Published online Nov 6, 2022. doi: 10.12998/wjcc.v10.i31.11466
Peer-review started: August 8, 2022
First decision: September 5, 2022
Revised: September 15, 2022
Accepted: September 23, 2022
Article in press: September 23, 2022
Published online: November 6, 2022
Processing time: 79 Days and 21.5 Hours
Polymyxin-induced nephrotoxicity is a major safety concern in clinical practice due to long-term adverse outcomes and high mortality.
To conducted a systematic review and meta-analysis of the prevalence and potential predictors of polymyxin-induced nephrotoxicity in adult intensive care unit (ICU) patients.
PubMed, EMBASE, the Cochrane Library and Reference Citation Analysis database were searched for relevant studies from inception through May 30, 2022. The pooled prevalence of polymyxin-induced nephrotoxicity and pooled risk ratios of associated factors were analysed using a random-effects or fixed-effects model by Stata SE ver. 12.1. Additionally, subgroup analyses and meta-regression were conducted to assess heterogeneity.
A total of 89 studies involving 12234 critically ill adult patients were included in the meta-analysis. The overall pooled incidence of polymyxin-induced nephrotoxicity was 34.8%. The pooled prevalence of colistin-induced nephrotoxicity was not higher than that of polymyxin B (PMB)-induced nephrotoxicity. The subgroup analyses showed that nephrotoxicity was significantly associated with dosing interval, nephrotoxicity criteria, age, publication year, study quality and sample size, which were confirmed in the univariable meta-regression analysis. Nephrotoxicity was significantly increased when the total daily dose was divided into 2 doses but not 3 or 4 doses. Furthermore, older age, the presence of sepsis or septic shock, hypoalbuminemia, and concomitant vancomycin or vasopressor use were independent risk factors for polymyxin-induced nephrotoxicity, while an elevated baseline glomerular filtration rate was a protective factor against colistin-induced nephrotoxicity.
Our findings indicated that the incidence of polymyxin-induced nephrotoxicity among ICU patients was high. It emphasizes the importance of additional efforts to manage ICU patients receiving polymyxins to decrease the risk of adverse outcomes.
Core Tip: Polymyxins have recently been reintroduced as a last-line option in chemotherapy for infections caused by multidrug-resistant gram-negative bacteria. However, these agents can cause nephrotoxicity. Notably, the prevalence of and risk factors for polymyxin-associated nephrotoxicity in adult intensive care unit (ICU) patients remain unclear. This is the first systematic review and meta-analysis to estimate the prevalence of and risk factors for polymyxin-induced nephrotoxicity in adult ICU patients. Based on the data collected and analysed, we conclude that the high incidence of polymyxin-induced nephrotoxicity is a primary safety concern and challenge in clinical practice. The avoidance of modifiable risk factors (such as nephrotoxic drugs and dosage regimens containing polymyxins) in adult ICU patients can likely reduce the risk of polymyxin-induced nephrotoxicity.
- Citation: Wang JL, Xiang BX, Song XL, Que RM, Zuo XC, Xie YL. Prevalence of polymyxin-induced nephrotoxicity and its predictors in critically ill adult patients: A meta-analysis. World J Clin Cases 2022; 10(31): 11466-11485
- URL: https://www.wjgnet.com/2307-8960/full/v10/i31/11466.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i31.11466
Acute kidney injury (AKI), a vastly complex heterogeneous syndrome, is associated with long-term adverse outcomes and high mortality[1]. Nearly 22% of hospitalized patients develop AKI, and the incidence of AKI in patients in intensive care units (ICUs) can reach 50%-70%[2-4]. Nephrotoxic drugs are considered the third most common aetiology for AKI following sepsis and hypovolemia and account for approximately 14% of cases in ICUs[4]. Drug-related risk hypervigilance and nephrotoxic drug stewardship are important strategies for the prevention of AKI in ICU patients[3,5].
Polymyxins (such as colistin and polymyxin B), were introduced into clinical practice in the 1950s but abandoned soon after due to broad toxicity, especially nephrotoxicity. Recently, polymyxins have been reintroduced as a final option in for the treatment of infections caused by carbapenem-resistant gram-negative bacteria in critically ill patients[6,7], though polymyxin-associated nephrotoxicity is still the primary safety concern and obstacle to their widespread clinical application[7,8]. A recent study suggested that among antibiotics, colistin had the highest AKI reporting odds ratios based on real-world data, including 2042801 reports from the Food and Drug Administration (FDA) Adverse Event Reporting System[9]. Hence, there is an urgent need to analyse the prevalence and potential risk factors for polymyxin-induced nephrotoxicity in critically ill patients.
Several systematic reviews and meta-analyses on polymyxin-induced nephrotoxicity and its predictors have been conducted[10-13]. The nephrotoxicity prevalence varies widely, ranging from 26.7% to 45%[10,12,13]. A meta-analysis of 237 studies that enrolled 35569 hospitalized patients treated with systemic or inhaled polymyxins was conducted. Patients receiving inhaled polymyxins showed a significantly lower nephrotoxicity rate than patients receiving systemic polymyxins (13.8% vs 29.5%; P < 0.001)[13]. Another recent meta-analysis of 48 studies involving 6,199 adult patients with at least 48 h of intravenous polymyxin exposure showed that older age, a high daily dose, accompanying diabetes, and concomitant nephrotoxic drugs uses were independent predictors of nephrotoxicity[12]. However, the incidence of nephrotoxicity was significantly higher in ICU patients than in non-ICU patients [odds ratio (OR) = 1.55; 95% confidence interval (CI), 1.02-2.37; P = 0.042][13]. In addition, the severity of patient illness was also reported to be a risk factor for colistin-related nephrotoxicity[14]. In general, previous meta-analyses pooled nephrotoxicity events and assessed risk factors for AKI in hospital patients receiving polymyxins; these data were insufficient to evaluate the prevalence and risk factors for polymyxin-associated nephrotoxicity in adult ICU patients, who account for the majority of the population using polymyxins in clinical practice.
This systematic review and meta-analysis were conducted and reported in accordance with the Meta-analysis of Observational Studies in Epidemiology guidelines and Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines, respectively.
We searched PubMed, EMBASE and the Cochrane Library from inception through May 30, 2022, and limited the search to English-language studies involving humans. When revising the manuscript, we searched the Reference Citation Analysis (RCA) database in order to supplement and improve the highlights of the latest frontier research results, but we did not find any potential articles to be included. Reference lists of the retrieved studies, systematic reviews, and meta-analyses pertaining to our study were also reviewed. The search strategy is provided in Supplementary Table 1.
Observational studies and randomized clinical trials (RCTs) were eligible for meta-analysis if they met the following criteria: (1) The incidence of nephrotoxicity induced by polymyxin B (PMB) or colistin in adult (age > 18 years) ICU patients was reported; (2) patients received at least 48 h of intravenous polymyxin exposure; and (3) risk factors associated with nephrotoxicity induced by either type of polymyxin were reported as ORs, relative risks (RRs) or hazard ratio (HRs) with 95%CIs. Studies were excluded if they met the following exclusion criteria: (1) Review, abstracts from conference proceedings, comments, or case reports; (2) missing full text or an inability to retrieve data required for analysis; or (3) a sample size of less than 10 patients.
Duplicates were detected and removed first. Then, two authors (Bi-Xiao Xiang and Yue-Liang Xie) independently screened the titles, abstracts and full texts based on the inclusion criteria and exclusion criteria. Two investigators (Jiang-Lin Wang and Bi-Xiao Xiang) independently extracted the data based on the predetermined selection criteria. Data extraction details are presented in the supplementary Method. All disagreements were resolved by consensus and, if not possible, by discussion with the remaining authors.
Two reviewers (Yue-Liang Xie and Jiang-Lin Wang) independently evaluated the risk of bias of the included studies using the Cochrane Collaboration risk of bias tool and the Newcastle–Ottawa Scale for RCTs and for observational studies (cohort and case–control studies), respectively. Discrepancies were resolved by a third investigator (Xiao-Cong Zuo). The scores on the Newcastle–Ottawa Scale range from 0 to 9. The included studies were classified into one of three categories based on the scores for each study: Low quality (score of less than 4), moderate quality (score of 5-7) and high quality (score of 8-9). The overall risk of bias for each included RCT was classified as low if the risk of bias was low in all domains, unclear if the risk of bias was unclear in one or more domains and with no judgement of high risk of bias, and high if the risk of bias was high in one or more domains[15].
Raw data including numbers of nephrotoxic events and total sample size were statistically pooled using a random effects model to calculate the overall event rate and 95%CI. The pooled ORs with 95%CIs of associated factors were calculated using a random-effects or fixed-effects model (I² < 50%). In addition, the RRs of colistin vs PMB as well as polymyxin treatment regimens vs nonpolymyxin-based treatment regimens were computed considering parallel design studies. Studies were weighted using the inverse variance method. We calculated the inconsistency index (I2) to measure heterogeneity. According to prespecified cut-off values, low heterogeneity was defined as an I2 < 50%, and high heterogeneity was defined as an I2 ≥ 50%. For each outcome, sensitivity analysis was performed by sequentially omitting each study from the pool; all studies were removed one at a time to analyse their influence on the pooled estimate and heterogeneity.
To account for potential sources of heterogeneity in the pooled incidence of polymyxin-induced nephrotoxicity, we performed several subgroup analyses detailed in the Supplementary Material. To further investigate potential sources of heterogeneity for the incidence of polymyxin-induced nephrotoxicity, we conducted several meta-regressions. In the first step, we performed univariable meta-regression analyses according to the mean age in each study, sex, study design, sample size, publication year, geographical location, definition of AKI and risk of bias. A multivariable meta-regression analysis was then conducted with the factors significantly associated with polymyxin-induced nephrotoxicity incidence in the univariable meta-regression analyses.
Publication bias was examined visually with the use of funnel plots and filled funnel plots and assessed with Egger’s test. When both indicators showed a significant result, it was assumed that publication bias was present. All statistical analyses were performed using Stata SE ver. 12.1 (StataCorp., College Station, TX, United States). Statistical tests were 2-sided, and P < 0.05 was considered statistically significant.
