therapy.
- Citation: Dall’Orto CC, Lopes RPF, Cancela MT, de Sales Padilha C, Pinto Filho GV, da Silva MR. Extensive right coronary artery thrombosis in a patient with COVID-19: A case report. World J Clin Cases 2022; 10(31): 11508-11516
- URL: https://www.wjgnet.com/2307-8960/full/v10/i31/11508.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i31.11508
Cardiovascular complications occurring in the course of coronavirus disease 2019 (COVID-19) cause morbidity and mortality affecting 30% of hospitalized patients[1-3]. One possible explanation for the damage caused to the myocardium by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) involves hypoxia following respiratory failure along with excessive inflammation, excess cytokine production, angiotensin-converting enzyme 2 receptor expression downregulation, platelet activation, coagulation cascade, endothelial cell injury, rupture of previously existing plaques [type 1 acute myocardial infarction (AMI)] and direct myocyte infiltration by the virus[4-6].
A 62-year-old woman with COVID-19 who presented with chest pain, dyspnea, nausea, and vomiting.
The patient initially presented with flu-like symptoms and was diagnosed with COVID-19 following a positive reading on a polymerase chain reaction (PCR) test. Ten days later, the patient presented with chest pain, dyspnea, nausea and vomiting.
The patient’s medical history included dyslipidemia and incipient atherosclerosis in the carotid and aortic territories, continuous use of nortriptyline for migraines and 9 years of tiboline [Libiam 1.25 mg once a day (Libbs-São Paulo–SP/BR)] use as menopausal hormone therapy. The patient had received three doses of a vaccine for SARS-CoV-2; two chimpanzee adenovirus vector vaccines (ChAdOx1 nCoV-19 AZD1222; Oxford/AstraZeneca/Fiocruz, Rio de Janeiro, Brazil) on April 12, 2021 and July 13, 2021, respectively, and one BNT162b2 mRNA COVID-19 vaccine (BioNTech/Pfizer, New York City, NY, United States) on December 13, 2021. Timeline is showed in Figure 1.
Upon physical examination, the vital signs were as follows: Body temperature 36.5 °C; blood pressure 122/78 mmHg; heart rate 82 beats per min; and respiratory rate 22 breaths per min. The patient’s clinical presentation was compatible with Killip-Kimball grade I classification.
Laboratory examination results were as follows: Blood cardiac biomarkers included a creatine kinase level of 6105 IU/L, a creatine kinase myocardial band fraction of 300 IU/L, a cardiac troponin I level of 25000 pg/mL, a C-reactive protein level of 75.8 mg/dL, a lactic dehydrogenase level of 1.510 U/L and a D-dimer level of 2.540 mcg/L FEU. In addition, transthoracic Doppler echocardiography revealed akinesis in the inferior mid-basal and apical infero-basal portions of the left ventricle.
