Published online Nov 6, 2022. doi: 10.12998/wjcc.v10.i31.11427
Peer-review started: July 13, 2022
First decision: July 31, 2022
Revised: August 16, 2022
Accepted: September 27, 2022
Article in press: September 27, 2022
Published online: November 6, 2022
Processing time: 105 Days and 5.2 Hours
Postoperative gastrointestinal function recovery is critical for rapid rehabilitation of patients with gastrointestinal tumors. Traditional Chinese medicine offers considerable advantages for gastrointestinal disease treatment. However, no study has reported the clinical efficacy of intradermal needle therapy (INT) at the Yuan-source, Luo-connecting, and He-sea points of the corresponding meridian for gastrointestinal function in patients following surgery for gastrointestinal tumors.
To investigate the effect of INT at combined acupoints on patients’ gastro
This randomized controlled trial was conducted at the Second Affiliated Hospital of Xi’an Jiaotong University on patients with diagnosed gastrointestinal cancer, no distant metastases or organ failure, and hospitalized for elective radical tumor resection, who did not receive preoperative radiotherapy or chemotherapy. Participants were randomly allocated to either the intervention (n = 32) or the control (n = 32) group. Participants in the control group received enhanced recovery care, while those in the intervention group received enhanced recovery care combined with INT at the Yuan-source, Luo-connecting, and He-sea points. After surgery, INT was performed immediately upon the patient's return to the ward, and continued for seven consecutive days. The independent samples t-test, chi-square test, and generalized estimating equations were used for data analysis.
The participants’ ages ranged from 40 to 80 years (average 63 ± 10.1 years). Most participants underwent surgery for either gastric (43.8%) or colon cancer (39.1%) and had adenocarcinoma (87.5%). Significant differences were noted in time to first postoperative flatus passage (66 ± 27 h vs 103 ± 41 h, P < 0.001), time to first defecation (106 ± 44 h vs 153 ± 50 h, P < 0.001), and time to first oral feeding (73 ± 30 h vs 115 ± 38 h, P < 0.001) between the intervention and control groups. Gastrointestinal symptoms, including abdominal distension, nausea, and fatigue 48 h and 72 h after surgery, were significantly alleviated in the intervention group compared with that observed in the control group (P < 0.05).
INT at the Yuan-source, Luo-connecting, and He-sea points can promote recovery of gastrointestinal function and ease gastrointestinal symptoms in patients following surgical resection of gastrointestinal tumors.
Core Tip: Postoperative gastrointestinal function recovery has been considered critical to the rapid rehabilitation of patients undergoing gastrointestinal tumor resection. In this randomized controlled trial, we selected distal acupoints of the corresponding meridian following the Traditional Chinese medicine theory (specifically, acupoints on the stomach, large intestine, liver, and spleen channels) as intradermal needle therapy treatment sites, and assessed the effects in a cohort of gastrointestinal tumor patients. We found that this intervention could promote recovery of gastrointestinal function and alleviate gastrointestinal symptoms. We provide preliminary evidence to support the integration of this intervention into the postoperative care of patients with gastrointestinal tumors to promote rapid recovery.
- Citation: Guo M, Wang M, Chen LL, Wei FJ, Li JE, Lu QX, Zhang L, Yang HX. Effect of intradermal needle therapy at combined acupoints on patients’ gastrointestinal function following surgery for gastrointestinal tumors. World J Clin Cases 2022; 10(31): 11427-11441
- URL: https://www.wjgnet.com/2307-8960/full/v10/i31/11427.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i31.11427
Gastric and colorectal cancers are common malignancies of the digestive tract. According to a global assessment of 36 types of cancer in 185 countries conducted in 2020, colorectal and gastric cancers ranked third and fifth in incidence, and second and fourth in mortality, respectively[1]. Surgical resection is currently the first-line treatment for gastrointestinal (GI) tumors. However, surgical trauma can trigger the release of inflammatory mediators that cause a stress response, leading to disturbances in metabolism and homeostasis, as well as abnormal secretion and regulation of postoperative GI hormones[2-4]. Surgery can also enhance the activity of the GI sympathetic nervous system through various sympathetic nerve activation mechanisms, thereby inhibiting the excitatory neurons of the myenteric plexus in the GI tract and further inhibiting GI motility, resulting in GI dysfunction[5]. This dysfunction often manifests as a series of GI symptoms, such as inability to pass flatus, nausea, vomiting, and abdominal distension, that can seriously affect patients’ recovery. In the consensus guidelines for Enhanced Recovery After Surgery (ERAS), a series of strategies, including reduced preoperative fasting time, selective indwelling nasogastric or drainage tubes, and early postoperative activity and oral feeding, are recommended to promote postoperative recovery of GI function[6,7]. However, most of these measures focus on optimizing patient care practices, and active intervention measures are still lacking. Traditional Chinese medicine (TCM) offers considerable advantages for treatment of GI diseases[8-11], but TCM decoctions are restricted during postoperative fasting. Thus, external treatment techniques based on TCM theories are widely applied to postoperative patients, with acupuncture being the most commonly used method[12,13].
Acupuncture can restore body homeostasis through its bidirectional ability to “adjust Xu and Shi and balance Yin and Yang.” The bidirectional regulation mechanism of acupuncture is based on the meridian theory of TCM and is affected by multiple factors, such as the status of the human body, specificity of the meridians or acupoints, and compatibility of the acupoints. In the human body, the meridians carry and circulate Qi and blood, link Zang-Fu organs, and connect the interior areas of the body, as well as connecting the interior to the exterior area. The meridian system also regulates body functions, transports Qi and blood, and harmonizes Yin and Yang through activities of Qi. The acupoints, which are widely distributed in the meridians, are special sites on the surface of the body formed by the infusion of Qi from Zang-Fu organs, and are thus closely related to the meridians, Zang-Fu organs, Qi, and blood. They are not only the locations where diseases are reflected but also the sites for treatments. Meridians and acupoints are two inseparable concepts; the acupoints are the strongholds of the meridians, while the meridians are the passages connecting the acupoints. The main function of the meridians is realized by the acupoints. The Yuan-source points are meridian points where the source Qi of the Zang-Fu organs passes and resides. They are considered to be the source points for life activities and the foundation for normal physiological functioning of the 12 meridians. According to TCM theory, “Yuan-source points are often used to treat diseases of the five Zang organs or the six Fu organs.” The Luo-connecting points are points where collaterals split off from the meridians; these function to connect the meridians and strengthen the therapeutic effects of the acupoints on the meridians. The He-sea points are located near the elbow and knee joints where the Qi channel is abundant, and are associated with the Zang-Fu organs. In the TCM theory, “He-sea points are often selected to treat Fu organ disorders.”
TCM theorizes that the symptoms of Qi stagnation, blood stasis, and obstruction of Fu-Qi following GI surgery result from deficiencies of fluid, Qi, and blood. Therefore, a treatment approach that focuses on strengthening the spleen, harmonizing the stomach, soothing the liver and Qi, and regulating the six Fu organs is recommended. While acupuncture is widely applied, it must be performed by a qualified TCM practitioner. Patients often find it difficult to accept the strong prickling sensation associated with traditional acupuncture treatment. In contrast, intradermal needle therapy (INT), a subtype of acupuncture, involves intradermal application of special small needles to the acupoints to achieve a prolonged retention period of continuous and enhanced stimulation to generate therapeutic effects [14]. This procedure can be performed by a nurse and is less painful, thus making it more acceptable for patients. Therefore, INT was adopted in this study.