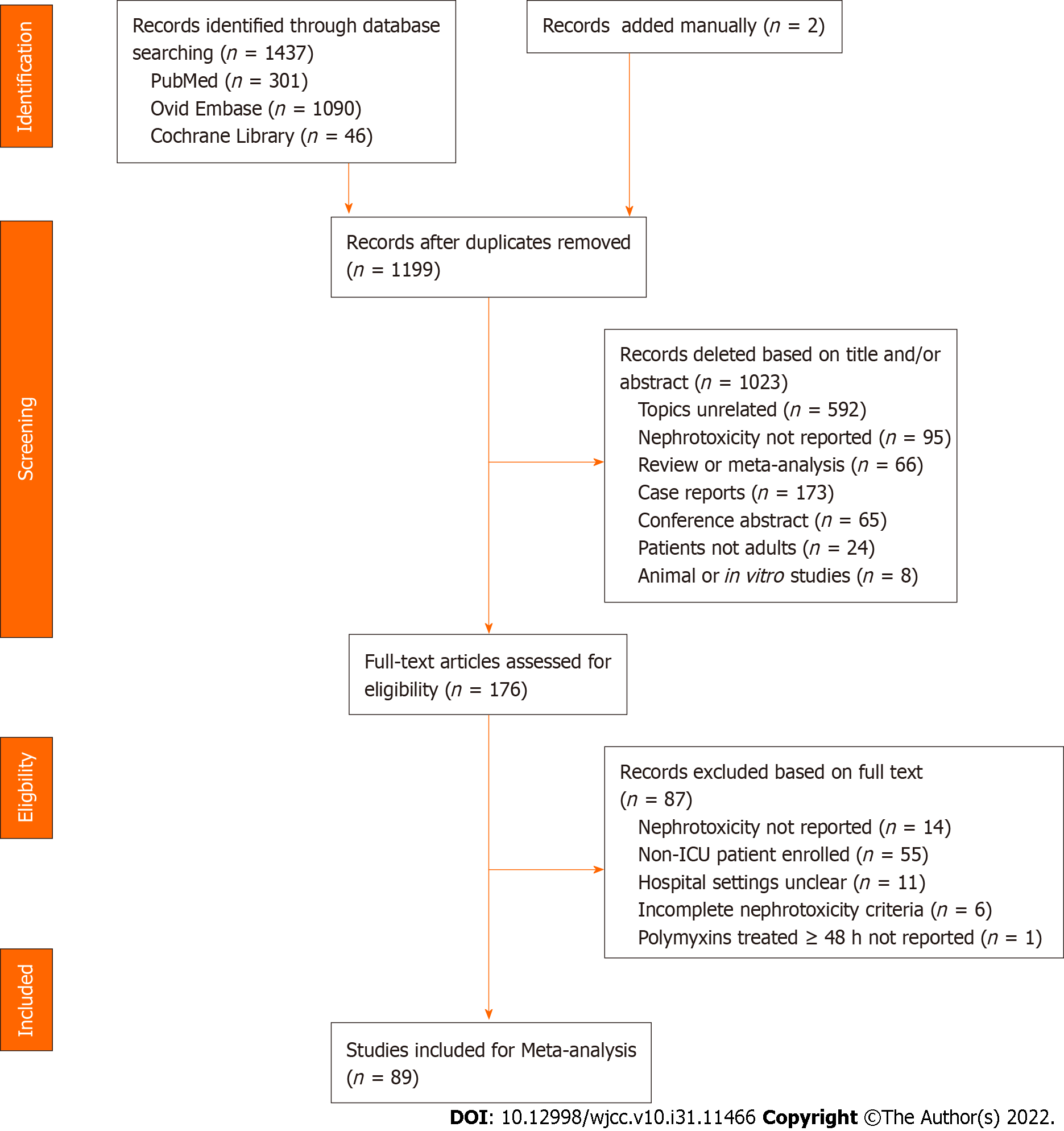
A total of 1437 results were retrieved from the search. After the removal of duplicates and title and abstract screening, 176 full texts were assessed for eligibility, and 89 studies were included for quantitative synthesis (Supplementary Table 1)[16-104]. The detailed study selection process is depicted in Figure 1.
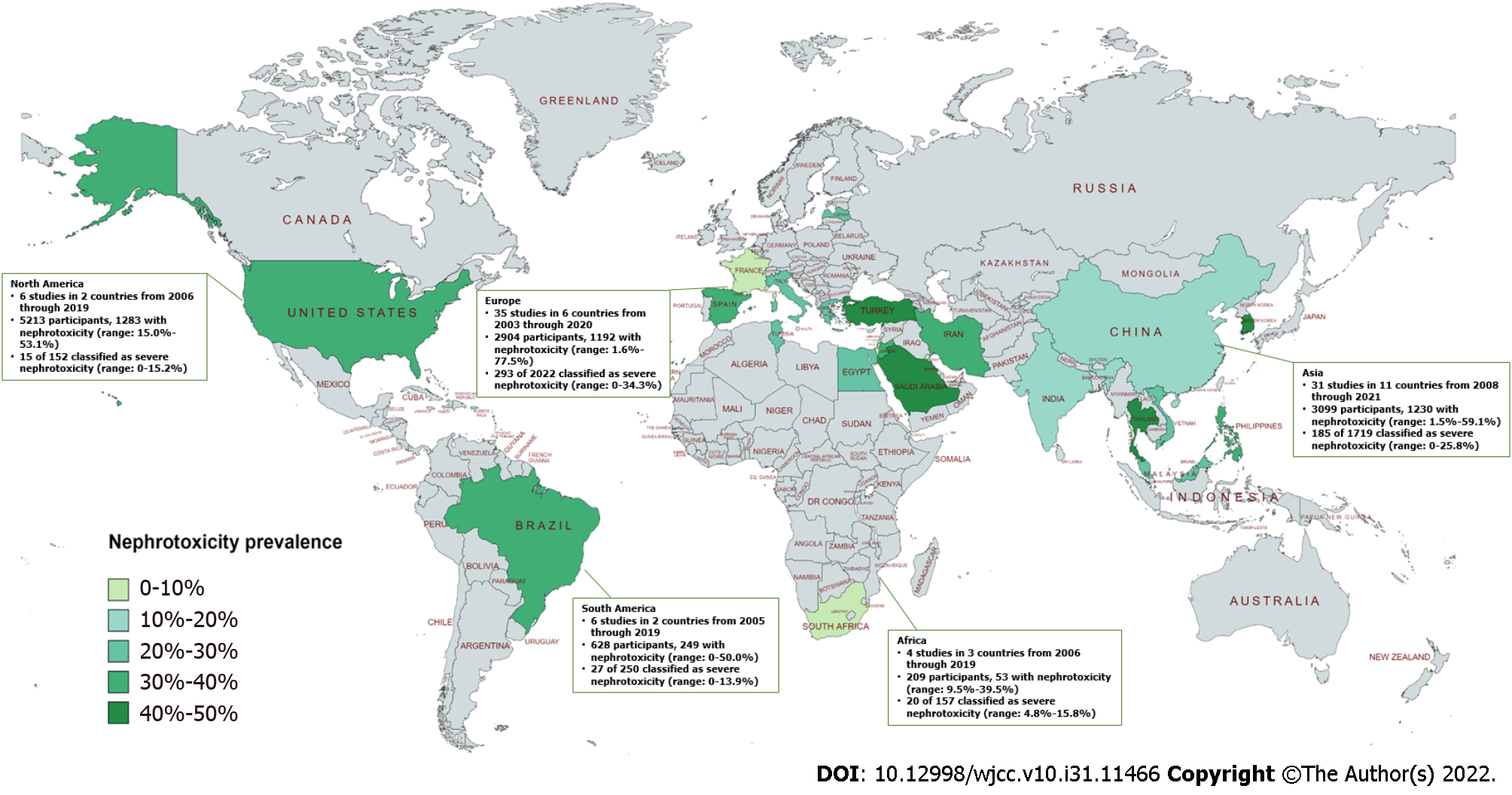
The characteristics of the included studies are shown in Supplementary Table 2. This systematic review and meta-analysis included 12234 adult critically ill patients receiving polymyxins, of whom 11211 were included in the colistin-treated groups and 903 were included in the PMB-treatment groups. All studies were published from 2003 to 2022. Among the included studies, 9 were RCTs[16,18,23,34,35,54,57,81,87], 5 were case–control studies[31,47,51,74,86] and 75 were cohort studies[17,19-22,24-30,32,33,36-46,48-50,52,53,55,56,58-73,75-80,82-85,88-104]. The sample sizes per study ranged from 11 to 4910 critically ill patients. This systematic review and meta-analysis included 3 parallel cohort studies that reported outcome measures associated with colistin and PMB use[62,76,80], 2 studies with colistin and PMB use[17,68], 75 studies with colistin use alone[16,18-40,42-57,59-61,64-67,70-75,78,81,83-99,101-102,104] and 9 studies with PMB use alone[41,58,63,69,77,79,82,100,103]. Regarding the geographical distribution, 35 studies were conducted in Europe, 31 in Asia, 6 in North America, 6 in South America and 4 in Africa. Regarding the definitions of AKI, 38, 10, and 8 studies relied on the Risk, Injury, Failure, Loss of kidney function, and End-stage kidney disease (RIFLE), Kidney Disease Improving Global Outcomes (KDIGO), and Acute Kidney Injury Network (AKIN) classification systems, respectively. The rest adopted self-reported definition of AKI. Only two studies reported median Acute Physiology and Chronic Health Evaluation (APACHE) II scores[44,100], whereas 53 studies reported mean APACHE II scores ranging from 11.8 (± 4.3)[58] to 30.4 (± 9.5)[91]. Nineteen studies and 62 studies described age as the median and the mean, respectively, with ages ranging from 40 to 73.8 years. Among the observational studies, there were 51 studies with a moderate risk of bias, 18 with a high risk of bias and 11 with a low risk of bias (Supplementary Table 3). Among the 9 RCTs, 1 was determined to have a high risk of bias[23], 7 had an unclear risk of bias[16,18,35,54,57,81,87], and 1 had a low risk of bias[34] (Supplementary Table 3).
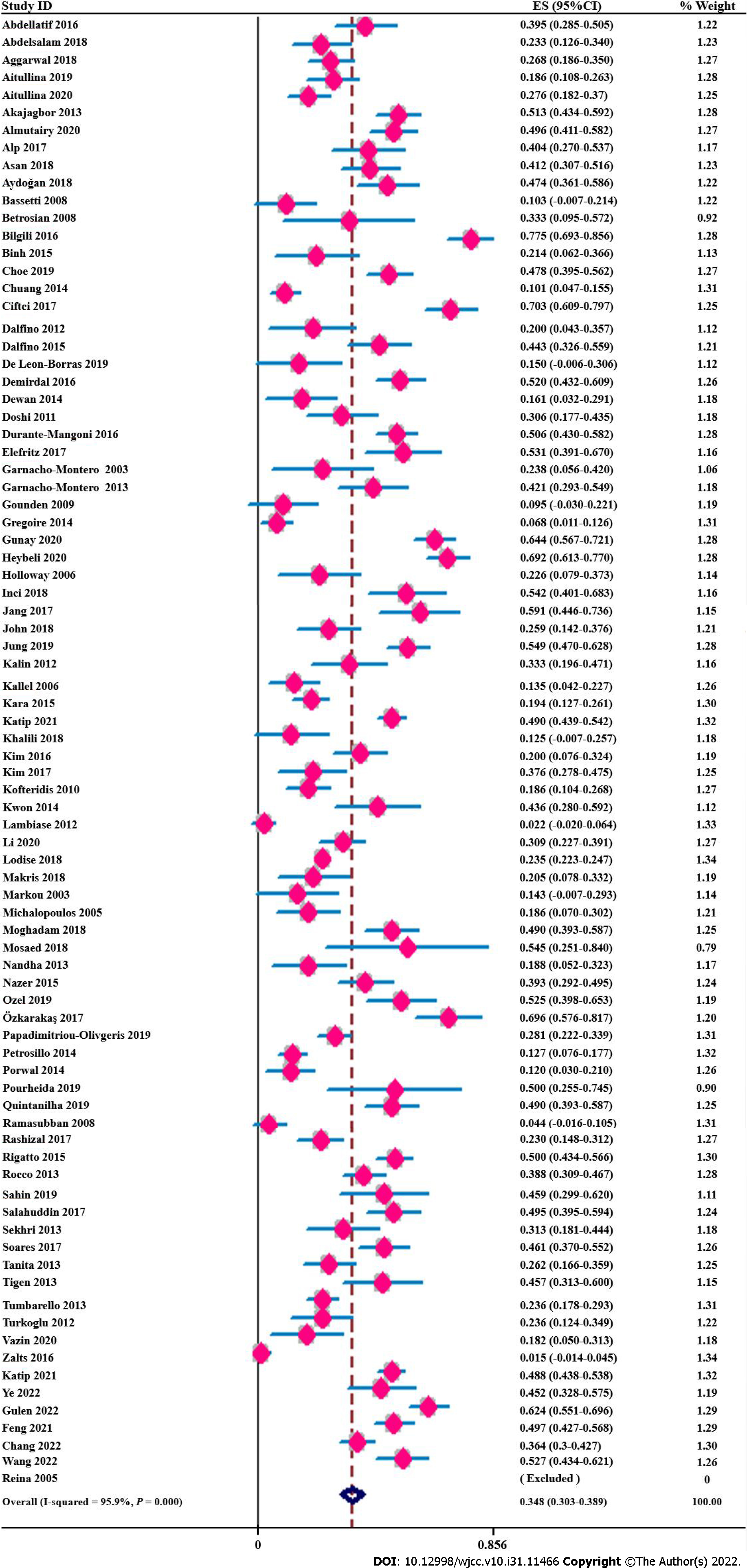
Across all studies, 4027 nephrotoxic events in 12234 critically ill adult patients receiving polymyxins were analysed. The pooled incidence of overall polymyxin-induced nephrotoxicity reached 34.8% (95%CI, 30.8%-38.9%, I2 = 95.90%) (Figure 2). In addition, we did not observe a significant influence of any study on the pooled estimates or heterogeneity (Supplementary Figure 1) in the sensitivity analyses. We classified nephrotoxicity into mild and severe (defined as a RIFLE grade of ‘failure’ or above, AKIN grade of 3 or KDIGO grade of 3 or required renal replacement therapy [RRT]) groups, and the incidence rates of mild and severe nephrotoxicity were 25.8% (95%CI, 21.6%-29.9%; I2 = 96.80%) and 12.7% (95%CI, 10.3%-15.0%; I2 = 89.60%), respectively (Table 1). In addition, ranking of the criteria used to obtain the AKI incidence indicated that the KDIGO criteria were the highest-ranked (46.5%; 95%CI, 35.9%-57.1%; I2 = 93.80%), followed by the RIFLE (39.6%; 95%CI, 33.9%-45.4%; I2 = 93.80%), AKIN (37.3%; 95%CI, 27.4%-47.3%; I2 = 87.40%) and finally other criteria (21.4%; 95%CI, 15.9%-26.9%; I2 = 94.20%). Since most studies used the standardized RIFLE criteria, we subsequently performed subgroup analyses, and the pooled incidence rates of polymyxin-induced nephrotoxicity classified as ‘risk’, ‘injury’ and ‘failure’ based on RIFLE criteria were 12.7% (95%CI, 9.6%-15.8%), 12.6% (95%CI, 10.0%-15.2%), and 14.9% (95%CI, 11.1%-18.6%), respectively (Supplementary Table 5).