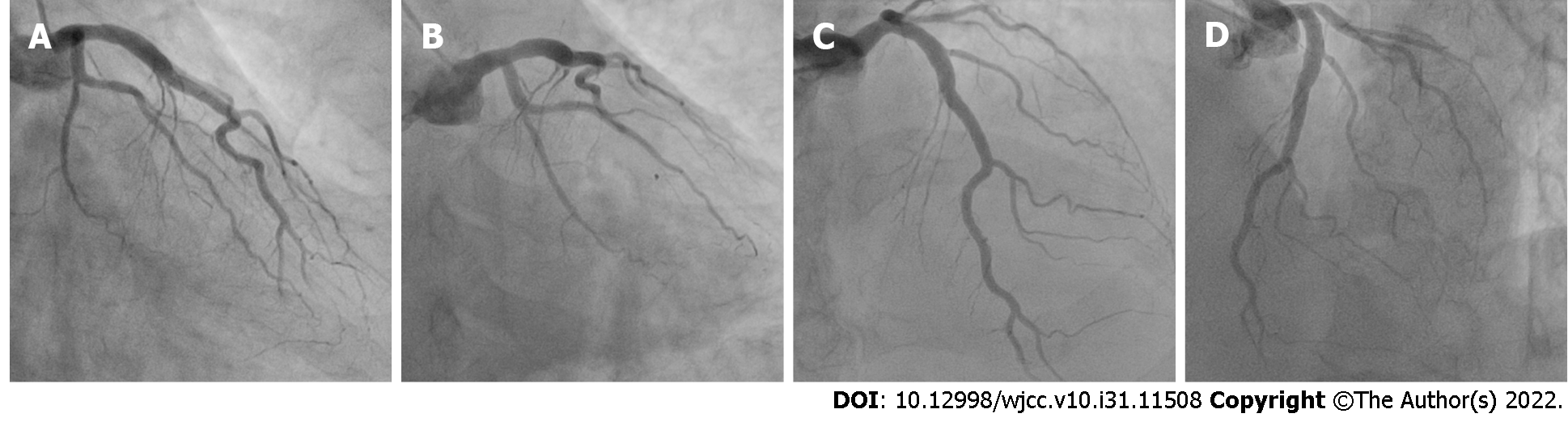
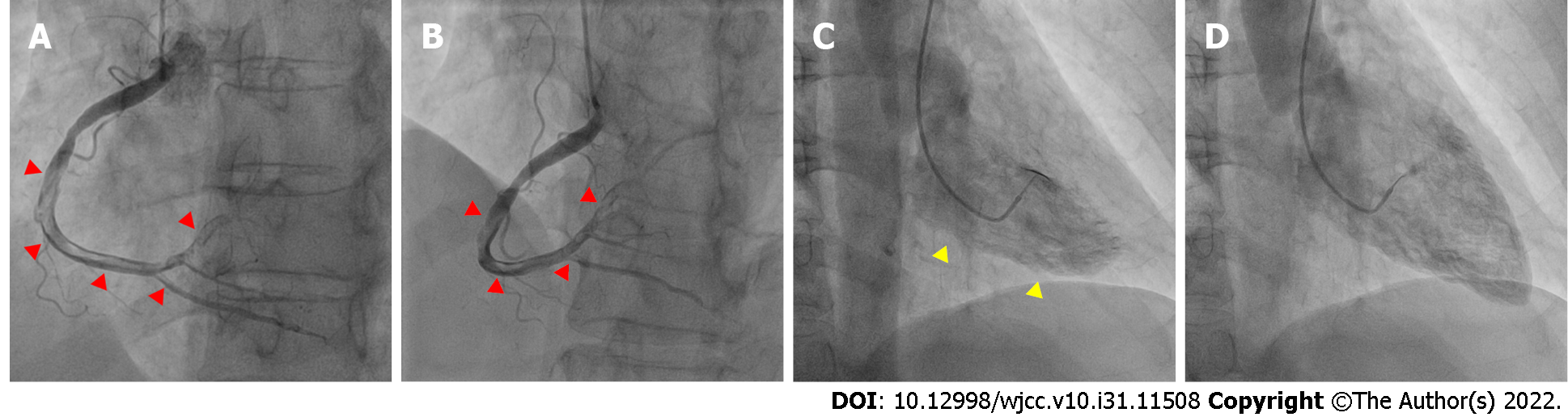
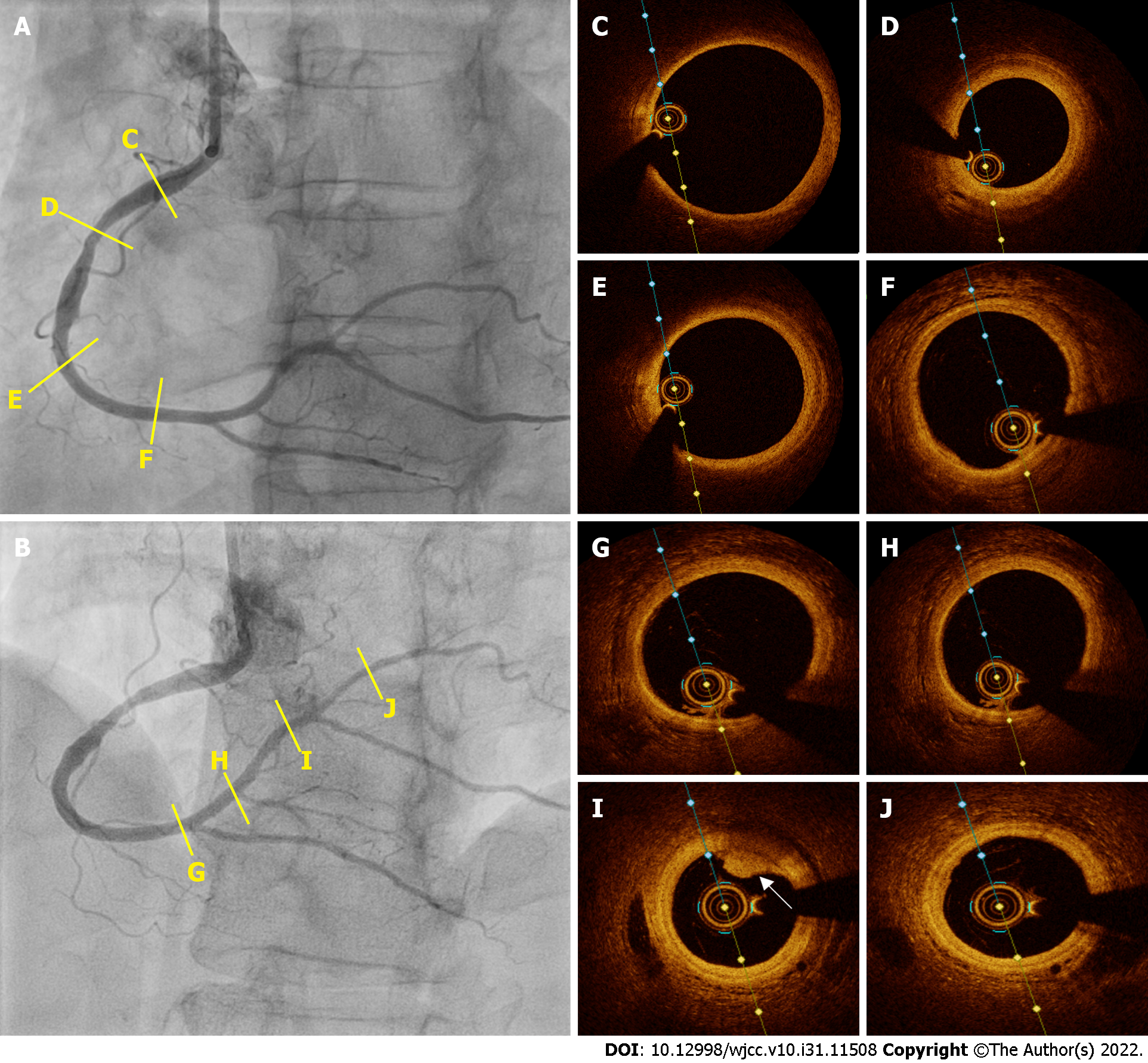
Emergency coronary angiography revealed that the anterior descending coronary artery and its diagonal branches and circumflex artery and its marginal branches were free of obstructive atherosclerotic lesions (Figure 2). However, images of the right coronary artery (RCA) and its right posterior descending (RPD) and right posterior ventricular (RPV) branches indicated defects in intraluminal filling, suggesting a thrombus (Figure 3).