Due to its notable therapeutic advantages, INT has recently been applied in the treatment of a range of conditions. A recent protocol developed a randomized controlled trial to examine whether INT at the Shenmen (HT36) and Sanyinjiao (SP6) acupoints would improve postoperative sleep quality in patients undergoing laparoscopic hysterectomy[15]. One study explored the effects of INT on hemiplegia recovery after stroke[16], while another study summarized the effectiveness of INT for treating gastric diseases by describing clinical cases[17]. Previous research applied INT in combination with pinaverium bromide in irritable bowel syndrome-diarrhea patients and found that this technique effectively regulated gastrointestinal hormone production and significantly improved gastrointestinal symptoms[18]. To date, to the best of our knowledge, the clinical efficacy of INT at the Yuan-source, Luo-connecting, and He-sea points on GI function in patients following surgery for GI tumors has not been reported. Thus, based on the TCM theory that “the points where the meridians pass through demand priority while considering treatments”, in combination with the known remote treatment effect of acupoints, the present study selected distal acupoints of the corresponding meridian (specifically, acupoints on the stomach, large intestine, liver, and spleen channels) as INT treatment sites, aiming to elucidate its effect in this cohort of patients.
This study was conducted as a randomized controlled trial with a parallel-group design.
Patients with gastric and/or colorectal cancer who underwent elective radical surgery at the Second Affiliated Hospital of Xi’an Jiaotong University in Shaanxi Province from April 2021 to December 2021 were recruited for this study. Patients: (1) With a diagnosis of gastric, colon, or rectal cancer who were hospitalized for elective radical tumor resection; (2) With no distant metastasis; (3) Who did not receive preoperative radiotherapy or chemotherapy; (4) Without organ failure; (5) Who were conscious; and (6) who were willing to participate in this study were included. Patients: (1) With concurrent preoperative ascites, obstruction, perforation, bleeding, etc. who needed urgent care; (2) With other preoperative diseases affecting GI motility; (3) Who received postoperative medications affecting GI motility; or (4) With skin allergies, injuries, infections, etc. At the acupoints making them unsuitable for needle embedding were excluded from the study.
The sample size was determined using the following formula: N = [2 × σ2 × (Zα+Zβ)2]/δ2. Zα is the critical value of the normal distribution at a significant level of α, (here, the α level is 0.05, two-tailed); Zβ is the critical value of normal distribution at β, [here, the power (1-β) is 90%]. σ is the standard deviation of the two populations, and δ is the difference between the mean of two different groups. Zα and Zβ in this study were 1.96 and 1.28, respectively. The sample size was calculated using the recorded time to first passage of flatus as the main indicator of outcomes. According to a previous study [19], the values of σ and δ were set to 7.56 and 6.5, respectively. The result of the calculation showed that the sample size in each group was at least 28. After a 15% expansion, a final sample size of 32 cases was required in each group.
Researchers unaffiliated with this study generated random numbers using the random number table. These random numbers were kept in sealed, light-tight envelopes labeled from 1–64. Among the 64 random numbers enclosed, the smaller 32 numbers were designated as the control group and the larger 32 numbers were designated as the intervention group. These envelopes were issued to recruited patients during the recruitment process, in order of enrollment, and participants were grouped based on the random numbers in their envelopes. During the study, the data collectors were blinded to the group assignment.
Control group: Patients received enhanced recovery care based on the ERAS protocol, including during the preoperative (counseling and patient education, preoperative optimization, preoperative nutritional care, preoperative fasting, and carbohydrate loading), intraoperative (optimization of anesthesia, tube placement, prevention of intraoperative hypothermia), and postoperative stages (postoperative analgesia, prevention of nausea and vomiting, tube management, postoperative early oral intake, postoperative nutritional care, early mobilization). Further details on these procedures are described in Table 1. Intervention group: in addition to the enhanced recovery care following the ERAS protocol described above, the Yuan-source, Luo-connecting, and He-sea points were selected together for INT. Information regarding acupoint locations and needling instruments are as follows, and further details on INT procedures are shown in Table 1.
| Components | Intervention group | Control group |
| Preoperative stage | ||
| Counseling and patient education | Patients were educated regarding knowledge of the disease and perioperative cautions, and key points to achieve better cooperation. | Same as the intervention group |
| Preoperative optimization | The use of tobacco and alcohol was restricted; patients were instructed to perform efficient cough and back percussion and were required to perform activities (e.g., blow balloons or climb stairs) to improve lung function. | |
| Preoperative nutritional care | Using the Nutritional Risk Screening score (NRS 2002) to assess nutritional risk. Patients at risk received oral nutritional supplements for 5–7 d prior to surgery. | |
| Preoperative fasting and carbohydrate loading | Fasting from solid foods for 6 h before surgery; 2–3 h before surgery, patients without diabetes received 200–350 mL of electrolyte drinks under the guidance of dietitians (ingredients: glucose 100 mmol/L, sodium 50 mmol, potassium 20 mmol/L, chloride 50 mmol/L, magnesium 1 mmol/L, phosphorus 2 mmol/L); water was prohibited during the 2 h before surgery. | |
| Intraoperative stage | ||
| Optimization of anesthesia | Medium- and short-acting anesthetics were recommended. | Same as the intervention group |
| Tube placement | Nasogastric tube, abdominal drainage tube or urinary catheter were selectively placed when indicated. | |
| Prevention of intraoperative hypothermia | Intraoperative normothermia was maintained at 36 °C or over, using the following methods. The operating room temperature was set at 26 ℃–27 ℃ before surgery and 30 min before the end of the operation; during the operation, the temperature was set at 22 ℃–23 ℃, and the exposed area of the patient’s body was wrapped with a quilt. The skin antiseptic agent was heated to 40 ℃, and the rinse solution and intravenous infusion fluid were heated to 37 ℃. | |