| Category | Subgroups | No. of studies | No. of nephrotoxicity patients | No. of patients | Events rate (95%CI) | Model | Heterogeneity (I2) | P value | |
| Overall | 83 | 4027 | 12234 | 0.348 (0.308-0.389) | Random | 95.90 | NA | ||
| Severity | Severe nephrotoxicitya | 47 | 648 | 5163 | 0.127 (0.103-0.150) | Random | 89.60 | NA | |
| Mild nephrotoxicity | 49 | 1378 | 5163 | 0.258 (0.216-0.299) | Random | 96.80 | |||
| Continent | Africa | 4 | 53 | 209 | 0.215 (0.087-0.343) | Random | 82.10 | 0.394 | |
| Asia | 31 | 1230 | 3099 | 0.340 (0.264-0.416) | Random | 96.30 | |||
| Europe | 35 | 1192 | 2904 | 0.364 (0.283-0.446) | Random | 97.00 | |||
| South America | 6 | 249 | 628 | 0.399 (0.295-0.503) | Random | 84.60 | |||
| North America | 6 | 1283 | 5213 | 0.329 (0.200-0.459) | Random | 92.40 | |||
| Polymyxins | Colistin | 72 | 3623 | 11211 | 0.355 (0.311-0.398) | Random | 96.10 | 0.151 | |
| Polymyxin B | 11 | 262 | 903 | 0.268 (0.171-0.364) | Random | 92.20 | |||
| Loading dose | Loading dose | 32 | 1305 | 3233 | 0.391 (0.321-0.461) | Random | 95.00 | 0.051 | |
| No loading dose | 48 | 2333 | 8340 | 0.309 (0.260-0.357) | Random | 95.20 | |||
| Maintenance doseb | Higher dose | 5 | 109 | 281 | 0.395 (0.302-0.487) | Random | 52.00 | 0.363 | |
| Normal dose | 62 | 2867 | 9588 | 0.324 (0.278-0.371) | Random | 96.00 | |||
| Dosing interval | Q12H or BID | 32 | 1494 | 3200 | 0.421 (0.356-0.485) | Random | 93.40 | 0.0011 | |
| Q8-6H or TID | 21 | 380 | 1322 | 0.251 (0.172-0.330) | Random | 93.90 | |||
| Nephrotoxicity Criteria | RIFLE | 38 | 1667 | 3985 | 0.396 (0.339-0.454) | Random | 93.80 | 0.0001 | |
| KDIGO | 10 | 627 | 1369 | 0.465 (0.359-0.571) | Random | 93.80 | |||
| AKIN | 8 | 284 | 761 | 0.373 (0.274-0.473) | Random | 87.40 | |||
| Others | 26 | 1473 | 6298 | 0.214 (0.159-0.269) | Random | 94.20 | |||
| Age (yr)c | < 65 yr | 59 | 2450 | 8980 | 0.307 (0.263-0.351) | Random | 95.10 | 0.0031 | |
| ≥ 65 yr | 19 | 1123 | 2328 | 0.451 (0.382-0.520) | Random | 91.40 | |||
| Sex proportion | Males > 50% | 60 | 3064 | 99908 | 0.344 (0.297-0.391) | Random | 96.10 | 0.417 | |
| Males ≤ 50% | 8 | 332 | 757 | 0.406 (0.243-0.569) | Random | 94.50 | |||
| APACH II score | < 20 | 23 | 653 | 1690 | 0.305 (0.209-0.401) | Random | 95.80 | 0.115 | |
| ≥ 20 | 29 | 1053 | 2601 | 0.384 (0.307-0.461) | Random | 95.00 | |||
| Publication yr | < 2010 | 10 | 42 | 333 | 0.138 (0.085-0.192) | Random | 44.10 | 0.0001 | |
| 2010-2015 | 25 | 612 | 2099 | 0.270 (0.205-0.334) | Random | 93.10 | |||
| 2015-2022 | 48 | 3386 | 9875 | 0.421 (0.365-0.476) | Random | 96.70 | |||
| Study design | Cohort study | 72 | 3685 | 11491 | 0.340 (0.296-0.384) | Random | 96.50 | 0.785 | |
| Case-control study | 5 | 185 | 457 | 0.410 (0.164-0.656) | Random | 96.70 | |||
| RCT | 8 | 158 | 407 | 0.341 (0.224 - 0.459) | Random | 82.70 | |||
| Study qualityd | Low | 11 | 1402 | 5715 | 0.215 (0.130-0.299) | Random | 97.30 | 0.0031 | |
| Fair | 50 | 1912 | 4925 | 0.363 (0.314-0.411) | Random | 93.70 | |||
| High | 17 | 599 | 1324 | 0.412 (0.329-0.494) | Random | 90.10 | |||
| Sample size | ≤ 50 | 30 | 284 | 1026 | 0.261 (0.199-0.323) | Random | 87.00 | 0.0001 | |
| 50-100 | 27 | 657 | 1965 | 0.341 (0.260-0.423) | Random | 95.40 | |||
| > 100 | 28 | 3130 | 9480 | 0.436 (0.370-0.501) | Random | 97.30 | |||
Moreover, to explore potential sources of heterogeneity, we performed several subgroup analyses (Table 1). Regarding specific medications, the pooled incidence of PMB-induced nephrotoxicity was 26.8% lower than that of colistin-induced nephrotoxicity (35.5%; 95%CI, 31.1%-39.8%; I2 = 96.1%) without significant difference. Furthermore, pairwise meta-analysis showed that adult patients treated with colistin (42.7%) had a higher incidence of AKI than those treated with PMB (21.3%), but this difference was not statistically significant (OR = 2.37; 95%CI, 0.62-9.07; P = 0.206) (Supplemen
Regarding geographical distribution, the highest pooled incidence of polymyxin-induced nephrotoxicity was 39.9% in South America, followed by 36.4% in Europe, 32.9% in North America, 34.0% in Asia and 21.5% in Africa (Figure 3 and Table 1). In the subgroup analysis of age, the overall pooled polymyxin-induced nephrotoxicity incidence was significantly lower in younger patients (aged < 65 years) than in older patients (aged ≥ 65 years). In the univariable meta-regression, we found a significant age trend associated with the incidence of AKI in adults (regression coefficient (Q) = -0.1427, P = 0.003) (Table 2 and Supplementary Figure 3). Furthermore, we did not observe a difference in the nephrotoxicity incidence between males > 50% (34.4%; 95%CI, 29.7%-39.1%; I2 = 96.1%) and males ≤ 50% (40.6%; 95%CI, 24.3%-56.9%; I2 = 94.5%) or between a mean or median APACHE II score ≥ 20 group (38.4%; 95%CI, 30.7%-46.1%; I2 = 95.0%) and a score < 20 group (30.5%; 95%CI, 20.9%-40.1%; I2 = 95.8%) .
| Variable | Coefficient | Standard error | Lower 95%CI | Upper 95%CI | P value | R2, % |
| Univariable analysis | ||||||
| Continent | 0.0148 | 0.0222 | -0.0294 | 0.0590 | 0.508 | -0.64 |
| Polymyxins | -0.0865 | 0.0597 | -0.2052 | 0.0322 | 0.151 | 1.41 |
| Loading dose | -0.0814 | 0.0412 | -0.1634 | 0.0005 | 0.051 | 3.62 |
| Maintenance dosea | -0.0845 | 0.0928 | -0.2703 | 0.1004 | 0.363 | -0.41 |
| Dosing interval | -0.1704 | 0.0476 | -0.2659 | -0.0749 | 0.0011 | 20.58 |
| Nephrotoxicity criteria | -0.0577 | 0.0141 | -0.0858 | -0.0295 | 0.0001 | 18.55 |
| Ageb | -0.1427 | 0.0470 | -0.2362 | -0.0492 | 0.0031 | 11.08 |
| Gender | 0.0627 | 0.0768 | -0.0907 | 0.2162 | 0.417 | -0.20 |
| APACH II score | 0.0812 | 0.0505 | -0.0204 | 0.1827 | 0.115 | 3.52 |
| Publication yr | 0.1377 | 0.0261 | 0.0857 | 0.1896 | 0.0001 | 26.95 |
| Study design | 0.0096 | 0.0351 | -0.0601 | 0.0794 | 0.785 | -1.15 |
| Study qualityc | 0.0966 | 0.0313 | 0.0343 | 0.1590 | 0.0031 | 10.66 |
| Sample size | 0.0873 | 0.0220 | 0.0435 | 0.1311 | 0.0001 | 17.08 |
| Multivariable analysis | ||||||
| Nephrotoxicity criteria | -0.0344 | 0.0141 | -0.0625 | -0.0064 | 0.017 | 40.00 |
| Publication yr | 0.0940 | 0.0278 | 0.0386 | 0.1495 | 0.001 | |
| Sample size | 0.0357 | 0.0203 | -0.047 | 0.0760 | 0.082 | |
| Study qualityc | 0.0387 | 0.0267 | -0.0144 | 0.0919 | 0.151 | |
Subgroup analysis based on publication year showed that the incidence of nephrotoxicity caused by polymyxins was highest between 2015 and 2022 (42.1%; 95%CI, 36.5%-47.6%; I2 = 96.7%), followed by 2010 to 2015 (27.0%; 95%CI, 20.5%-33.4%; I2 = 93.1%) and before 2010 (13.8%; 95%CI, 8.5%-19.2%; I2 = 44.1%). A significant time trend of nephrotoxicity incidence with year of study publication was observed in the univariable meta-regression analysis (regression coefficient (Q) = 0.1337, P < 0.001) (Table 2 and Supplementary Figure 4). In addition, the nephrotoxicity incidence varied by sample sizes of the studies (P < 0.001 for subgroup analysis). Smaller studies (< 50 participants) showed the lowest incidence of nephrotoxicity compared to medium (50-100 participants) and large (≥ 100 participants) studies (Table 1). We excluded the study with the largest sample size[53] and performed a univariable meta-regression analysis according to the sample size of the studies. The results showed an association between the sample size of the studies and the incidence of nephrotoxicity caused by polymyxins (regression coefficient (Q) = 0.0873, P < 0.001) (Table 2 and Supplementary Figure 5). In addition, we found that the type of study did not impact the incidence of polymyxin-associated nephrotoxicity (Table 1). However, the pooled incidence rates of nephrotoxicity differed significantly among quality subgroups (P = 0.001). The highest pooled incidence of nephrotoxicity was 41.2% in high-quality studies, followed by 36.3% in moderate-quality studies and 21.5% in low-quality studies (Table 1). Univariable meta-regression indicated that studies with a higher risk of bias reported significantly lower rates of polymyxin-induced nephrotoxicity (regression coefficient (Q) = 0.0966, P = 0.003) (Table 2 and Supplementary Figure 6). Although we performed subgroup analyse, the heterogeneity of each group remained high. The final multivariable meta-regression model, which included the sample sizes of studies, publication year, study quality and definitions of AKI, was able to explain a significant proportion of the heterogeneity reported (R2 = 40.00%; P = 0.001) (Table 2).