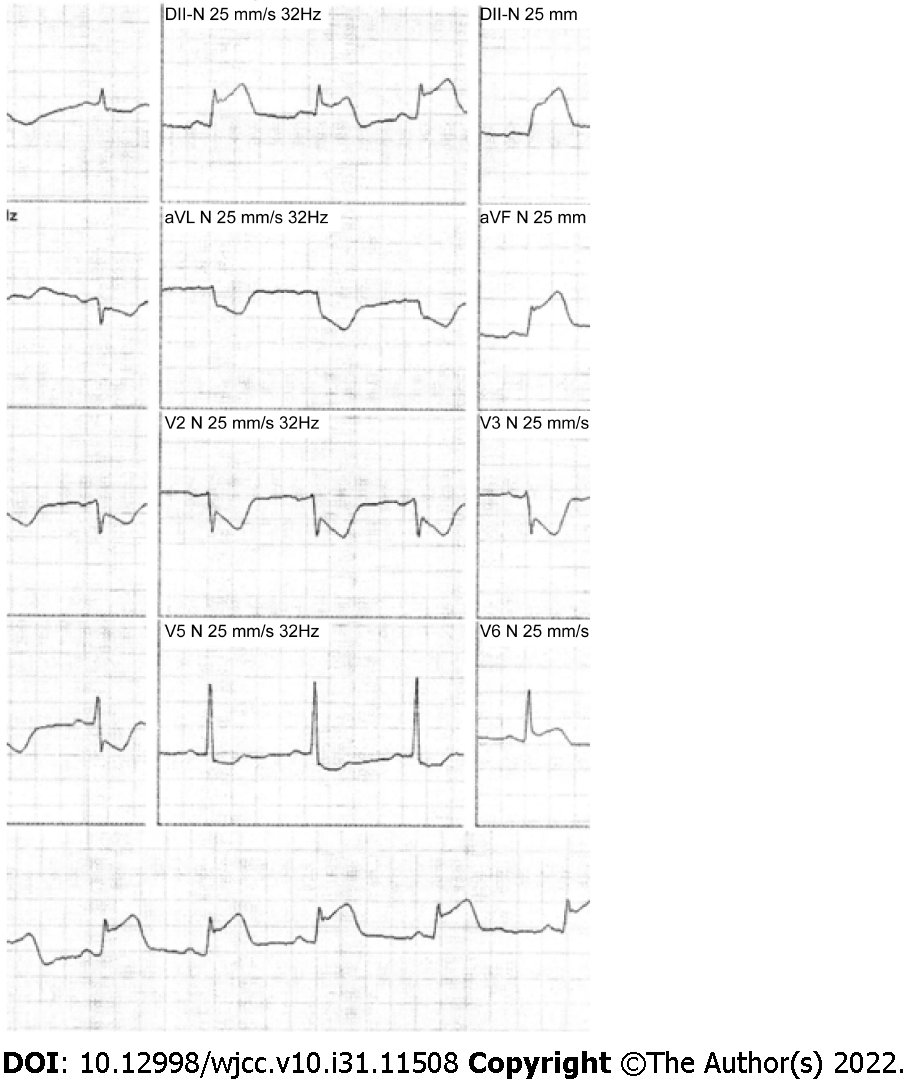
Electrocardiography showed sinus rhythm, inferior ST-segment elevations and reciprocal changes in the anterolateral leads (Figure 4). The patient was referred for emergency angiography.
We evaluated the RCA by intracoronary ultrasound. Intravascular ultrasound (IVUS) pullbacks were performed using a 40 MHz IVUS OPTICROSS catheter (Boston Scientific, Natick, MA, United States) at 0.5 mm/s. The images suggested a subacute, homogeneous, echolucent thrombus in a large extension of the RCA, RPD branch, and RPV branch. Additionally, they showed an acute thrombus with a bright aspect, clear outline and no signal attenuation in the RPV branch (Figure 5)[7]. We believe that the presence of thrombi on IVUS in the acute and subacute stages was due to the fact that at the time the patient was studied, the process had already been evolving for over 24 h and the thrombotic process (when it does not culminate with vessel occlusion) is a continuum of thrombus stages. We also identified mild to moderate atherosclerosis in the middle third of the RCA (Figure 5).