| Postoperative stage | ||
| Postoperative analgesia | Multimodal analgesia: Intravenous patient-controlled analgesia and NSAIDs. | Same as the intervention group |
| Prevention of nausea and vomiting | Antiemetics were appropriately administered to patients based on their number of risk factors. | |
| Tube management | Avoidance or early removal of nasogastric tube, abdominal drainage tube or urinary catheter. | |
| Postoperative early oral intake | Patients were encouraged to drink when they were awake after the operation. If there was no discomfort, a liquid diet was allowed after the first postoperative flatus passage, and the semi flow and general diet were gradually recovered. | |
| Postoperative nutritional care | According to the NRS-2002, oral nutritional supplementation (or additional parenteral nutrition when indicated) was provided for malnourished patients. | |
| Early mobilization | Six hours after recovering from anesthesia, the patients were restricted to the lateral or semi-recumbent position; the patients were allowed to turn over in the bed on the first day, move out of bed on the second day, and stand and walk around on the third day after the operation. | |
| INT | Disinfecting the bilateral acupoints LI-4, PC-6, LR-3, SP-4, ST-36, ST-37, and ST-39 with 75% medical alcohol. Removing the sealing paper from the press needle, then attaching the adhesive tape with press needle to the acupoints. Pressure was applied to the acupoints until the patients experienced local soreness, numbness, and/or pain. The patient or caregiver was instructed to press each acupoint for 2 min at 4–6 h intervals. The press needles were changed every 24 h. INT was performed immediately upon the patient's return to the ward after surgery and continued for seven consecutive days. | The patients in the control group did not receive INT. However, adhesive tapes were attached at their corresponding acupoints with the same appearance as the adhesive tapes with needles applied to the acupoints of the patients in the intervention group. |
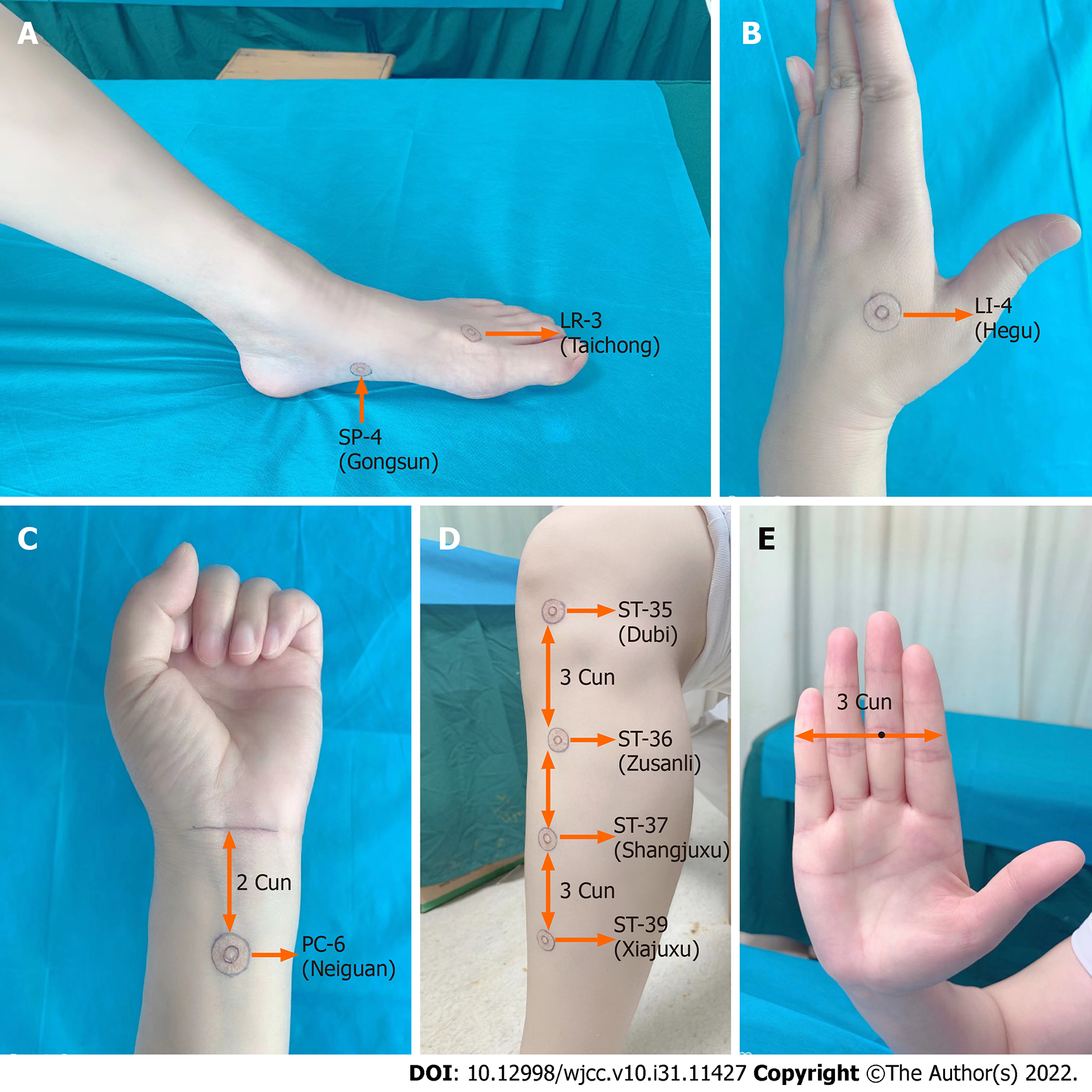
The acupoints were located by referencing descriptions provided by “Acupuncture and Moxibustion”[14]. In brief, LR-3, the Yuan-source point on the liver channel of Foot Jueyin, is located at the dorsum of the foot, in the depression distal to the junction of the first and second metatarsal bones, where the arterial pulse is sensible (Figure 1A). SP-4, the Luo-connecting point on the spleen meridian of Foot Taiyin, is located in the depression distal and inferior to the base of the first metatarsal at the junction of the red and white skin (Figure 1A). LI-4, the Yuan-source point of the large intestine channel of the Hand Yangming, is located on the dorsum of the hand, in the middle of the second metacarpal bone on the radial side (Figure 1B). This acupoint can be easily located by placing the horizontal crease of the thumb’s interphalangeal joint of one hand against the edge of the web between the thumb and index finger of the other hand, and the acupoint is located at the tip of the thumb. PC-6, the Luo-connecting point on the pericardium channel of the Hand Jueyin and one of the eight extraordinary confluence points connected to the Yin Linking vessel, is located at the anterior forearm, two cuns above the distal wrist crease, the tendons of palmaris longus, and the flexor carpi radialis muscles (Figure 1C). ST-36, the lower-He-sea point on the stomach channel of the Foot Yangming, is located on the anterior aspect of the lower leg, three cuns below Dubi (ST-35), and one finger breadth lateral from the anterior tibia crest (Figure 1D). ST-37, the lower-He-sea point on the large intestine channel, is located on the anterior aspect of the lower leg, six cuns below ST-35 (Figure 1D). ST-39, the lower-He-sea point on the small intestine channel, is located on the anterior aspect of the lower leg, nine cuns below ST-35 (Figure 1D). This study adopted the four-finger-breadth measurement, in which the breadth of four of the patient’s closed fingers at the level of the proximal interphalangeal crease of the middle finger was considered three cuns (Figure 1E).
Needling instrument: Sterilized disposable Seirin press needles (Seirin Corporation, Shizuoka-shi, Japan) were used for the INT (Medical Device Registration Certificate of the People’s Republic of China, No: Guo Xie Zhu Jin 20162271259, size: 0.20 mm × 1.50 mm).
The primary outcome was postoperative GI function recovery. The secondary outcomes were GI symptoms: (1) Postoperative indices reflective of GI function recovery, including time to first flatus and stool passages and time to first oral feeding, were recorded and compared between the intervention and control groups. During the preoperative counseling and patient education, the patients or their families were taught how to identify and record the time to first flatus and stool passages and the time to first oral feeding. Patients were prompted to inform nurses immediately after their first postoperative flatus passages. On the first day after the operation, the nurse interviewed the patient or their family regarding the time of first flatus and stool passages every 4 h; (2) Symptom scores of abdominal distension, nausea, vomiting, and fatigue were recorded at 24 h (T1), 48 h (T2), and 72 h (T3) post-operation for patients in both groups. The symptom scores were determined on a scale of 0–3 points using the TCM syndrome scoring method[20], wherein higher scores indicated more severe symptoms. For example, an abdominal distension score of 0 indicated minor symptoms unnoticed by the patients, while a score of 3 indicated unbearable and persistent abdominal distension, possibly with vomiting, that required drugs to soothe Qi and remove stagnation.
Data were analyzed using SPSS (Version 24, SPSS Inc., Chicago, IL, United States). Continuous variables are presented as mean and standard deviation, and categorical variables as frequency and percentage. The independent samples t-test was used to examine continuous variables, and the chi-square test or Fisher’s exact test was used to examine categorical variables. The generalized estimating equation was used to evaluate the effects of the interventions on the outcomes. Subgroup analysis was performed based on cancer type. P values were two-tailed, and P < 0.05 was considered statistically significant.