Visual inspection of the funnel plots revealed potential asymmetry for the incidence of nephrotoxicity caused by polymyxins (Supplementary Figure 7A), with a significant Egger’s test result (P = 0.001). The trim-and-fill method also showed significant publication bias for the incidence of polymyxin-induced nephrotoxicity (Supplementary Figure 7B).
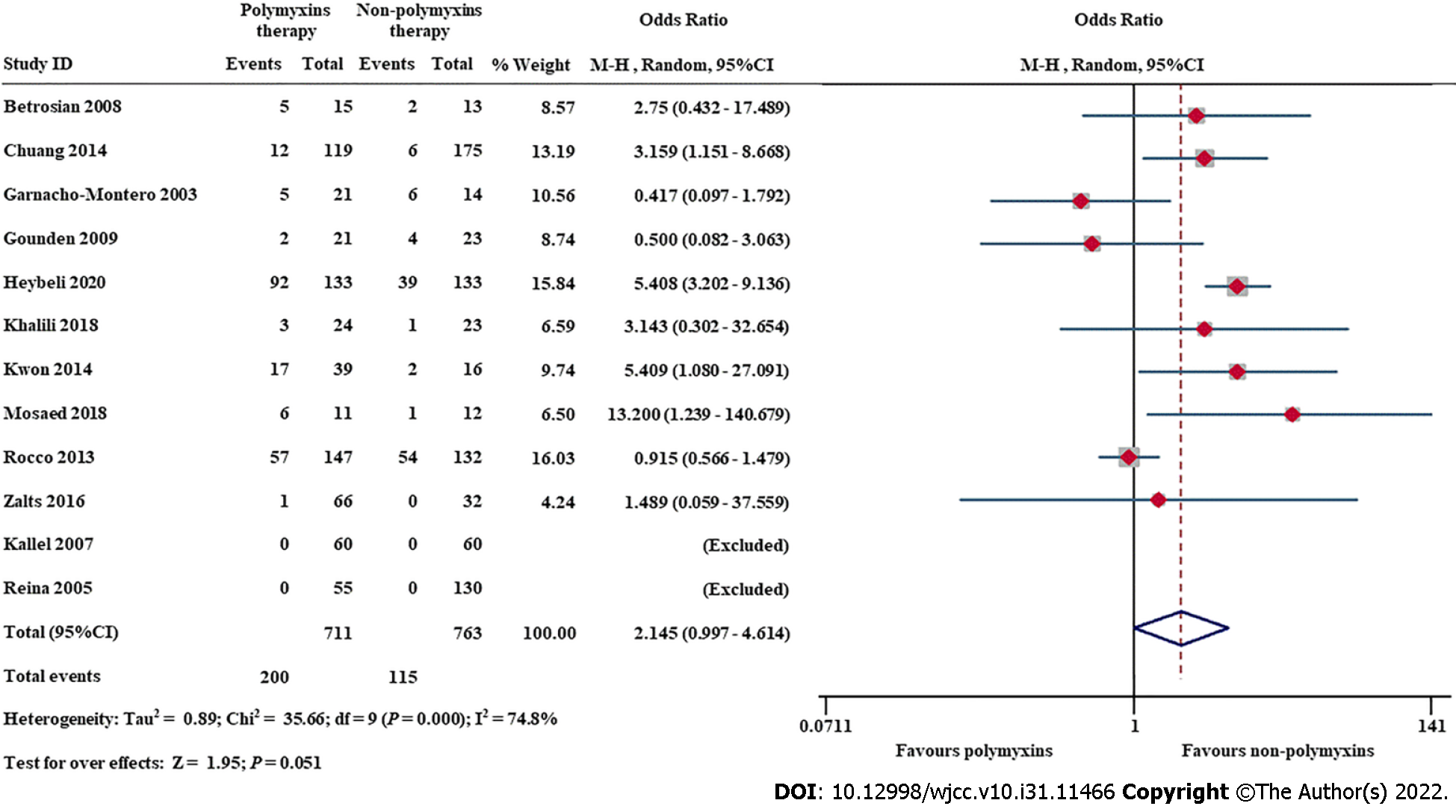
Twelve studies compared nephrotoxicity rates in critically ill adult patients with and without polymyxin use. Meta-analysis showed that polymyxin therapy was associated with a higher prevalence of nephrotoxicity than nonpolymyxin therapies (OR = 2.145; 95%CI, 0.997-4.614, I2 = 74.8%) (Figure 4), but the difference was not statistically significant. We performed sensitivity analysis and found that the exclusion of Rocco et al[66], Gounden et al[39] or Garnacho-Montero et al[37] changed the magnitude of the summary effect (Supplementary Figure 8). The funnel plots (Supplementary Figure 9) and Egger’s regression asymmetry tests (P = 0.686) suggested that there was no significant publication bias in this meta-analysis.
Given the potential predictors of polymyxin-induced nephrotoxicity (Table 3 and Supple
| Variables | Effect size | Heterogeneity | ||||
| OR (95%CI) | Model | Z | P value | I2 (%) | P value | |
| Pooled OR, RR, HR based on univariate analysis | ||||||
| Male gender | 1.570 (0.930-2.660) | Fixed | 1.67 | 0.095 | 0 | 0.964 |
| Female gender | 0.773 (0.418-1.427) | Random | 0.82 | 0.410 | 65.6 | 0.033 |
| Age (yr) | 1.025 (1.012-1.039) | Random | 3.65 | 0.0001 | 51.9 | 0.052 |
| Age (> 61 yr) | 2.072 (1.092-3.933) | Fixed | 2.23 | 0.0261 | 0 | 0.576 |
| APACHE II score | 1.036 (1.002-1.071) | Fixed | 2.06 | 0.0391 | 14.7 | 0.319 |
| Sepsisa | 2.114 (1.412-3.164) | Fixed | 3.64 | < 0.0011 | 45.3 | 0.139 |
| Baseline eGFR (mL/min/1.73 m2) | 0.883 (0.820-0.952) | Fixed | 3.26 | 0.0011 | 0 | 0.696 |
| Albumin (hypoalbuminemia)b | 2.795 (1.620-4.825) | Fixed | 3.69 | < 0.0011 | 0 | 0.565 |
| Diabetes mellitus | 1.229 (0.624-2.421) | Fixed | 0.60 | 0.550 | 0 | 0.542 |
| Concomitant diuretics | 0.853 (0.252-2.890) | Random | 0.26 | 0.799 | 77.2 | 0.004 |
| Concomitant vancomycin | 2.110 (1.190-3.730) | Fixed | 2.55 | 0.0111 | 0 | 0.667 |
| Concomitant vasopressor | 1.496 (1.007-2.222) | Random | 2.00 | 0.0461 | 72.0 | 0.013 |
| Daily dose (mg/kg/d) | 0.996 (0.991-1.000) | Fixed | 1.77 | 0.077 | 0 | 0.523 |
| Duration of therapy (d) | 1.019 (0.979-1.061) | Fixed | 0.92 | 0.359 | 34.5 | 0.191 |
| Pooled OR, RR, HR based on multivariate analysis | ||||||
| Age (yr) | 1.035 (1.021-1.050) | Fixed | 4.95 | < 0.0011 | 0 | 0.863 |
| APACHE II score | 1.031 (1.017-1.045) | Fixed | 4.36 | 0.000 | 26.1 | 0.255 |
| Concomitant vasopressor | 3.099 (1.169-8.219) | Random | 2.27 | 0.0231 | 80.4 | 0.002 |
| Concomitant diuretics | 2.979 (1.290-6.882) | Fixed | 2.56 | 0.0111 | 2.6 | 0.358 |
Nephrotoxicity due to polymyxin use is a major safety concern in clinical practice. In contrast to other meta-analyses[10,13], our study did not find that polymyxin therapy was associated with a higher risk of nephrotoxicity than other therapies (OR = 2.145; 95%CI, 0.997-4.614) in adult critically ill patients (Figure 4). Antimicrobial drugs, which are used in at least 70% of critically ill patients[105], include a wide range of medications that can cause nephrotoxicity, e.g., vancomycin, aminoglycosides, polymyxins, etc.[106]. I has been shown that earlier administration of appropriate antimicrobials for sepsis or septic shock can decrease mortality[107], and early use of PMB-based combination therapy is associated with a significant decrease in mortality compared with delayed administration[108]. Therefore, polymyxin-based combination therapy regimens for carbapenem-resistant gram-negative bacterial infections should be administered early despite the concern about nephrotoxicity in adult critically ill patients[109-111].
The overall prevalence of polymyxin-induced nephrotoxicity was 34.8% (95%CI, 30.8%-38.9%, I2 = 95.9%) in our study, which was slightly lower than that in other meta-analyses[12,13]. This result was consistent with other analyses showing that the nephrotoxicity rate was associated with the definition of nephrotoxicity[11,13]. The pooled prevalence of nephrotoxicity using standardized international criteria, such as the AKIN, KDIGO and RIFLE criteria, was similar, ranging from 37.3% to 46.5% (Table 1), which was similar to that in a previous meta-analysis[10,13] and may be the true prevalence of polymyxin-induced nephrotoxicity. Furthermore, we also evaluated the degree of nephrotoxicity using the RIFLE criteria, which were used by most of the studies in this meta-analysis. The prevalence of polymyxin-associated nephrotoxicity classified as failure (F) was 14.9%, higher than the 10% reported in the previous literature[12] (Supplementary Table 5). This finding indicated that 15 of 100 patients experienced acute renal failure during polymyxin treatment and developed AKI, resulting in 67% mortality and a higher risk of death than that in non-AKI patients[112]. Thus, nephrotoxicity caused by polymyxins and early identification of potential risk factors should be of great concern among ICU patients.
Among the potential predictors of polymyxin-induced nephrotoxicity, older age, the presence of sepsis or septic shock, hypoalbuminemia and concomitant vancomycin or vasopressor use were independent potential risk factors for polymyxin-associated AKI among ICU patients. Studies in sepsis patients showed a 2-fold higher risk of nephrotoxicity (OR = 2.114; 95%CI, 1.412-3.164), and patients with concomitant vasopressor use showed a 3-fold higher risk of nephrotoxicity than that in patients without sepsis (OR = 3.099; 95%CI, 1.169-8.219), which indicates that prompt treatment of sepsis and timely withdrawal of vasoactive drugs may be potential protective factors against polymyxin-induced nephrotoxicity. Sepsis is the most common aetiology of AKI in critically ill patients[113,114]. Multiple pathophysiological pathways of sepsis-associated AKI have been shown to be involved in the complex mechanism of polymyxin-induced damage in renal tubular cells[113-115].