The patient was diagnosed with AMI with inferior ST-segment elevations, Killip grade I heart failure and COVID-19.
On angiography, the patient was pain-free and had thrombolysis in myocardial infarction grade 3 flow, despite extensive thrombotic burden in the RCA. Therefore, we did not perform primary angioplasty and, instead, opted for dual antiplatelet therapy with ticagrelor and aspirin and anticoagulation therapy with enoxaparin 1 mg/kg twice a day. Additionally, we administered the pharmacology recommended by current guidelines for patients with AMI with ST-segment elevation, including statins, a beta-blocker, an angiotensin-converting enzyme or angiotensin II receptor blocker and a mineralocorticoid receptor antagonist[8-10].
On angiography and IVUS, we were unable to identify any culprit lesions. Therefore, we used optical coherence tomography (OCT) for confirmation to look for signs of erosion or plaque rupture, which could have explained the condition and guided treatment; for example, if there was a need for mechanical passivation of the plate with a stent. However, we did not find plaque erosion or rupture on OCT.
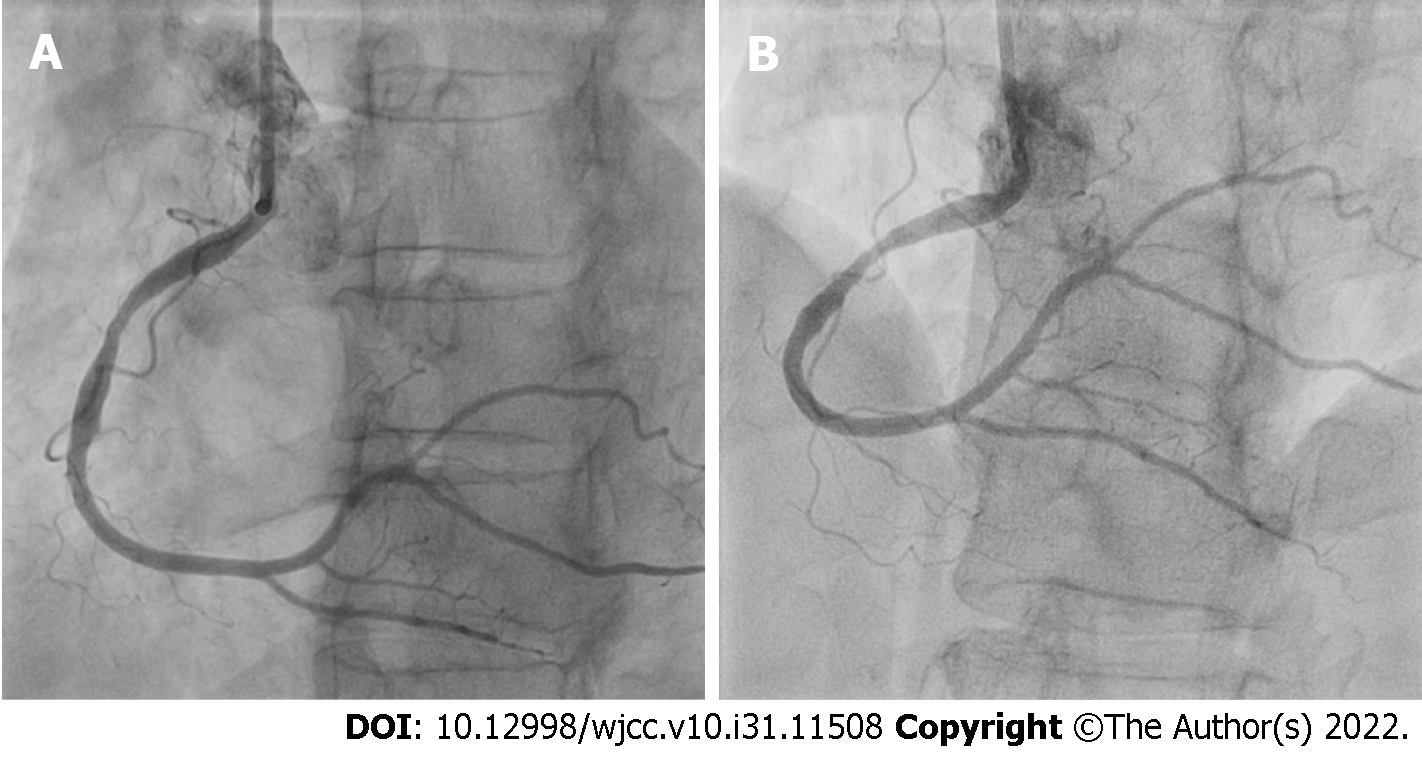
After 7 d, repeat coronary angiography showed complete disappearance of the thrombi located in the RCA and its branches (Figure 6). Therefore, we performed intravascular OCT for confirmation, using the ILUMIENTM OPISTM, OPTIS Integrated, and OPTIS Mobile systems (Abbott Vascular, Santa Clara, CA, United States) with a rapid exchange catheter (DragonflyTM DUO, DragonflyTM OPISTM, and Dra
The patient recovered without further event and was discharged on the 11th day of hospitalization. After showing good tolerance to medications with no adverse effects, she was prescribed ticagrelor, aspirin, statins, beta-blockers and angiotensin-converting enzyme inhibitors.
The patient has been under follow-up until the present day by our team, with a 6 mo follow-up completed thus far, and she remains asymptomatic without any other clinical conditions since then.
In the present case, the diagnosis of AMI with ST-segment elevation was confirmed by clinical findings, electrocardiogram, laboratory blood tests and coronary angiography. The latter showed extensive failure of intraluminal filling suggesting a thrombus, which was then confirmed by IVUS and OCT findings. The plaque in the middle third of the RCA, which we believe was not responsible for the event, did not cause any hemodynamic repercussions, a fact confirmed by the assessment of invasive physiology.