A total of 64 participants (32 patients each in the intervention and control groups) were included in this study. The participants’ ages ranged from 40 to 80 years (average 63 ± 10.1 years), and the majority were male (57.8%). The proportion of patients with a bachelor's degree was relatively low (28.1%); most patients were farmers (37.5%) and retirees (34.4%); the vast majority lived in urban areas (62.5%), were married (85.9%), had no smoking history (73.4%), and consumed alcohol occasionally (59.4%). Most participants underwent surgery for either gastric cancer (43.8%) or colon cancer (39.1%), had adenocarcinoma (87.5%), and had stage II disease progression (70.3%). No significant differences were noted in baseline characteristics between the two groups (P > 0.05) (Table 2).
| Variable | mean ± SD or n (%) | t /x2 | P value | |
| Intervention group (n = 32) | Control group (n = 32) | |||
| Age (years) | 65 ± 9.3 | 61 ± 10.6 | 1.696 | 0.10 |
| Gender | ||||
| Male | 18 (56.3) | 19 (59.4) | 0.064 | 0.80 |
| Female | 14 (43.8) | 13 (40.6) | ||
| Education level | 0.309 | 0.86 | ||
| Middle school or below | 12 (37.5) | 11 (34.4) | ||
| High school or junior college | 12 (37.5) | 11 (34) | ||
| Baccalaureate or above | 8 (25.0) | 10 (31.3) | ||
| Occupations1 | 3.442 | 0.50 | ||
| Farmer | 12 (37.5) | 12 (37.5) | ||
| Worker | 4 (12.5) | 2 (6.3) | ||
| Government official | 1 (3.1) | 5 (15.6) | ||
| Retiree | 12 (37.5) | 10 (31.3) | ||
| Other | 3 (9.4) | 3 (9.4) | ||
| Residence | 0.000 | 1.00 | ||
| Rural areas | 12 (37.5) | 12 (37.5) | ||
| Urban areas | 20 (62.5) | 20 (62.5) | ||
| Marital status1 | 0.408 | 1.00 | ||
| Married | 28 (87.5) | 27 (84.4) | ||
| Widowed | 2 (6.3) | 2 (6.3) | ||
| Divorced | 2 (6.3) | 3 (9.4) | ||
| Smoking | 0.080 | 0.78 | ||
| Yes | 8 (25.0) | 9 (28.1) | ||
| No | 24 (75.0) | 23 (71.9) | ||
| Drinking1 | 0.554 | 0.83 | ||
| Often | 8 (25.0) | 8 (25.0) | ||
| Occasionally | 18 (56.2) | 20 (62.5) | ||
| Never | 6 (18.8) | 4 (12.5) | ||
| Cancer types1 | 1.059 | 0.64 | ||
| Gastric cancer | 12 (37.5) | 16 (50.0) | ||
| Colon cancer | 14 (43.7) | 11 (34.4) | ||
| Rectal cancer | 6 (18.8) | 5 (15.6) | ||
| Pathological type1 | 0.75 | 0.71 | ||
| Adenocarcinoma | 27 (84.4) | 29 (90.6) | ||
| Squamous cell carcinoma | 5 (15.6) | 3 (9.4) | ||
| Stages of disease1 | 0.877 | 0.67 | ||
| Ⅰ | 2 (6.3) | 1 (3.1) | ||
| Ⅱ | 21 (65.6) | 24 (75.0) | ||
| Ⅲ | 9 (28.1) | 7 (21.9) | ||
| Surgical approach | 0.577 | 0.45 | ||
| Traditional radical resection | 20 (62.5) | 17 (53.1) | ||
| Laparoscopy | 12 (37.5) | 15 (46.9) | ||
Four of the ERAS components (preoperative optimization, postoperative tube management, postoperative early oral intake, and early mobilization) had compliance rates ranging from 71.9% to 84.4%. The other ERAS components had compliance rates of 85% or greater. Specifically, the compliance rates of preoperative optimization in the intervention and control groups were 84.4% and 81.2%, respectively. Meanwhile, the compliance rates of postoperative tube management in the intervention and control groups were 84.4% and 75.0%, respectively. The compliance rates of postoperative early oral intake in the intervention and control groups were 78.1% and 71.9%, respectively. The compliance rates of early mobilization in the intervention and control groups were 71.9% and 75.0%, respectively. No statistical differences were observed between the two groups in terms of the compliance rates of preoperative optimization, postoperative tube management, postoperative early oral intake, or early mobilization.
The effect of INT on recovery of postoperative GI functions is summarized in Table 3. Significant differences were noted in the time to first postoperative flatus passage (66 ± 27 h vs 103 ± 41 h, P < 0.001), time to first defecation (106 ± 44 h vs 153 ± 50 h, P < 0.001), and time to first oral feeding (73 ± 30 h vs 115 ± 38 h, P < 0.001) between the intervention and control groups. Subgroup analysis indicated that the differences in the time to first postoperative flatus passage and time to first defecation between the two groups were significant in subjects with colon cancer (P < 0.05), while they were not statistically significant in those with gastric cancer (P > 0.05). The differences between the groups in terms of the time to first oral feeding was significant in both subjects with gastric and with colon cancer (P < 0.05).
| Outcomes | Group | Merged | Subgroup | |||||||
| Gastric cancer | Colon cancer | |||||||||
| n | mean ± SD | P value | n | mean ± SD | P value | n | mean ± SD | P value | ||
| Time to first flatus passage (h) | Intervention group | 32 | 66 ± 27 | < 0.001 | 12 | 83 ± 32 | 0.06 | 20 | 56 ± 17 | 0.002 |
| Control group | 32 | 103 ± 41 | 16 | 110 ± 37 | 16 | 97 ± 44 | ||||
| Time to first defecation (h) | Intervention group | 32 | 106 ± 44 | < 0.001 | 12 | 125 ± 36 | 0.09 | 20 | 94 ± 44 | 0.001 |
| Control group | 32 | 153 ± 50 | 16 | 155 ± 48 | 16 | 150 ± 53 | ||||
| Time to first oral feeding (h) | Intervention group | 32 | 73 ± 30 | < 0.001 | 12 | 94 ± 30 | 0.02 | 20 | 61 ± 22 | < 0.001 |
| Control group | 32 | 115 ± 38 | 16 | 126 ± 35 | 16 | 105 ± 40 | ||||
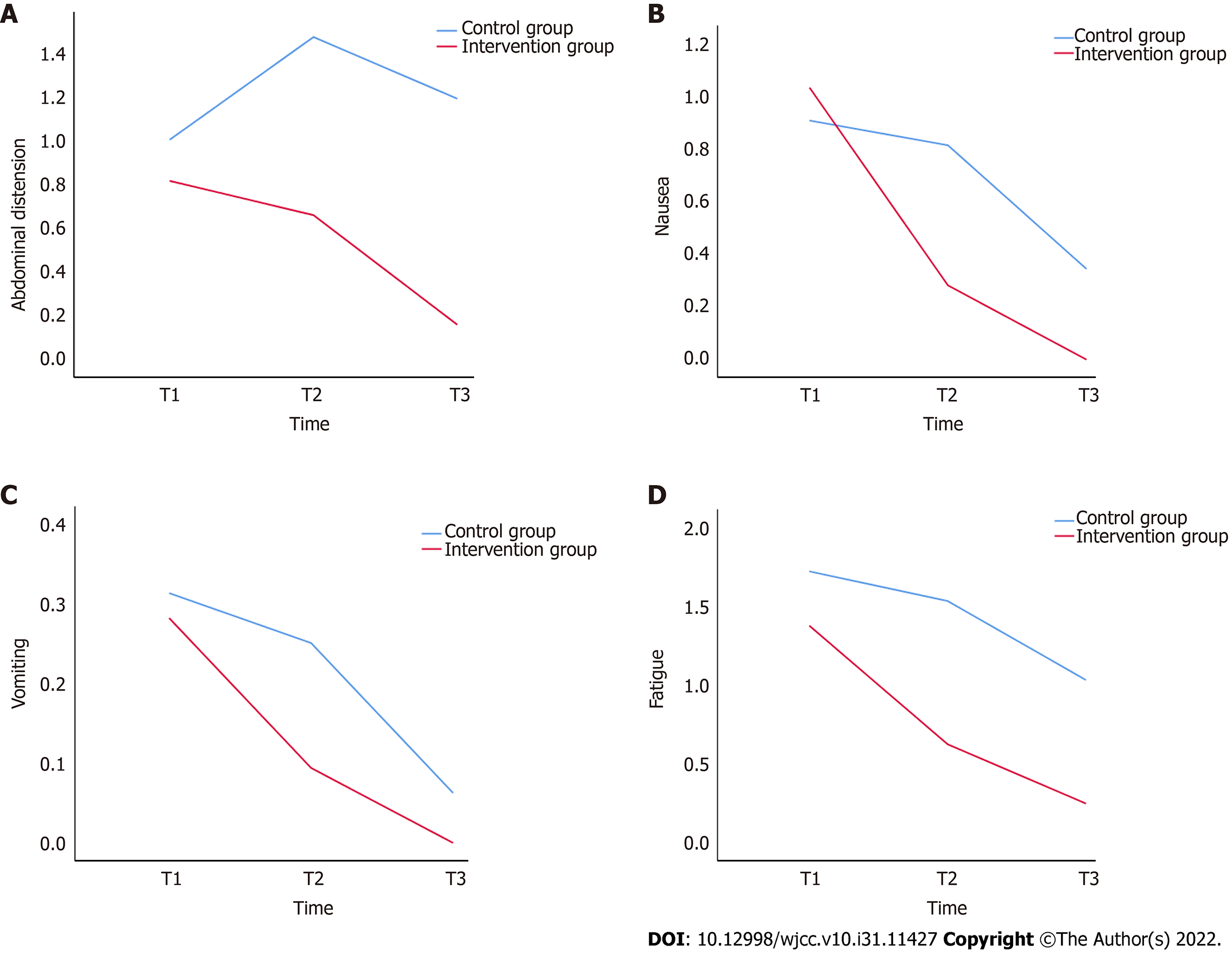