Moreover, interactions between polymyxins and the cell membrane are also responsible for nephrotoxicity due to the amphipathic nature and accumulation of polymyxins in renal proximal tubular cells[116,117]. Therefore, nephrotoxicity caused by polymyxins has been reported to vary with pharmacokinetics and renal disposal mechanisms. Colistin methane sulfonate is a prodrug with approximately 40%-70% of the dose excreted in urine and ongoing conversion to colistin in the kidneys and bladder[118]. PMB is eliminated mainly by the nonrenal pathway with very low urinary recovery (approximately 4%)[7]. In our study, the prevalence of colistin-induced nephrotoxicity (35.5%; 95%CI, 31.1%-39.8%) was slightly higher than that of PMB-induced nephrotoxicity (26.8%; 95%CI, 17.1%-36.4%) without statistical significance (P = 0.151), similar to previous results[11-13]. Conversely, we found that nephrotoxicity was significantly increased when the total daily dose was divided into 2 doses but not 3 or 4 doses. Manchandani et al[119] emphasized that the steady-state trough concentration (Css trough) and average plasma concentration (Css avg) were higher in those receiving a dosing regimen of Q12H than in those receiving a dosing regimen of Q8H, which were confirmed as independent risk factors for nephrotoxicity[120], and it was demonstrated that a higher baseline estimated glomerular filtration rate was associated with a reduced risk of AKI (Table 3). This partly explains why hypoalbuminemia was also a risk factor for polymyxin-induced nephrotoxicity in our and previous meta-analyses[12]. Zavascki et al[121] indicated that plasma protein binding of PMB was higher in critically ill patients (ranging from 78.5% to 92.4%) than in healthy participants (approximately 50%). Hence, unbound plasma concentrations of PMB increased, and extensive accumulation of PMB inside tubular epithelial cells may, at least in part, explain the potential nephrotoxicity in critically ill individuals. Accordingly, albumin infusion was described to play a potential nephroprotective role in critically ill patients[122].
In addition, megalin, a crucial endocytic receptor highly expressed in the apical membranes of proximal renal epithelial cells, has been implicated in contributing to the nephrotoxicity of polymyxins[123]. Megalin plays a dual role in AKI, initially mediating nephrotoxins (e.g., polymyxin, vancomycin, aminoglycosides, etc.) in proximal renal epithelial cells, which induce the development or progression of AKI and mediate a variety of endogenous substances (e.g., vitamins and proteins, etc.) involved in AKI recovery[124]. Therefore, we speculated that megalin would be saturated by a variety of nephrotoxic drugs, such as aminoglycosides, vancomycin, and other nephrotoxins, leading to insufficient uptake of endogenous nephroprotective substances and increased nephrotoxicity. This is supported by a previous study in which polymyxin nephrotoxicity increased with the number of concomitant nephrotoxins[13]. In the present meta-analysis, vancomycin exposure significantly increased the odds of nephrotoxicity (OR = 2.110; 95%CI, 1.190-3.730; P = 0.011).
Some limitations should be considered in the interpretation of the findings of the current meta-analysis. First, although we established strict inclusion and exclusion criteria for the literature, our meta-analysis revealed high heterogeneity, which is a common concern in epidemiological meta-analyses[125] and is consistent with a previous systematic review that estimated the prevalence of polymyxin-induced nephrotoxicity in the general population[10-13]. The high between-study variability was associated with a single cohort in most studies and influenced to a greater extent by other factors, such as the numbers of enrolled patients, study quality and heterogeneous nephrotoxicity criteria. Then, we performed subgroup analyses and a meta-regression analysis to identify potential heterogeneity factors, and the multivariable meta-regression analysis explained almost 40% of the observed heterogeneity (Table 3). Moreover, to weaken the effects of diagnostic criteria on outcomes, we explored the incidence and severity of polymyxin-induced nephrotoxicity using only the RIFLE criteria, but high heterogeneity remained, in accordance with a previous study[12]. AKI in critically ill patients is a complicated heterogeneous syndrome[113]; hence, inconsistency among patient populations was also a potential source of heterogeneity.
Second, to comprehensively evaluate the incidence of polymyxin-associated nephrotoxicity, we made an effort to include all relevant studies, including not only studies in ICU patients but also those including subgroups of ICU patients[76,79,80,89,96,103] in this analysis. Nevertheless, it is possible that some potentially eligible studies were not captured by our search strategy. Third, during the pooled prevalence meta-analysis, zero-event studies were automatically excluded. Although this strategy is widely accepted, there is no consensus concerning whether it is the most reliable methodology, and the effect on pooled estimates is unclear. In addition, considering the limited data, other potential risk factors for polymyxin-induced nephrotoxicity that we could not capture may exist. For the above reasons, our findings should be interpreted with caution, and further studies are required to strengthen our results.
In conclusion, the present meta-analysis showed that the prevalence of nephrotoxicity during polymyxin treatment in the ICU was 34.8%, similar to that in the non-ICU setting, but the incidence of severe renal injury was higher in ICU patients. Older age (particularly > 65 years), the presence of sepsis or septic shock, hypoalbuminemia and concomitant vancomycin or vasopressor use were potential independent predictors of nephrotoxicity. Furthermore, a dosage regimen of 3 or 4 doses per day and dosage adjustment of colistin based on the renal baseline estimated glomerular filtration rate were associated with lower nephrotoxicity rates. Therefore, it is beneficial to adjust colistin doses in critically ill adult patients with renal impairment.
The prevalence of and risk factors for polymyxin-associated nephrotoxicity in intensive care unit (ICU) adult patients remain unclear.
The incidence of nephrotoxicity among polymyxin-treated patients is common and is one of the reasons why the use of polymyxins has been restricted. Nevertheless, the prevalence of and potential risk factors for polymyxin-induced nephrotoxicity in adult ICU patients are controversial. Therefore, a meta-analysis was carried out to assess the prevalence of and potential risk factors for polymyxin-induced nephrotoxicity.
This study aimed to meta-analyse reports evaluating the prevalence and potential predictors of polymyxin-induced nephrotoxicity in adult ICU patients.
We performed a systematic literature search in PubMed, EMBASE, the Cochrane Library and RCA database from inception to May 30, 2022 and included eligible randomized clinical trials and observational studies in a meta-analysis evaluating the prevalence and potential predictors of polymyxin-induced nephrotoxicity in adult ICU patients.
The overall pooled incidence of polymyxin-induced nephrotoxicity was 34.8%. Older age (particularly > 65 years), the presence of sepsis or septic shock, hypoalbuminemia and concomitant vancomycin or vasopressor use were risk factors for polymyxin-induced nephrotoxicity. In addition, our findings showed that a dosage regimen of 3 or 4 doses per day and dosage adjustment of colistin based on the renal baseline estimated glomerular filtration rate were associated with a lower nephrotoxicity rate.
The incidence of polymyxin-induced nephrotoxicity was high in ICU adult patients. Patients with older age, the presence of sepsis or septic shock, and a decreased baseline glomerular filtration rate had a potentially higher risk of polymyxin-induced nephrotoxicity. A polymyxin dosage regimen of 3 or 4 doses per day, dosage adjustment of colistin based on the renal baseline estimated glomerular filtration rate, and avoidance of other nephrotoxic drugs (vancomycin or vasopressors) were helpful in decreasing the risk of polymyxin-induced nephrotoxicity.
Exploring alternative treatments in patients with clinical or microbiologic carbapenem-resistant gram-negative bacterial infection treatment failures is required in the future to increase treatment efficacy and reduce adverse outcomes.
| 1. | Hoste EAJ, Kellum JA, Selby NM, Zarbock A, Palevsky PM, Bagshaw SM, Goldstein SL, Cerdá J, Chawla LS. Global epidemiology and outcomes of acute kidney injury. Nat Rev Nephrol. 2018;14:607-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 362] [Cited by in RCA: 962] [Article Influence: 137.4] [Reference Citation Analysis (0)] |
| 2. | Ortega-Loubon C, Martínez-Paz P, García-Morán E, Tamayo-Velasco Á, López-Hernández FJ, Jorge-Monjas P, Tamayo E. Genetic Susceptibility to Acute Kidney Injury. J Clin Med. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 3. | Kane-Gill SL. Nephrotoxin Stewardship. Crit Care Clin. 2021;37:303-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 4. | Hoste EA, Bagshaw SM, Bellomo R, Cely CM, Colman R, Cruz DN, Edipidis K, Forni LG, Gomersall CD, Govil D, Honoré PM, Joannes-Boyau O, Joannidis M, Korhonen AM, Lavrentieva A, Mehta RL, Palevsky P, Roessler E, Ronco C, Uchino S, Vazquez JA, Vidal Andrade E, Webb S, Kellum JA. Epidemiology of acute kidney injury in critically ill patients: the multinational AKI-EPI study. Intensive Care Med. 2015;41:1411-1423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2063] [Cited by in RCA: 1992] [Article Influence: 181.1] [Reference Citation Analysis (0)] |
| 5. | Kellum JA, Romagnani P, Ashuntantang G, Ronco C, Zarbock A, Anders HJ. Acute kidney injury. Nat Rev Dis Primers. 2021;7:52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 1081] [Article Influence: 216.2] [Reference Citation Analysis (0)] |
| 6. | Tsuji BT, Pogue JM, Zavascki AP, Paul M, Daikos GL, Forrest A, Giacobbe DR, Viscoli C, Giamarellou H, Karaiskos I, Kaye D, Mouton JW, Tam VH, Thamlikitkul V, Wunderink RG, Li J, Nation RL, Kaye KS. International Consensus Guidelines for the Optimal Use of the Polymyxins: Endorsed by the American College of Clinical Pharmacy (ACCP), European Society of Clinical Microbiology and Infectious Diseases (ESCMID), Infectious Diseases Society of America (IDSA), International Society for Anti-infective Pharmacology (ISAP), Society of Critical Care Medicine (SCCM), and Society of Infectious Diseases Pharmacists (SIDP). Pharmacotherapy. 2019;39:10-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 345] [Cited by in RCA: 688] [Article Influence: 114.7] [Reference Citation Analysis (0)] |
| 7. | Nang SC, Azad MAK, Velkov T, Zhou QT, Li J. Rescuing the Last-Line Polymyxins: Achievements and Challenges. Pharmacol Rev. 2021;73:679-728. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 264] [Cited by in RCA: 306] [Article Influence: 61.2] [Reference Citation Analysis (0)] |