The diagnosis that proved to be feasible in this case was AMI with ST-segment elevation type 2; that is, AMI not related to the instability of atherosclerotic plaque in the coronary artery. Faced with this diagnosis, it is necessary to reflect on the possible causes to guide us towards the most appropriate treatment. A possible cause suggested in this patient could be hormone replacement therapy for menopause. The patient was chronically using tibolone, which is a synthetic steroid whose metabolites have estrogenic, androgenic and progestogenic properties. In the Long-term Intervention on Fracture with Tibolone study, tibolone in postmenopausal women was linked to an excessive risk of stroke in women receiving tibolone compared with placebo. However, there were no significant differences in the risk of coronary heart disease or venous thromboembolism between the two groups[11].
The hypothesis of a post-vaccination reaction to the vaccine for COVID-19 could also be raised. However, we did not emphasize the possibility that the patient's clinical condition was due to an adverse effect of the vaccine because the event occurred more than 30 d after the third dose; additionally, she had a positive PCR test result indicating active infection.
In view of the abovementioned findings, with a positive PCR test result for SARS-CoV-2, in addition to inflammatory markers, with very high serum (lactic dehydrogenase, C-reactive protein and D-dimer) levels corroborating active infection, the most plausible cause we found for the etiology of AMI with ST-segment elevation type 2 in this case was COVID-19.
The common occurrence of extra-respiratory involvement in SARS-CoV-2 infections has become more evident over time. AMI with ST-segment elevation is observed with a pattern on angiography, and extensive thrombosis can affect one or more coronary arteries and different vascular territories simultaneously, not caused by rupture of atherosclerotic plaques. These occurrences present new challenges to treating and managing this viral infection[10,12]. The increased incidence of stent thrombosis may be associated with these phenomena, and severe inflammation with consequent hypercoagulation is another primary pathology associated with SARS-CoV-2[13].