The effect of INT on abdominal distension in the merged sample is presented in Table 4. The abdominal distension scores at T3 were not statistically different from those at T1 in the control group (P > 0.05), while the abdominal distention scores at T3 were significantly lower than those at T1 in the intervention group (P < 0.001). In addition, no significant difference was noted in the abdominal distension scores between the two groups at T1 (P > 0.05), while the abdominal distension scores at T2 and T3 were significantly lower in the intervention group than those in the control group (P < 0.001). The subgroup analysis of gastric and colon cancer showed the same results as the analysis of the merged sample (Table 5). As shown in Table 6, comparison of the T2-T1 abdominal distension score differences between the intervention and control groups revealed a difference of -0.6 (P < 0.001), which indicated that from T1 to T2, the intervention group achieved a significantly better outcome in terms of alleviation of abdominal distension than the control group did. Comparison of the T3-T1 abdominal distension score differences between the intervention and control groups revealed a difference of -0.8 (P < 0.001), which indicates that from T1 to T3, the intervention group demonstrated significantly reduced abdominal distension compared with that of the control group. A line graph was adopted for an intuitive presentation of the changing trend in abdominal distension levels among patients (Figure 2A).
| Measure | Intervention group (n = 32) | Control group (n = 32) | Between-group difference | |||
| Mean difference (95%CI) | P value | Mean difference (95%CI) | P value | Mean difference (95%CI) | P value | |
| Abdominal distension | ||||||
| T1 | Reference | N/A | Reference | N/A | -0.2 (-0.5, 0.1) | 0.26 |
| T2 | -0.2 (-0.5, -0.2) | 0.72 | 0.5 (0.2, 0.7) | <0.001 | -0.8 (-1.1, -0.5) | < 0.001 |
| T3 | -0.7 (-1.0, -0.4) | < 0.001 | 0.2 (-0.3, 0.6) | 0.97 | -1.0 (-1.3, -0.7) | < 0.001 |
| Nausea | ||||||
| T1 | Reference | N/A | Reference | N/A | 0.1 (-0.2, 0.5) | 0.46 |
| T2 | -0.6 (-0.9, -0.6) | < 0.001 | -0.1 (-0.4, 0.2) | 1.00 | -0.5 (-0.8, -0.3) | < 0.001 |
| T3 | -1.0 (-1.3, -0.8) | < 0.001 | -0.6 (-1.0, -0.2) | 0.002 | -0.3 (-0.5, -0.2) | < 0.001 |
| Vomiting | ||||||
| T1 | Reference | N/A | Reference | N/A | 0 (-0.3, 0.3) | 0.83 |
| T2 | -0.2 (-0.4, 0) | 0.02 | -0.1 (-0.2, 0.1) | 1.00 | -0.2 (-0.4, 0.1) | 0.19 |
| T3 | -0.3 (-0.5, 0) | 0.02 | -0.3 (-0.5, 0) | 0.06 | -0.1 (-0.2, 0) | 0.14 |
| Fatigue | ||||||
| T1 | Reference | N/A | Reference | N/A | -0.3 (-0.7, 0) | 0.04 |
| T2 | -0.8 (-1.0, -0.5) | < 0.001 | -0.2 (-0.4, 0) | 0.13 | -0.9 (-1.2, -0.6) | < 0.001 |
| T3 | -1.1 (-1.4, -0.9) | < 0.001 | -0.7 (-0.9, -0.5) | < 0.001 | -0.8 (-1.1, -0.5) | < 0.001 |
| Subgroup/measure | Intervention group | Control group | Between-group difference | ||||
| Mean difference (95%CI) | P value | Mean difference (95%CI) | P value | Mean difference (95%CI) | P value | ||
| Abdominal distension | |||||||
| Gastric cancer (n = 28) | T1 | Reference | N/A | Reference | N/A | 0 (-0.5, 0.5) | 1.00 |
| T2 | -0.1 (-0.7, 0.5) | 1.00 | 0.4 (0.1, 0.8) | 0.01 | -0.5 (-1.0, 0) | 0.04 | |
| T3 | -0.7 (-1.3, -0.1) | 0.02 | 0.2 (-0.4, 0.8) | 1.00 | -0.9 (-1.3, -0.4) | < 0.001 | |
| Colon cancer (n = 36) | T1 | Reference | N/A | Reference | N/A | -0.3 (-0.7, 0.1) | 0.17 |
| T2 | -0.2 (-0.6, 0.2) | 0.56 | 0.5 (0.1, 0.9) | 0.003 | -1.0 (-1.4, -0.6) | < 0.001 | |
| T3 | -0.7 (-1.0, -0.3) | < 0.001 | 0.2 (-0.5, 0.9) | 1.00 | -1.1 (-1.5, -0.7) | < 0.001 | |
| Nausea | |||||||
| Gastric cancer (n = 28) | T1 | Reference | N/A | Reference | N/A | 0.6 (0.1, 1.0) | 0.01 |
| T2 | -0.9 (-1.1, -0.7) | < 0.001 | 0.2 (-0.2, 0.6) | 0.71 | -0.5 (-0.9, -0.1) | 0.009 | |
| T3 | -1.3 (-1.8, -0.9) | < 0.001 | -0.4 (-0.9, 0.2) | 0.32 | -0.4 (-0.7, -0.1) | 0.01 | |
| Colon cancer (n = 36) | T1 | Reference | N/A | Reference | N/A | -0.2 (-0.7, 0.3) | 0.38 |
| T2 | -0.9 (-1.2, -0.5) | < 0.001 | -0.4 (-0.7, 0) | 0.04 | -0.5 (-0.8, -0.2) | 0.004 | |
| T3 | -0.7 (-0.9, -0.4) | < 0.001 | -0.8 (-1.3, -0.2) | 0.003 | -0.3 (-0.5, -0.1) | 0.007 | |
| Vomiting | |||||||
| Gastric cancer (n = 28) | T1 | Reference | N/A | Reference | N/A | 0.2 (-0.3, 0.7) | 0.43 |
| T2 | -0.3 (-0.7, 0) | 0.04 | 0.1 (-0.2, 0.3) | 1.00 | -0.2 (-0.7, 0.2) | 0.35 | |
| T3 | -0.5 (-1.0, -0.1) | 0.02 | -0.2 (-0.6, 0.2) | 0.71 | -0.1 (-0.3, 0) | 0.13 | |
| Colon cancer (n = 36) | T1 | Reference | N/A | Reference | N/A | -0.2 (-0.5, 0.2) | 0.37 |
| T2 | -0.1 (-0.3, 0.1) | 0.067 | -0.2 (-0.4, 0.1) | 0.10 | -0.1 (-0.3, 0.1) | 0.44 | |
| T3 | -0.2 (-0.4, 0.1) | 0.11 | -0.3 (-0.7, 0.1) | 0.15 | 0 (0, 0) | 1.00 | |
| Fatigue | |||||||
| Gastric cancer (n = 28) | T1 | Reference | N/A | Reference | N/A | -0.5 (-1.1, 0) | 0.06 |
| T2 | -0.5 (-1.0, 0.1) | 0.07 | -0.1 (-0.5, 0.2) | 1.00 | -0.9 (-1.3, -0.5) | < 0.001 | |
| T3 | -0.8 (-1.2, -0.5) | < 0.001 | -0.8 (-1.1, -0.5) | < 0.001 | -0.5 (-0.9, -0.2) | 0.003 | |
| Colon cancer (n = 36) | T1 | Reference | N/A | Reference | N/A | -0.3 (-0.7, 0.2) | 0.28 |
| T2 | -0.9 (-1.2, -0.6) | < 0.001 | -0.3 (-0.5, 0) | 0.06 | -0.9 (0.4, 1.4) | < 0.001 | |
| T3 | -1.3 (-1.6, -1.0) | < 0.001 | -0.6 (-0.9, -0.3) | < 0.001 | -1.0 (-1.4, -0.6) | < 0.001 | |
| Measure | Parameters | B | 95%CI lower | 95%CI upper | P value |
| Abdominal distension | (group = 1) × (time = 3) | -0.8 | -1.3 | -0.4 | < 0.001 |
| (group = 1) × (time = 2) | -0.6 | -1.0 | -0.3 | < 0.001 | |
| Nausea | (group = 1) × (time = 3) | -0.5 | -0.9 | -0.1 | 0.02 |
| (group = 1) × (time = 2) | -0.7 | -0.9 | -0.4 | 0.001 | |
| Vomiting | (group = 1) × (time = 3) | 0.1 | -0.3 | 0.3 | 0.83 |
| (group = 1) × (time = 2) | -0.1 | -0.3 | 0.1 | 0.22 | |
| Fatigue | (group = 1) × (time = 3) | -0.4 | -0.7 | -0.2 | 0.003 |
| (group = 1) × (time = 2) | -0.6 | -0.9 | -0.3 | < 0.001 |