| 8. | Wang J, Niu H, Wang R, Cai Y. Safety and efficacy of colistin alone or in combination in adults with Acinetobacter baumannii infection: A systematic review and meta-analysis. Int J Antimicrob Agents. 2019;53:383-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 9. | Patek TM, Teng C, Kennedy KE, Alvarez CA, Frei CR. Comparing Acute Kidney Injury Reports Among Antibiotics: A Pharmacovigilance Study of the FDA Adverse Event Reporting System (FAERS). Drug Saf. 2020;43:17-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 10. | Chien HT, Lin YC, Sheu CC, Hsieh KP, Chang JS. Is colistin-associated acute kidney injury clinically important in adults? Int J Antimicrob Agents. 2020;55:105889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 11. | Oliota AF, Penteado ST, Tonin FS, Fernandez-Llimos F, Sanches AC. Nephrotoxicity prevalence in patients treated with polymyxins: a systematic review with meta-analysis of observational studies. Diagn Microbiol Infect Dis. 2019;94:41-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 68] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 12. | Sisay M, Hagos B, Edessa D, Tadiwos Y, Mekuria AN. Polymyxin-induced nephrotoxicity and its predictors: a systematic review and meta-analysis of studies conducted using RIFLE criteria of acute kidney injury. Pharmacol Res. 2021;163:105328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 70] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 13. | Wagenlehner F, Lucenteforte E, Pea F, Soriano A, Tavoschi L, Steele VR, Henriksen AS, Longshaw C, Manissero D, Pecini R, Pogue JM. Systematic review on estimated rates of nephrotoxicity and neurotoxicity in patients treated with polymyxins. Clin Microbiol Infect. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 107] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 14. | Ordooei Javan A, Shokouhi S, Sahraei Z. A review on colistin nephrotoxicity. Eur J Clin Pharmacol. 2015;71:801-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 200] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 15. | Alhazzani W, Alshamsi F, Belley-Cote E, Heels-Ansdell D, Brignardello-Petersen R, Alquraini M, Perner A, Møller MH, Krag M, Almenawer S, Rochwerg B, Dionne J, Jaeschke R, Alshahrani M, Deane A, Perri D, Thebane L, Al-Omari A, Finfer S, Cook D, Guyatt G. Efficacy and safety of stress ulcer prophylaxis in critically ill patients: a network meta-analysis of randomized trials. Intensive Care Med. 2018;44:1-11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 114] [Cited by in RCA: 111] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 16. | Abdellatif S, Trifi A, Daly F, Mahjoub K, Nasri R, Ben Lakhal S. Efficacy and toxicity of aerosolised colistin in ventilator-associated pneumonia: a prospective, randomised trial. Ann Intensive Care. 2016;6:26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 95] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 17. | Aggarwal R, Dewan A. Comparison of nephrotoxicity of Colistin with Polymyxin B administered in currently recommended doses: a prospective study. Ann Clin Microbiol Antimicrob. 2018;17:15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 58] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 18. | Abdelsalam MFA, Abdalla MS, El-Abhar HSE. Prospective, comparative clinical study between high-dose colistin monotherapy and colistin-meropenem combination therapy for treatment of hospital-acquired pneumonia and ventilator-associated pneumonia caused by multidrug-resistant Klebsiella pneumoniae. J Glob Antimicrob Resist. 2018;15:127-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 19. | Aitullina A, Krūmiņa A, Svirskis Š, Purviņa S. Colistin Use in Patients with Extreme Renal Function: From Dialysis to Augmented Clearance. Medicina (Kaunas). 2019;55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 20. | Alp E, Eren E, Elay G, Cevahir F, Esmaoğlu A, Rello J. Efficacy of loading dose of colistin in Acinetobacter baumannii ventilator-associated pneumonia. Infez Med. 2017;25:311-319. [PubMed] |
| 21. | Aydoğan BB, Yıldırım F, Zerman A, Gönderen K, Türkoğlu M, Aygencel G. Colistin nephrotoxicity in the ICU: Is it different in the geriatric patients? Aging Clin Exp Res. 2018;30:573-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | Bassetti M, Repetto E, Righi E, Boni S, Diverio M, Molinari MP, Mussap M, Artioli S, Ansaldi F, Durando P, Orengo G, Bobbio Pallavicini F, Viscoli C. Colistin and rifampicin in the treatment of multidrug-resistant Acinetobacter baumannii infections. J Antimicrob Chemother. 2008;61:417-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 130] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 23. | Betrosian AP, Frantzeskaki F, Xanthaki A, Douzinas EE. Efficacy and safety of high-dose ampicillin/sulbactam vs. colistin as monotherapy for the treatment of multidrug resistant Acinetobacter baumannii ventilator-associated pneumonia. J Infect. 2008;56:432-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 170] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 24. | Bilgili B, Halilolu M, Gül F, Cinel I. Septic shock is an independent risk factor for colistin-induced severe acute kidney injury: a retrospective cohort study. Int J Clin Exp Med. 2016;9:14649-14655. [RCA] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 25. | Binh NG, Hayakawa K, Co DX, Tuan ND, Anh NH, Thuy NT, Phuong DM, Huong NT, Thuy PT, Chau NQ, Nhung PH, Gam do TH, Hai DT, Huong TT, Van Anh L, Takeshita N, Ohmagari N. The efficacy and nephrotoxicity associated with colistin use in an intensive care unit in Vietnam: Use of colistin in a population of lower body weight. Int J Infect Dis. 2015;35:18-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 26. | Choe J, Sohn YM, Jeong SH, Park HJ, Na SJ, Huh K, Suh GY, Jeon K. Inhalation with intravenous loading dose of colistin in critically ill patients with pneumonia caused by carbapenem-resistant gram-negative bacteria. Ther Adv Respir Dis. 2019;13:1753466619885529. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 27. | Chuang YC, Cheng CY, Sheng WH, Sun HY, Wang JT, Chen YC, Chang SC. Effectiveness of tigecycline-based vs colistin- based therapy for treatment of pneumonia caused by multidrug-resistant Acinetobacter baumannii in a critical setting: a matched cohort analysis. BMC Infect Dis. 2014;14:102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 103] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 28. | Ciftci A, Izdes S, Altintas ND. Factors Determining Nephrotoxicity and Mortality in Critical Care Patients Receiving Colistin. J Infect Dev Ctries. 2018;11:912-918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 29. | Dalfino L, Puntillo F, Mosca A, Monno R, Spada ML, Coppolecchia S, Miragliotta G, Bruno F, Brienza N. High-dose, extended-interval colistin administration in critically ill patients: is this the right dosing strategy? Clin Infect Dis. 2012;54:1720-1726. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 184] [Cited by in RCA: 189] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 30. | Dalfino L, Puntillo F, Ondok MJ, Mosca A, Monno R, Coppolecchia S, Spada ML, Bruno F, Brienza N. Colistin-associated Acute Kidney Injury in Severely Ill Patients: A Step Toward a Better Renal Care? Clin Infect Dis. 2015;61:1771-1777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 76] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 31. | Demirdal T, Sari US, Nemli SA. Is inhaled colistin beneficial in ventilator associated pneumonia or nosocomial pneumonia caused by Acinetobacter baumannii? Ann Clin Microbiol Antimicrob. 2016;15:11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 32. | Dewan A, Shoukat M. Evaluation of risk of nephrotoxicity with high dose, extended-interval colistin administration. Indian J Crit Care Med. 2014;18:427-430. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 33. | Doshi NM, Mount KL, Murphy CV. Nephrotoxicity associated with intravenous colistin in critically ill patients. Pharmacotherapy. 2011;31:1257-1264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 57] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 34. | Durante-Mangoni E, Signoriello G, Andini R, Mattei A, De Cristoforo M, Murino P, Bassetti M, Malacarne P, Petrosillo N, Galdieri N, Mocavero P, Corcione A, Viscoli C, Zarrilli R, Gallo C, Utili R. Colistin and rifampicin compared with colistin alone for the treatment of serious infections due to extensively drug-resistant Acinetobacter baumannii: a multicenter, randomized clinical trial. Clin Infect Dis. 2013;57:349-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 323] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 35. | Durante-Mangoni E, Andini R, Signoriello S, Cavezza G, Murino P, Buono S, De Cristofaro M, Taglialatela C, Bassetti M, Malacarne P, Petrosillo N, Corcione A, Viscoli C, Utili R, Gallo C. Acute kidney injury during colistin therapy: a prospective study in patients with extensively-drug resistant Acinetobacter baumannii infections. Clin Microbiol Infect. 2016;22:984-989. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 36. | Elefritz JL, Bauer KA, Jones C, Mangino JE, Porter K, Murphy CV. Efficacy and Safety of a Colistin Loading Dose, High-Dose Maintenance Regimen in Critically Ill Patients With Multidrug-Resistant Gram-Negative Pneumonia. J Intensive Care Med. 2017;32:487-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 37. | Garnacho-Montero J, Ortiz-Leyba C, Jiménez-Jiménez FJ, Barrero-Almodóvar AE, García-Garmendia JL, Bernabeu-WittelI M, Gallego-Lara SL, Madrazo-Osuna J. Treatment of multidrug-resistant Acinetobacter baumannii ventilator-associated pneumonia (VAP) with intravenous colistin: a comparison with imipenem-susceptible VAP. Clin Infect Dis. 2003;36:1111-1118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 366] [Cited by in RCA: 355] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 38. | Garnacho-Montero J, Amaya-Villar R, Gutiérrez-Pizarraya A, Espejo-Gutiérrez de Tena E, Artero-González ML, Corcia-Palomo Y, Bautista-Paloma J. Clinical efficacy and safety of the combination of colistin plus vancomycin for the treatment of severe infections caused by carbapenem-resistant Acinetobacter baumannii. Chemotherapy. 2013;59:225-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 77] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 39. | Gounden R, Bamford C, van Zyl-Smit R, Cohen K, Maartens G. Safety and effectiveness of colistin compared with tobramycin for multi-drug resistant Acinetobacter baumannii infections. BMC Infect Dis. 2009;9:26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 60] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 40. | Grégoire N, Mimoz O, Mégarbane B, Comets E, Chatelier D, Lasocki S, Gauzit R, Balayn D, Gobin P, Marchand S, Couet W. New colistin population pharmacokinetic data in critically ill patients suggesting an alternative loading dose rationale. Antimicrob Agents Chemother. 2014;58:7324-7330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 68] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 41. | Holloway KP, Rouphael NG, Wells JB, King MD, Blumberg HM. Polymyxin B and doxycycline use in patients with multidrug-resistant Acinetobacter baumannii infections in the intensive care unit. Ann Pharmacother. 2006;40:1939-1945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 62] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 42. | Inci A, Toker MK, Bicer IG, Derbent A, Salihoglu Z. Determination of colistin-related nephrotoxicity and risk factors in intensive care unit. North Clin Istanb. 2018;5:120-124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 43. | Jang JY, Kwon HY, Choi EH, Lee WY, Shim H, Bae KS. Efficacy and toxicity of high-dose nebulized colistin for critically ill surgical patients with ventilator-associated pneumonia caused by multidrug-resistant Acinetobacter baumannii. J Crit Care. 2017;40:251-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 44. | Kalin G, Alp E, Akin A, Coskun R, Doganay M. Comparison of colistin and colistin/sulbactam for the treatment of multidrug resistant Acinetobacter baumannii ventilator-associated pneumonia. Infection. 2014;42:37-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 71] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 45. | Kalin G, Alp E, Coskun R, Demiraslan H, Gündogan K, Doganay M. Use of high-dose IV and aerosolized colistin for the treatment of multidrug-resistant Acinetobacter baumannii ventilator-associated pneumonia: do we really need this treatment? J Infect Chemother. 2012;18:872-877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 80] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 46. | Kallel H, Bahloul M, Hergafi L, Akrout M, Ketata W, Chelly H, Hamida CB, Rekik N, Hammami A, Bouaziz M. Colistin as a salvage therapy for nosocomial infections caused by multidrug-resistant bacteria in the ICU. Int J Antimicrob Agents. 2006;28:366-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 86] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 47. | Kallel H, Hergafi L, Bahloul M, Hakim A, Dammak H, Chelly H, Hamida CB, Chaari A, Rekik N, Bouaziz M. Safety and efficacy of colistin compared with imipenem in the treatment of ventilator-associated pneumonia: a matched case-control study. Intensive Care Med. 2007;33:1162-1167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 115] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 48. | Kara I, Yildirim F, Bilaloglu B, Karamanlioglu D, Kayacan E, Dizbay M, Turkouglu M, Aygencel G. Comparison of the efficacy of colistin monotherapy and colistin combination therapies in the treatment of nosocomial pneumonia and ventilator-associated pneumonia caused by Acinetobacter baumannii. S Afr J Crit Care. 2015;31:51-58. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 49. | Kim WY, Moon JY, Huh JW, Choi SH, Lim CM, Koh Y, Chong YP, Hong SB. Comparable Efficacy of Tigecycline vs Colistin Therapy for Multidrug-Resistant and Extensively Drug-Resistant Acinetobacter baumannii Pneumonia in Critically Ill Patients. PLoS One. 2016;11:e0150642. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 55] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 50. | Kim YK, Lee JH, Lee HK, Chung BC, Yu SJ, Lee HY, Park JH, Kim S, Kim HK, Kiem S, Jang HJ. Efficacy of nebulized colistin-based therapy without concurrent intravenous colistin for ventilator-associated pneumonia caused by carbapenem-resistant Acinetobacter baumannii. J Thorac Dis. 2017;9:555-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 51. | Kofteridis DP, Alexopoulou C, Valachis A, Maraki S, Dimopoulou D, Georgopoulos D, Samonis G. Aerosolized plus intravenous colistin vs intravenous colistin alone for the treatment of ventilator-associated pneumonia: a matched case-control study. Clin Infect Dis. 2010;51:1238-1244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 140] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 52. | Lambiase A, Piazza O, Rossano F, Del Pezzo M, Tufano R, Catania MR. Persistence of carbapenem-resistant Acinetobacter baumannii strains in an Italian intensive care unit during a forty-six month study period. New Microbiol. 2012;35:199-206. [PubMed] |