There is no specific finding that confirms the relationship between COVID-19 infection and AMI other than the known extensive thrombotic burden, which is not a specific finding. The high thrombotic load in patients with AMI and COVID is known. In addition to the findings in other studies in the literature[14,15], there are also other important reports addressing this issue, such as a statement from the American College of Cardiology in which the authors concluded that patients with ST-segment elevation myocardial infarction and concurrent COVID-19 experienced a higher thrombotic burden than those without concurrent COVID-19[13].
AMI without plaque rupture (type 2) can indeed occur in addition to COVID-19; however, we did not identify any other causes of type 2 AMI, such as coronary dissection, vasospasm, emboli, microvascular dysfunction or increases in demand with or without underlying coronary artery disease.
The difference between this case and those previously published is that we were able to document the absence of plaque rupture or erosion in a patient with coronary artery disease and to demonstrate that the plaque had no hemokinetic repercussions, shown via invasive physiology. We believe that our case corroborates a body of evidence that has been building toward an understanding of COVID and AMI.
While the importance of differentiating between type I and type II AMI and myocarditis in patients with COVID-19 presenting with acute coronary syndrome (ACS) is established, there is no consensus on the ideal management approach for ACS in this scenario. In patients with known or suspected COVID-19, treatment of ST-segment elevation myocardial infarction is similar to that for patients without COVID-19, using aspirin, nitrate, beta-blockers, anticoagulation, antiplatelet aggregation with a P2Y12 agent, statins, and reperfusion therapy with fibrinolytics or primary angioplasty. Percutaneous coronary intervention, aspiration and antiplatelet thrombectomy are options, with the latter being the most generally agreed upon for treating these patients[16]. However, in this study, the thrombi disappeared after dual antiplatelet therapy, anticoagulation therapy and traditional post-myocardial infarction pharmaceutical interventions were administered.
This was the first report of extensive RCA thrombosis in a patient with COVID-19 evaluated by intracoronary imaging and intracoronary invasive physiology.
| 1. | Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054-1062. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17476] [Cited by in RCA: 18403] [Article Influence: 3067.2] [Reference Citation Analysis (13)] |
| 2. | Arentz M, Yim E, Klaff L, Lokhandwala S, Riedo FX, Chong M, Lee M. Characteristics and Outcomes of 21 Critically Ill Patients With COVID-19 in Washington State. JAMA. 2020;323:1612-1614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1511] [Cited by in RCA: 1637] [Article Influence: 272.8] [Reference Citation Analysis (0)] |
| 3. | Guo T, Fan Y, Chen M, Wu X, Zhang L, He T, Wang H, Wan J, Wang X, Lu Z. Cardiovascular Implications of Fatal Outcomes of Patients With Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020;5:811-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2516] [Cited by in RCA: 2874] [Article Influence: 479.0] [Reference Citation Analysis (1)] |
| 4. | Stefanini GG, Montorfano M, Trabattoni D, Andreini D, Ferrante G, Ancona M, Metra M, Curello S, Maffeo D, Pero G, Cacucci M, Assanelli E, Bellini B, Russo F, Ielasi A, Tespili M, Danzi GB, Vandoni P, Bollati M, Barbieri L, Oreglia J, Lettieri C, Cremonesi A, Carugo S, Reimers B, Condorelli G, Chieffo A. ST-Elevation Myocardial Infarction in Patients With COVID-19: Clinical and Angiographic Outcomes. Circulation. 2020;141:2113-2116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 278] [Cited by in RCA: 329] [Article Influence: 54.8] [Reference Citation Analysis (0)] |