In the analysis of the merged sample, nausea scores at T3 were significantly lower than those at T1 in both the intervention and control groups (P < 0.05) (Table 4). As shown in Table 5, in the subgroup analysis of patients with gastric cancer, there was no significant difference in nausea scores in the control group at the three time points (P > 0.05). The nausea scores at T3 and T2 were significantly lower than those at T1 in the intervention group (P < 0.05). In the subgroup analysis of colon cancer, the nausea scores at T3 and T2 were significantly lower than those at T1 in both the intervention and control groups (P < 0.05). In both the merged sample and subgroup analyses, no significant differences were noted in nausea scores between the two groups at T1 (P > 0.05), and the nausea scores were significantly lower in the intervention group than in the control group at both T2 and T3 (P < 0.05). As shown in Table 6, comparison of the T2-T1 nausea score differences between the intervention and control groups revealed a difference of -0.7 (P = 0.001), which suggested that from T1 to T2, the intervention group achieved significantly better relief from nausea than the control group did. In addition, comparison of the T3-T1 nausea score differences between the intervention and control groups revealed a difference of -0.5 (P = 0.019), suggesting that from T1 to T3, the intervention group had a significantly greater reduction in nausea compared with the control group. A line graph was generated to visualize the changing trend of the patients’ nausea scores (Figure 2B).
As shown in Tables 4 and 5, in both the merged sample and subgroup analyses of patients with gastric cancer, no significant difference was noted in vomiting scores across all the three time points in the control group (P > 0.05). Additionally, the vomiting scores at T3 were significantly lower than those at T1 in the intervention group (P < 0.05). The subgroup analysis of patients with colon cancer showed no significant differences in the change in vomiting scores at the three time points between the control and intervention groups (P > 0.05). In addition, in the merged sample and subgroup analyses, no significant differences were found in vomiting scores between the two groups at all three time points (P > 0.05). As shown in Table 6, there were no significant differences in the T2-T1 vomiting score differences, nor in the T3-T1 vomiting score differences between groups (P > 0.05). A line graph was drawn to intuitively display the trends in the patients’ vomiting scores (Figure 2C).
As shown in Table 4, in the analysis of the merged sample, the fatigue scores at T3 were significantly lower than those at T1 in both the control and intervention groups (P < 0.001). In addition, the fatigue scores at T2 and T3 were significantly lower in the intervention group than those in the control group (P < 0.001). The subgroup analysis of gastric and colon cancer showed the same results as the analysis of the merged sample (Table 5). As shown in Table 6, comparisons of the T2-T1 and T3-T1 fatigue score differences between the intervention and control groups revealed differences of -0.6 (P < 0.001) and -0.4 (P = 0.003), respectively, indicating that from T1 to T2 and from T1 to T3, respectively, the intervention group achieved significantly more relief from fatigue than the control group did. A line graph was generated to visually reflect this trend in the patients’ fatigue scores (Figure 2D).
As the most common complication following abdominal surgery, postoperative GI dysfunction comprises a series of signs and symptoms caused by impaired recovery of the postoperative GI function[21]. This study demonstrated that INT at the patients’ Yuan-source, Luo-connecting, and He-sea points could decrease their time to first postoperative flatus passage, oral feeding, and defecation and alleviate their symptoms, including abdominal distension, nausea, and fatigue 48 h and 72 h after surgery. Nonetheless, the effect of INT on vomiting remains unclear and requires further investigation in future research. According to the TCM theory, both the spleen and stomach are contained in the middle Jiao and are very closely related. The stomach is responsible for receiving victuals, while the spleen dominates their transportation and transformation. These two organs collaborate to digest, absorb, and transport food and drink. The spleen is one of the five Zang organs responsible for transmitting essential substances to other vital organs via a process known as ascending lucidity, while the stomach and intestines are Fu organs that send digested food and wastes downward, which is referred to as descending turbidity. The liver controls the rise and free flow of Qi; thus, it can regulate the activities of Qi in the spleen and stomach. If the liver Qi is regulated and functions in harmony, then Qi movement in the spleen and stomach will be smooth, as will the processes of ascending lucidity and descending turbidity, ensuring that the GI function remains normal. In patients with GI cancers, Qi stagnation and blood stasis are considered to be the result of the long-term GI tract occupation by tumors before surgery, and the damaged meridian-collateral system and largely consumed Qi and blood due to anesthesia and/or surgical procedures. Therefore, the patient’s spleen and stomach are not properly nourished, causing the spleen to lose its healthy transporting function, the stomach to lose its ability to move digested food and wastes, and the liver to lose its Qi regulative capability. Consequently, the spleen and stomach cannot fulfill their normal functions of ascending lucidity and descending turbidity, the flow of Qi in the patient’s body is blocked, and the GI function is inhibited. A previous study[22] investigated the TCM syndrome elements after GI surgery using factor analysis and suggested that lesions in these patients are located in the spleen, stomach, and intestine and are characterized by several deficiency syndromes, including Qi deficiency, spleen deficiency, and liver deficiency, and are accompanied by pathological products, such as blood stasis, phlegm dampness, Qi stagnation, or reversed Qi flow. Moreover, they exhibited the pathological features of Qi deficiency in the spleen, as well as the accumulation of phlegm and phlegm-stagnation-derived toxins. In other words, it is an intermingled condition of deficiency accompanied by excessiveness, wherein the healthy Qi is asthenic, and the pathogenic factors are sthenic. Thus, the major goals of treatment are to strengthen the body resistance, consolidate the constitution, regulate the Qi flow, ascend lucidity, and descend turbidity.