| 53. | Lodise TP, Fan W, Griffith DC, Dudley MN, Sulham KA. A Retrospective Cohort Analysis Shows that Coadministration of Minocycline with Colistin in Critically Ill Patients Is Associated with Reduced Frequency of Acute Renal Failure. Antimicrob Agents Chemother. 2018;62. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 54. | Makris D, Petinaki E, Tsolaki V, Manoulakas E, Mantzarlis K, Apostolopoulou O, Sfyras D, Zakynthinos E. Colistin vs Colistin Combined with Ampicillin-Sulbactam for Multiresistant Acinetobacter baumannii Ventilator-associated Pneumonia Treatment: An Open-label Prospective Study. Indian J Crit Care Med. 2018;22:67-77. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 69] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 55. | Markou N, Apostolakos H, Koumoudiou C, Athanasiou M, Koutsoukou A, Alamanos I, Gregorakos L. Intravenous colistin in the treatment of sepsis from multiresistant Gram-negative bacilli in critically ill patients. Crit Care. 2003;7:R78-R83. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 189] [Cited by in RCA: 202] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 56. | Moghadam OM, Ghanbarpour R, Niakan M, Taher MT, Hassani V, Dadashi A, Shiri E. Assessment of renal damage in patients with multi-drug resistant strains of pneumonia treated with colistin. T Trauma Mon. 2018;23:e60002. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 57. | Mosaed R, Haghighi M, Kouchak M, Miri MM, Salarian S, Shojaei S, Javadi A, Taheri S, Nazirzadeh P, Foroumand M, Sistanizad M. Interim Study: Comparison Of Safety And Efficacy of Levofloxacin Plus Colistin Regimen With Levofloxacin Plus High Dose Ampicillin/Sulbactam Infusion In Treatment of Ventilator-Associated Pneumonia Due To Multi Drug Resistant Acinetobacter. Iran J Pharm Res. 2018;17:206-213. [PubMed] |
| 58. | Nandha R, Sekhri K, Mandal AK. To study the clinical efficacy and nephrotoxicity along with the risk factors for acute kidney injury associated with parenteral polymyxin B. Indian J Crit Care Med. 2013;17:283-287. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 59. | Nazer LH, Rihani S, Hawari FI, Le J. High-dose colistin for microbiologically documented serious respiratory infections associated with carbapenem-resistant Acinetobacter baummannii in critically ill cancer patients: a retrospective cohort study. Infect Dis (Lond). 2015;47:755-760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 60. | Özkarakaş H, Köse I, Zincircioğlu Ç, Ersan S, Ersan G, Şenoğlu N, Köse Ş, Erbay RH. Risk factors for colistin-associated nephrotoxicity and mortality in critically ill patients. Turk J Med Sci. 2017;47:1165-1172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 61. | Petrosillo N, Giannella M, Antonelli M, Antonini M, Barsic B, Belancic L, Inkaya A C, De Pascale G, Grilli E, Tumbarello M, Akova M. Clinical experience of colistin-glycopeptide combination in critically ill patients infected with Gram-negative bacteria. Antimicrob Agents Chemother. 2014;58:851-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 83] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 62. | Quintanilha JCF, Duarte NDC, Lloret GR, Visacri MB, Mattos KPH, Dragosavac D, Falcão ALE, Moriel P. Colistin and polymyxin B for treatment of nosocomial infections in intensive care unit patients: pharmacoeconomic analysis. Int J Clin Pharm. 2019;41:74-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 63. | Ramasubban S, Majumdar A, Das PS. Safety and efficacy of polymyxin B in multidrug resistant Gram-negative severe sepsis and septic shock. Indian J Crit Care Med. 2008;12:153-157. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 64. | Rashizal Sazli MR, Syed Mohamed AF, Wan Mazuan WM, Ling SM, Mahmud A, Amin Nordin S. Colistin-associated nephrotoxicity among patients in intensive care units (ICU) of hospitals in Selangor. Med J Malaysia. 2017;72:100-105. [PubMed] |
| 65. | Reina R, Estenssoro E, Sáenz G, Canales HS, Gonzalvo R, Vidal G, Martins G, Das Neves A, Santander O, Ramos C. Safety and efficacy of colistin in Acinetobacter and Pseudomonas infections: a prospective cohort study. Intensive Care Med. 2005;31:1058-1065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 143] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 66. | Rocco M, Montini L, Alessandri E, Venditti M, Laderchi A, De Pascale G, Raponi G, Vitale M, Pietropaoli P, Antonelli M. Risk factors for acute kidney injury in critically ill patients receiving high intravenous doses of colistin methanesulfonate and/or other nephrotoxic antibiotics: a retrospective cohort study. Crit Care. 2013;17:R174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 69] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 67. | Salahuddin N, Sammani M, Hamdan A, Joseph M, Al-Nemary Y, Alquaiz R, Dahli R, Maghrabi K. Fluid overload is an independent risk factor for acute kidney injury in critically Ill patients: results of a cohort study. BMC Nephrol. 2017;18:45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 75] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 68. | Sekhri K, Nandha R, Mandal A, Bhasin D, Singh H. Parenteral polymyxins: assessing efficacy and safety in critically ill patients with renal dysfunction. Indian J Pharmacol. 2013;45:608-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 69. | Soares DS, Reis ADF, Silva Junior GBD, Leite TT, Parente Filho SLA, Rocha CVO, Daher EF. Polymyxin-B and vancomycin-associated acute kidney injury in critically ill patients. Pathog Glob Health. 2017;111:137-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 70. | Tanita MT, Carrilho CM, Garcia JP, Festti J, Cardoso LT, Grion CM. Parenteral colistin for the treatment of severe infections: a single center experience. Rev Bras Ter Intensiva. 2013;25:297-305. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 71. | Tigen ET, Koltka EN, Dogru A, Gura M, Vahabaoglu H. The risk factors of colistin methanesulfonate associated nephrotoxicity. Indian J Crit Care Med. 2016;20:353-356. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 72. | Tigen ET, Koltka EN, Dogru A, Orhon ZN, Gura M, Vahaboglu H. Impact of the initiation time of colistin treatment for Acinetobacter infections. J Infect Chemother. 2013;19:703-708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 73. | Tumbarello M, De Pascale G, Trecarichi EM, De Martino S, Bello G, Maviglia R, Spanu T, Antonelli M. Effect of aerosolized colistin as adjunctive treatment on the outcomes of microbiologically documented ventilator-associated pneumonia caused by colistin-only susceptible gram-negative bacteria. Chest. 2013;144:1768-1775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 119] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 74. | Turkoglu M, Dizbay M, Ciftçi A, Aksakal FN, Aygencel G. Colistin therapy in critically ill patients with chronic renal failure and its effect on development of renal dysfunction. Int J Antimicrob Agents. 2012;39:142-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 75. | Zalts R, Neuberger A, Hussein K, Raz-Pasteur A, Geffen Y, Mashiach T, Finkelstein R. Treatment of Carbapenem-Resistant Acinetobacter baumannii Ventilator-Associated Pneumonia: Retrospective Comparison Between Intravenous Colistin and Intravenous Ampicillin-Sulbactam. Am J Ther. 2016;23:e78-e85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 76. |
Akajagbor DS, Wilson SL, Shere-Wolfe KD, Dakum P, Charurat ME, Gilliam BL Higher incidence of acute kidney injury with intravenous colistimethate sodium compared with polymyxin B in critically ill patients at a tertiary care medical center.
|
| 77. | John JF, Falci DR, Rigatto MH, Oliveira RD, Kremer TG, Zavascki AP. Severe Infusion-Related Adverse Events and Renal Failure in Patients Receiving High-Dose Intravenous Polymyxin B. Antimicrob Agents Chemother. 2018;62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 78. | Kwon SH, Ahn HL, Han OY, La HO. Efficacy and safety profile comparison of colistin and tigecycline on the extensively drug resistant Acinetobacter baumannii. Biol Pharm Bull. 2014;37:340-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 79. | Rigatto MH, Behle TF, Falci DR, Freitas T, Lopes NT, Nunes M, Costa LW, Zavascki AP. Risk factors for acute kidney injury (AKI) in patients treated with polymyxin B and influence of AKI on mortality: a multicentre prospective cohort study. J Antimicrob Chemother. 2015;70:1552-1557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 97] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 80. | Rigatto MH, Oliveira MS, Perdigão-Neto LV, Levin AS, Carrilho CM, Tanita MT, Tuon FF, Cardoso DE, Lopes NT, Falci DR, Zavascki AP. Multicenter Prospective Cohort Study of Renal Failure in Patients Treated with Colistin vs Polymyxin B. Antimicrob Agents Chemother. 2016;60:2443-2449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 108] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 81. | Khalili H, Shojaei L, Mohammadi M, Beigmohammadi MT, Abdollahi A, Doomanlou M. Meropenem/colistin vs meropenem/ampicillin-sulbactam in the treatment of carbapenem-resistant pneumonia. J Comp Eff Res. 2018;7:901-911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 82. | De León-Borrás R, Sánchez-Sergentón C, Mayor-Becerra A, Laureano-Cuadrado AF. Polymyxin B for Gram Negative Multidrug Resistant Bacteria in a Hispanic Population. P R Health Sci J. 2019;38:15-21. [PubMed] |
| 83. | Michalopoulos AS, Tsiodras S, Rellos K, Mentzelopoulos S, Falagas ME. Colistin treatment in patients with ICU-acquired infections caused by multiresistant Gram-negative bacteria: the renaissance of an old antibiotic. Clin Microbiol Infect. 2005;11:115-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 214] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 84. | Papadimitriou-Olivgeris M, Assimakopoulos SF, Kolonitsiou F, Solomou A, Vamvakopoulou S, Spyropoulou A, Karamouzos V, Anastassiou ED, Papachristou E, Spiliopoulou I, Christofidou M, Fligou F, Marangos M. Risk factors for acute kidney injury in critically ill patients with bacteraemia by carbapenem non-susceptible Gram negative bacteria. Infez Med. 2019;27:380-392. [PubMed] |