| 5. | Bois MC, Boire NA, Layman AJ, Aubry MC, Alexander MP, Roden AC, Hagen CE, Quinton RA, Larsen C, Erben Y, Majumdar R, Jenkins SM, Kipp BR, Lin PT, Maleszewski JJ. COVID-19-Associated Nonocclusive Fibrin Microthrombi in the Heart. Circulation. 2021;143:230-243. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 123] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 6. | Masi P, Hékimian G, Lejeune M, Chommeloux J, Desnos C, Pineton De Chambrun M, Martin-Toutain I, Nieszkowska A, Lebreton G, Bréchot N, Schmidt M, Edouard Luyt C, Combes A, Frere C. Systemic Inflammatory Response Syndrome Is a Major Contributor to COVID-19-Associated Coagulopathy: Insights From a Prospective, Single-Center Cohort Study. Circulation. 2020;142:611-614. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 105] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 7. | Groenland FTW, Ligthart JMR, Witberg KT, Daemen J. Patterns of intracoronary thrombus by high-definition intravascular ultrasound. EuroIntervention. 2022;18:e158-e159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, Caforio ALP, Crea F, Goudevenos JA, Halvorsen S, Hindricks G, Kastrati A, Lenzen MJ, Prescott E, Roffi M, Valgimigli M, Varenhorst C, Vranckx P, Widimský P; ESC Scientific Document Group. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2018;39:119-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7073] [Cited by in RCA: 6925] [Article Influence: 865.6] [Reference Citation Analysis (1)] |
| 9. | Mahmud E, Dauerman HL, Welt FGP, Messenger JC, Rao SV, Grines C, Mattu A, Kirtane AJ, Jauhar R, Meraj P, Rokos IC, Rumsfeld JS, Henry TD. Management of acute myocardial infarction during the COVID-19 pandemic: A Consensus Statement from the Society for Cardiovascular Angiography and Interventions (SCAI), the American College of Cardiology (ACC), and the American College of Emergency Physicians (ACEP). Catheter Cardiovasc Interv. 2020;96:336-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 101] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 10. | Task Force for the management of COVID-19 of the European Society of Cardiology. ESC guidance for the diagnosis and management of cardiovascular disease during the COVID-19 pandemic: part 2-care pathways, treatment, and follow-up. Eur Heart J. 2022;43:1059-1103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 98] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 11. | Cummings SR, Ettinger B, Delmas PD, Kenemans P, Stathopoulos V, Verweij P, Mol-Arts M, Kloosterboer L, Mosca L, Christiansen C, Bilezikian J, Kerzberg EM, Johnson S, Zanchetta J, Grobbee DE, Seifert W, Eastell R; LIFT Trial Investigators. The effects of tibolone in older postmenopausal women. N Engl J Med. 2008;359:697-708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 292] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 12. | Kermani-Alghoraishi M. A Review of Coronary Artery Thrombosis: A New Challenging Finding in COVID-19 Patients and ST-elevation Myocardial Infarction. Curr Probl Cardiol. 2021;46:100744. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 13. | Choudry FA, Hamshere SM, Rathod KS, Akhtar MM, Archbold RA, Guttmann OP, Woldman S, Jain AK, Knight CJ, Baumbach A, Mathur A, Jones DA. High Thrombus Burden in Patients With COVID-19 Presenting With ST-Segment Elevation Myocardial Infarction. J Am Coll Cardiol. 2020;76:1168-1176. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 155] [Cited by in RCA: 212] [Article Influence: 35.3] [Reference Citation Analysis (0)] |
| 14. | Pandit BN, Shrivastava A, Nath RK, Kuber D, Sinha SK, Aggarwal P. Impact of COVID-19 on Thrombus Burden and Outcome in Acute Myocardial Infarction. Cureus. 2021;13:e16817. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 15. | Trivi M, Lalor N, Spaletra P, Raffaeli A, Costabel J, Belardi J. [Acute myocardial infarction in patients recovering from COVID-19 pneumonia]. Medicina (B Aires). 2020;80 Suppl 6:97-99. [PubMed] |
| 16. | Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, Mehra MR, Schuepbach RA, Ruschitzka F, Moch H. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417-1418. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4227] [Cited by in RCA: 4668] [Article Influence: 778.0] [Reference Citation Analysis (2)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: Brazil
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D, D
Grade E (Poor): 0
P-Reviewer: Ito S, Japan; Kharlamov AN, Netherlands S-Editor: Fan JR L-Editor: Filipodia P-Editor: Fan JR