LR-3, the Yuan-source point on the liver channel of the Foot Jueyin, is mainly responsible for regulating blood and descending turbidity. It can also clear liver-fire, drain dampness-heat of the lower Jiao, and disperse stagnated liver Qi. LI-4, the Yuan-source point on the large intestine channel of the Hand Yangming, is mainly responsible for regulating Qi and ascending lucidity. It also has the functions of clearing the GI tract and regulating its Qi flow. These two acupoints are highly compatible, and they represent Yin and Yang, Zang and Fu, and blood and Qi. These acupoints are interdependent and mutually utilized. In combination, they can coordinate the ascending and descending processes, dredge meridians, and reconcile Qi and blood flows. PC-6, the Luo-connecting point on the pericardium channel of the Hand Jueyin, enables communication between the pericardium and San Jiao meridian, and connects to the Yin Linking vessel. It can regulate Qi flows and is mainly used to treat symptoms caused by the imbalance of the ascending and descending Qi flows in the middle Jiao. Studies have shown that acupuncture of PC-6 can prevent postoperative nausea and vomiting[23,24]. SP-4, the Luo-connecting point on the spleen meridian of Foot Taiyin, supports the communication between the spleen and stomach meridians and connects to the thoroughfare vessel. It can regulate Qi and blood, harmonizing the thoroughfare and conception vessels, while supporting the spleen and stomach functions[25]. Both PC-6 and SP-4 are connected to the stomach and intestines, and when they are both treated, they can exert spasmolytic and analgesic effects, strengthen the spleen, and regulate the stomach. The addition of treatments at the Luo-connecting points enhances the therapeutic effect of the Yuan-source points. This combined therapeutic strategy treats both the cause and the symptoms of the disease and harmonizes the Zang and Fu organs, alleviating the GI symptoms.
The Yangming meridian contains abundant Qi and blood, and it has the function of stimulating the Qi movement of the spleen and stomach. The lower-He-sea points of the stomach, large intestine, and small intestine are all located on the stomach channel of the Foot Yangming. ST-36 is a lower-He-sea point of the stomach that regulates the spleen and stomach, clears and activates the meridian-collateral system, strengthens the spleen, and removes dampness. It is a common acupoint used for treating GI diseases. A previous study reported that acupuncture at ST-36 could increase the expression levels of angiotensin and nitric oxide and regulate the activity of endogenous opioid peptides to enhance GI motility[26]. Moreover, acupuncture at ST-36 can further increase the plasma motilin level while reducing the postoperative plasma gastrin level, which contributes to decreasing the gastric emptying time and accelerating intestinal movement[27,28]. ST-37 and ST-39 are the lower-He-sea points of the large and small intestines, respectively. They can regulate the Qi of the intestines to unblock the intestines and help them to descend turbidity. In conclusion, the combined treatment of the Yuan-source, Luo-connecting, and He-sea points in this study generated a synergistic effect by regulating Zang and Fu organs simultaneously and treating symptoms and root causes concurrently. Therefore, the combined treatment of these acupoints could achieve the goals of tonifying the spleen and stomach, unblocking and nourishing the intestines, soothing the liver, and regulating the Qi movement. A previous study suggested that acupuncture at the acupoints on the limbs promotes GI motility, while acupuncture at abdominal acupoints inhibits GI motility[29]. Similarly, in this study, we successfully stimulated GI motility and promoted the recovery of GI function in patients by embedding the acupuncture needles at acupoints in their limbs. Following GI surgery, patients had syndromes of intermingled asthenia and sthenia, with asthenia being the predominant syndrome. In INT, very fine needles are applied to stimulate acupoints continuously and gently. Therefore, INT can repel the pathogenic factors without harming the vital Qi, enhancing the efficacy of treating Qi deficiency, and making it more beneficial to postoperative patients.
In this randomized controlled trial based on the TCM theories, we innovatively selected distal acupoints of the corresponding meridian (specifically, acupoints on the stomach, large intestine, liver, and spleen channels) as INT treatment sites and elucidated the effects on GI function in patients following surgery for GI tumors. We provided potential explanations for understanding how this approach can promote rapid recovery in patients after surgery. However, this study has limitations. First, the small sample size must be acknowledged. Although we conducted a subgroup analysis based on the type of cancer, the reliability of the subgroup analysis results might be limited by the relatively insufficient sizes of each of the subgroups. Therefore, further studies focusing on a specific type of cancer should be conducted to validate the findings. Second, self-reported indicators were used as the outcomes in this study, and this could have resulted in self-reported bias. In future studies, the effect of INT at the Yuan-source, Luo-connecting, and He-sea points will be further explored using larger sample sizes and collecting more objective outcome measures.
INT at the Yuan-source, Luo-connecting, and He-sea points can promote recovery of GI function and alleviate GI symptoms in patients following surgical resection of GI tumors. This technique is easy to perform and can be implemented by nurses in clinical practices. We provide preliminary evidence to support the integration of this intervention into the postoperative care of patients with gastrointestinal tumors to promote rapid recovery.
Postoperative gastrointestinal function recovery is considered critical to the rapid rehabilitation of patients who have undergone gastrointestinal tumor resection. However, active intervention to promote the rapid recovery of gastrointestinal function after surgery remains insufficient. Therefore, finding an effective method to promote postoperative gastrointestinal function recovery is essential.
Traditional Chinese medicine offers considerable advantages for the treatment of gastrointestinal diseases. Acupuncture based on Traditional Chinese medicine theories is widely applied to postoperative patients. However, compared to traditional acupuncture, intradermal needle therapy (INT) is easier to perform and less painful for patients.
We selected distal acupoints of the corresponding meridian (specifically, acupoints on the stomach, large intestine, liver, and spleen channels) as intradermal needle therapy treatment sites and aimed to elucidate the effect of this treatment in a cohort of gastrointestinal tumor patients.
In this randomized controlled trial, patients diagnosed with gastrointestinal cancer were randomly allocated to either the intervention or the control group. Participants in the control group received enhanced recovery care, while those in the intervention group received enhanced recovery care combined with INT at the Yuan-source, Luo-connecting, and He-sea points.
INT at the Yuan-source, Luo-connecting, and He-sea points decreased patients’ time to the first postoperative flatus passage, oral feeding, and defecation, and alleviated their symptoms, including abdominal distension, nausea, and fatigue 48 h and 72 h after surgery.
INT at the Yuan-source, Luo-connecting, and He-sea points can promote recovery of gastrointestinal function and ease gastrointestinal symptoms in patients following surgical resection of gastrointestinal tumors.
This study provided preliminary evidence to support the integration of this intervention into the postoperative care of patients with gastrointestinal tumors to promote rapid recovery. In future studies, the effect of INT at the Yuan-source, Luo-connecting, and He-sea points needs to be further explored by focusing on a specific tumor type and using larger sample sizes.
The authors thank all the participants in this study and are grateful to Professor Zhang YP for assistance conducting and designing the study.