| 85. | Şahin AZ, Şimşek KB. Colistin-induced nephrotoxicity and risk factors in intensive care unit: estimating from the routine laboratory findings. Medical Science and Discovery. 2019;6:210-220. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 86. | Heybeli C, Canaslan K, Oktan MA, Yıldız S, Arda HÜ, Çavdar C, Çelik A, Gökmen N, Cömert B. Acute kidney injury following colistin treatment in critically-ill patients: may glucocorticoids protect? J Chemother. 2021;33:85-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 87. | Pourheidar E, Haghighi M, Kouchek M, Miri MM, Shojaei S, Salarian S, Hassanpour R, Sistanizad M. Comparison of Intravenous Ampicillin-sulbactam Plus Nebulized Colistin with Intravenous Colistin Plus Nebulized Colistin in Treatment of Ventilator Associated Pneumonia Caused by Multi Drug Resistant Acinetobacter Baumannii: Randomized Open Label Trial. Iran J Pharm Res. 2019;18:269-281. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 88. | Aitullina A, Purviņa S, Krūmiņa A. Colistin co-administration with other nephrotoxins: experience of teaching hospital of Latvia. Int J Clin Pharm. 2021;43:509-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 89. | Almutairy R, Aljrarri W, Noor A, Elsamadisi P, Shamas N, Qureshi M, Ismail S. Impact of Colistin Dosing on the Incidence of Nephrotoxicity in a Tertiary Care Hospital in Saudi Arabia. Antibiotics (Basel). 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 90. | Asan A, Karasu D, Gül C, Akinoglu G, Koca N, Akca MO, Yilmaz C, Karaduman I, Kose S. Nephrotoxicity rates related to colistin and evaluation of risk factors. EuRJ. 2020;6:62-66. [DOI] [Full Text] |
| 91. | Gunay E, Kaya S, Baysal B, Yuksel E, Arac E. Evaluation of prognosis and nephrotoxicity in patients treated with colistin in intensive care unit. Ren Fail. 2020;42:704-709. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 92. | Ozel AS, Ergönül Ö, Korten V. Colistin nephrotoxicity in critically ill patients after implementation of a new dosing strategy. J Infect Dev Ctries. 2019;13:877-885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 93. | Vazin A, Malek M, Karimzadeh I. Evaluation of colistin nephrotoxicity and urinary level of kidney injury molecule-1 in hospitalized adult ICU patients. J Renal Inj Prev. 2020;9:e13. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 94. | Jung S, Chung EK, Jun MS, Son ES, Rhie SJ. Differences in Colistin Administration and Bacterial and Treatment Outcomes in Critically Ill Patients. Sci Rep. 2019;9:8781. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 95. | Katip W, Oberdorfer P. Clinical Efficacy and Nephrotoxicity of Colistin Alone vs Colistin Plus Vancomycin in Critically Ill Patients Infected with Carbapenem-Resistant Acinetobacter baumannii: A Propensity Score-Matched Analysis. Pharmaceutics. 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 96. | Li KL, Abad CLR. The clinical profile and outcomes of adult patients given intravenous colistin for multidrug-resistant gram negative infections in a Philippine tertiary hospital. Int J Infect Dis. 2020;93:9-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 97. | Porwal R, Gopalakrishnan R, Rajesh NJ, Ramasubramanian V. Carbapenem resistant Gram-negative bacteremia in an Indian intensive care unit: A review of the clinical profile and treatment outcome of 50 patients. Indian J Crit Care Med. 2014;18:750-753. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 98. | Katip W, Uitrakul S, Oberdorfer P. A Comparison of Colistin vs Colistin Plus Meropenem for the Treatment of Carbapenem-Resistant Acinetobacter baumannii in Critically Ill Patients: A Propensity Score-Matched Analysis. Antibiotics (Basel). 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 53] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 99. | Katip W, Uitrakul S, Oberdorfer P. Clinical Efficacy and Nephrotoxicity of the Loading Dose Colistin for the Treatment of Carbapenem-Resistant Acinetobacter baumannii in Critically Ill Patients. Pharmaceutics. 2021;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 100. | Feng JY, Lee YT, Pan SW, Yang KY, Chen YM, Yen DH, Li SY, Wang FD. Comparison of colistin-induced nephrotoxicity between two different formulations of colistin in critically ill patients: a retrospective cohort study. Antimicrob Resist Infect Control. 2021;10:111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 101. | Ye Q, Wang Q, Chen Z, Chen W, Zhan Q, Wang C. Effectiveness, nephrotoxicity, and therapeutic drug monitoring of polymyxin B in nosocomial pneumonia among critically ill patients. Clin Respir J. 2022;16:402-412. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 102. | Gulen TA, Imre A, Kayabas, U. Factors affecting the colistin nephrotoxicity: advanced age and/or other factors? Mediterr J Infect Microb Antimicrob. 2022;11:13. [DOI] [Full Text] |
| 103. | Chang K, Wang H, Zhao J, Yang X, Wu B, Sun W, Huang M, Cheng Z, Chen H, Song Y, Chen P, Chen X, Gan X, Ma W, Xing L, Wang Y, Cao B. Risk factors for polymyxin B-associated acute kidney injury. Int J Infect Dis. 2022;117:37-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 104. | Wang SH, Yang KY, Sheu CC, Chen WC, Chan MC, Feng JY, Chen CM, Wu BR, Zheng ZR, Chou YC, Peng CK; T. -CARE (Taiwan Critical Care, Infection) Group. The necessity of a loading dose when prescribing intravenous colistin in critically ill patients with CRGNB-associated pneumonia: a multi-center observational study. Crit Care. 2022;26:91. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 105. | Vincent JL, Sakr Y, Singer M, Martin-Loeches I, Machado FR, Marshall JC, Finfer S, Pelosi P, Brazzi L, Aditianingsih D, Timsit JF, Du B, Wittebole X, Máca J, Kannan S, Gorordo-Delsol LA, De Waele JJ, Mehta Y, Bonten MJM, Khanna AK, Kollef M, Human M, Angus DC; EPIC III Investigators. Prevalence and Outcomes of Infection Among Patients in Intensive Care Units in 2017. JAMA. 2020;323:1478-1487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 601] [Article Influence: 100.2] [Reference Citation Analysis (0)] |
| 106. | Mehta RL, Awdishu L, Davenport A, Murray PT, Macedo E, Cerda J, Chakaravarthi R, Holden AL, Goldstein SL. Phenotype standardization for drug-induced kidney disease. Kidney Int. 2015;88:226-234. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 131] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 107. | Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, Kumar A, Sevransky JE, Sprung CL, Nunnally ME, Rochwerg B, Rubenfeld GD, Angus DC, Annane D, Beale RJ, Bellinghan GJ, Bernard GR, Chiche JD, Coopersmith C, De Backer DP, French CJ, Fujishima S, Gerlach H, Hidalgo JL, Hollenberg SM, Jones AE, Karnad DR, Kleinpell RM, Koh Y, Lisboa TC, Machado FR, Marini JJ, Marshall JC, Mazuski JE, McIntyre LA, McLean AS, Mehta S, Moreno RP, Myburgh J, Navalesi P, Nishida O, Osborn TM, Perner A, Plunkett CM, Ranieri M, Schorr CA, Seckel MA, Seymour CW, Shieh L, Shukri KA, Simpson SQ, Singer M, Thompson BT, Townsend SR, Van der Poll T, Vincent JL, Wiersinga WJ, Zimmerman JL, Dellinger RP. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Med. 2017;43:304-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3352] [Cited by in RCA: 4152] [Article Influence: 461.3] [Reference Citation Analysis (8)] |
| 108. | Liang Q, Huang M, Xu Z. Early use of polymyxin B reduces the mortality of carbapenem-resistant Klebsiella pneumoniae bloodstream infection. Braz J Infect Dis. 2019;23:60-65. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 109. | Jacobs DM, Safir MC, Huang D, Minhaj F, Parker A, Rao GG. Triple combination antibiotic therapy for carbapenemase-producing Klebsiella pneumoniae: a systematic review. Ann Clin Microbiol Antimicrob. 2017;16:76. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 110. | Hou SY, Wu D, Feng XH. Polymyxin monotherapy vs polymyxin-based combination therapy against carbapenem-resistant Klebsiella pneumoniae: A systematic review and meta-analysis. J Glob Antimicrob Resist. 2020;23:197-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 111. | Kengkla K, Kongpakwattana K, Saokaew S, Apisarnthanarak A, Chaiyakunapruk N. Comparative efficacy and safety of treatment options for MDR and XDR Acinetobacter baumannii infections: a systematic review and network meta-analysis. J Antimicrob Chemother. 2018;73:22-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 124] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 112. | Folkestad T, Brurberg KG, Nordhuus KM, Tveiten CK, Guttormsen AB, Os I, Beitland S. Acute kidney injury in burn patients admitted to the intensive care unit: a systematic review and meta-analysis. Crit Care. 2020;24:2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 82] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 113. | Petejova N, Martinek A, Zadrazil J, Kanova M, Klementa V, Sigutova R, Kacirova I, Hrabovsky V, Svagera Z, Stejskal D. Acute Kidney Injury in Septic Patients Treated by Selected Nephrotoxic Antibiotic Agents-Pathophysiology and Biomarkers-A Review. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 53] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 114. | Pickkers P, Darmon M, Hoste E, Joannidis M, Legrand M, Ostermann M, Prowle JR, Schneider A, Schetz M. Acute kidney injury in the critically ill: an updated review on pathophysiology and management. Intensive Care Med. 2021;47:835-850. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 341] [Article Influence: 68.2] [Reference Citation Analysis (0)] |
| 115. | Azad MAK, Nation RL, Velkov T, Li J. Mechanisms of Polymyxin-Induced Nephrotoxicity. Adv Exp Med Biol. 2019;1145:305-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 116. | Jiang X, Zhang S, Azad MAK, Roberts KD, Wan L, Gong B, Yang K, Yuan B, Uddin H, Li J, Thompson PE, Velkov T, Fu J, Wang L. Structure-Interaction Relationship of Polymyxins with the Membrane of Human Kidney Proximal Tubular Cells. ACS Infect Dis. 2020;6:2110-2119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 117. | Azad MA, Roberts KD, Yu HH, Liu B, Schofield AV, James SA, Howard DL, Nation RL, Rogers K, de Jonge MD, Thompson PE, Fu J, Velkov T, Li J. Significant accumulation of polymyxin in single renal tubular cells: a medicinal chemistry and triple correlative microscopy approach. Anal Chem. 2015;87:1590-1595. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 63] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 118. | Sandri AM, Landersdorfer CB, Jacob J, Boniatti MM, Dalarosa MG, Falci DR, Behle TF, Bordinhão RC, Wang J, Forrest A, Nation RL, Li J, Zavascki AP. Population pharmacokinetics of intravenous polymyxin B in critically ill patients: implications for selection of dosage regimens. Clin Infect Dis. 2013;57:524-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 296] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 119. | Manchandani P, Thamlikitkul V, Dubrovskaya Y, Babic JT, Lye DC, Lee LS, Tam VH. Population Pharmacokinetics of Polymyxin B. Clin Pharmacol Ther. 2018;104:534-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 65] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 120. | Sorlí L, Luque S, Grau S, Berenguer N, Segura C, Montero MM, Alvarez-Lerma F, Knobel H, Benito N, Horcajada JP. Trough colistin plasma level is an independent risk factor for nephrotoxicity: a prospective observational cohort study. BMC Infect Dis. 2013;13:380. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 186] [Cited by in RCA: 183] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 121. | Zavascki AP, Goldani LZ, Cao G, Superti SV, Lutz L, Barth AL, Ramos F, Boniatti MM, Nation RL, Li J. Pharmacokinetics of intravenous polymyxin B in critically ill patients. Clin Infect Dis. 2008;47:1298-1304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 172] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 122. | Wiedermann CJ, Joannidis M. Nephroprotective Potential of Human Albumin Infusion: A Narrative Review. Gastroenterol Res Pract. 2015;2015:912839. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 123. | Suzuki T, Yamaguchi H, Ogura J, Kobayashi M, Yamada T, Iseki K. Megalin contributes to kidney accumulation and nephrotoxicity of colistin. Antimicrob Agents Chemother. 2013;57:6319-6324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 94] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 124. | Nielsen R, Christensen EI, Birn H. Megalin and cubilin in proximal tubule protein reabsorption: from experimental models to human disease. Kidney Int. 2016;89:58-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 375] [Article Influence: 41.7] [Reference Citation Analysis (0)] |
| 125. | Colditz GA, Burdick E, Mosteller F. Heterogeneity in meta-analysis of data from epidemiologic studies: a commentary. Am J Epidemiol. 1995;142:371-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 163] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Infectious diseases
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Mohapatra S, India; Sultan AAEA, Egypt S-Editor: Liu JH L-Editor: A P-Editor: Liu JH