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 68648] [Article Influence: 13729.6] [Reference Citation Analysis (201)] |
| 2. | Venara A, Neunlist M, Slim K, Barbieux J, Colas PA, Hamy A, Meurette G. Postoperative ileus: Pathophysiology, incidence, and prevention. J Visc Surg. 2016;153:439-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 216] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 3. | Long AJ, Burton PR, De Veer MJ, Ooi GJ, Laurie CP, Nottle PD, Watt MJ, Brown WA. Radical gastric cancer surgery results in widespread upregulation of pro-tumourigenic intraperitoneal cytokines. ANZ J Surg. 2018;88:E370-E376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 4. | Miedema BW, Johnson JO. Methods for decreasing postoperative gut dysmotility. Lancet Oncol. 2003;4:365-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 113] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 5. | Carroll J, Alavi K. Pathogenesis and management of postoperative ileus. Clin Colon Rectal Surg. 2009;22:47-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 42] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 6. | Mortensen K, Nilsson M, Slim K, Schäfer M, Mariette C, Braga M, Carli F, Demartines N, Griffin SM, Lassen K; Enhanced Recovery After Surgery (ERAS®) Group. Consensus guidelines for enhanced recovery after gastrectomy: Enhanced Recovery After Surgery (ERAS®) Society recommendations. Br J Surg. 2014;101:1209-1229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 386] [Cited by in RCA: 547] [Article Influence: 45.6] [Reference Citation Analysis (0)] |
| 7. | Gustafsson UO, Scott MJ, Hubner M, Nygren J, Demartines N, Francis N, Rockall TA, Young-Fadok TM, Hill AG, Soop M, de Boer HD, Urman RD, Chang GJ, Fichera A, Kessler H, Grass F, Whang EE, Fawcett WJ, Carli F, Lobo DN, Rollins KE, Balfour A, Baldini G, Riedel B, Ljungqvist O. Guidelines for Perioperative Care in Elective Colorectal Surgery: Enhanced Recovery After Surgery (ERAS®) Society Recommendations: 2018. World J Surg. 2019;43:659-695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1071] [Cited by in RCA: 1388] [Article Influence: 198.3] [Reference Citation Analysis (0)] |
| 8. | Wu F, Liu W, Feng H, Long L, Hou L, Hou C. Application of Traditional Chinese Medicines in Postoperative Abdominal Adhesion. Evid Based Complement Alternat Med. 2020;2020:8073467. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 9. | Cao LX, Chen ZQ, Jiang Z, Chen QC, Fan XH, Xia SJ, Lin JX, Gan HC, Wang T, Huang YX. Rapid rehabilitation technique with integrated traditional Chinese and Western medicine promotes postoperative gastrointestinal function recovery. World J Gastroenterol. 2020;26:3271-3282. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 22] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 10. | Dai L, Zhou WJ, Zhong LLD, Tang XD, Ji G. Chinese medicine formulas for nonalcoholic fatty liver disease: Overview of systematic reviews. World J Clin Cases. 2021;9:102-117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 4] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 11. | Yang L, Li Z, Li W, Zeng L, Bian Y. Effects of moxibustion on gastrointestinal function recovery in preventing early postoperative small-bowel obstruction: a meta-analysis. Ann Palliat Med. 2021;10:3988-3999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 12. | Shah S, Godhardt L, Spofford C. Acupuncture and Postoperative Pain Reduction. Curr Pain Headache Rep. 2022;26:453-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 45] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 13. | Zhang W, Zhang H, Wang SM, Guo J, Ma Y, Li Y, Su F, Chi Y. Perioperative Acupuncture Optimizes Surgical Outcomes: Theory, Clinical Practice and Future Perspectives. Am J Chin Med. 2022;50:961-978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 14. | Wang L. Acupuncture and Moxibustion. Higher Education Press, 2016. [RCA] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 15. | He GL, Gong XZ, He JL, Yang Q, Mai JY, Wu SN, Guo QH. Evaluation of the efficacy and safety of intradermal needle therapy on the sleep quality of patients following laparoscopic hysterectomy: study protocol for a randomized controlled trial. Ann Transl Med. 2022;10:808. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 16. | Yan R, Zhang Y, Lim J, Yang F, Zhou L, Lyu D, Wang Y, Zou Y, Li Z. The effect and biomechanical mechanisms of intradermal needle for post-stroke hemiplegia recovery: Study protocol for a randomized controlled pilot trial. Medicine (Baltimore). 2018;97:e0448. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 17. | Ding X. Intradermal needle therapy and its application in treating gastric diseases. J Acupunct Tuina Sci. 2012;10:120-124. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 18. | Zhang X, Gu H, Shi Z, Li X. Effects of intradermal needle therapy plus pinaverium bromide on gastrointestinal hormone levels in irritable bowel syndrome-diarrhea patients. J Acupunct Tuina Sci. 2020;18:431-437. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 19. | Jung SY, Chae HD, Kang UR, Kwak MA, Kim IH. Effect of Acupuncture on Postoperative Ileus after Distal Gastrectomy for Gastric Cancer. J Gastric Cancer. 2017;17:11-20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 20. | Luo H, Li H, Wang Y, Yao S, Xu W. Clinical practice guidelines for treating headache with Traditional Chinese Medicine: quality assessment with the appraisal of guidelines for research and evaluation Ⅱ instrument. J Tradit Chin Med. 2018;38:339-350. [PubMed] |
| 21. | Mazzotta E, Villalobos-Hernandez EC, Fiorda-Diaz J, Harzman A, Christofi FL. Postoperative Ileus and Postoperative Gastrointestinal Tract Dysfunction: Pathogenic Mechanisms and Novel Treatment Strategies Beyond Colorectal Enhanced Recovery After Surgery Protocols. Front Pharmacol. 2020;11:583422. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 95] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 22. | Jiao J, Li DF, Fan JL, HE PL, Cai JL, Tang JY, Hu Y. The Factor Analysis on Postoperative Gastric Cancer Syndromes Elements. Guiding Journal of TCM. 2014;20:30-33. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 23. | Frey UH, Funk M, Löhlein C, Peters J. Effect of P6 acustimulation on post-operative nausea and vomiting in patients undergoing a laparoscopic cholecystectomy. Acta Anaesthesiol Scand. 2009;53:1341-1347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 24. | Lee A, Chan SK, Fan LT. Stimulation of the wrist acupuncture point PC6 for preventing postoperative nausea and vomiting. Cochrane Database Syst Rev. 2015;CD003281. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 111] [Cited by in RCA: 92] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 25. | Zhang PM, Zhang W, Tan ZG, Tang YN, Liu XJ, Pan T, Xiao D, Xie ZR, Chen DZ. [Application of special acupoints for chronic gastritis in ancient literature of acupuncture and moxibustion]. Zhongguo Zhen Jiu. 2020;40:1018-1023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 26. | Shen GM, Zhou MQ, Xu GS, Xu Y, Yin G. Role of vasoactive intestinal peptide and nitric oxide in the modulation of electroacupucture on gastric motility in stressed rats. World J Gastroenterol. 2006;12:6156-6160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 16] [Cited by in RCA: 22] [Article Influence: 1.1] [Reference Citation Analysis (1)] |
| 27. | Hou L, Xu L, Shi Y, Gu F. Effect of electric acupoint stimulation on gastrointestinal hormones and motility among geriatric postoperative patients with gastrointestinal tumors. J Tradit Chin Med. 2016;36:450-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 28. | Zhou D, Hu B, He S, Li X, Gong H, Li F, Wang Q. Transcutaneous Electrical Acupoint Stimulation Accelerates the Recovery of Gastrointestinal Function after Cesarean Section: A Randomized Controlled Trial. Evid Based Complement Alternat Med. 2018;2018:7341920. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 29. | Takahashi T. Acupuncture for functional gastrointestinal disorders. J Gastroenterol. 2006;41:408-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 168] [Article Influence: 8.4] [Reference Citation Analysis (1)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: Shaanxi Province Nursing Association, Shaanxi Integrated Traditional Chinese and Western Medicine Association.
Specialty type: Surgery
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Gupta N, India; Liu YW, China; Teragawa H, Japan S-Editor: Xing YX L-Editor: A P-Editor: Xing YX